Abstract
Nonerythroid α-spectrin (αIISp) is a structural protein involved in repair of DNA interstrand cross-links and is deficient in cells from patients with Fanconi anemia (FA), which are defective in ability to repair cross-links. In order to further demonstrate the importance of the role that αIISp plays in normal human cells and in the repair defect in FA, αIISp was knocked down in normal cells using siRNA. Depletion of αIISp in normal cells by siRNA resulted in chromosomal instability and cellular hypersensitivity to DNA interstrand cross-linking agents. An increased number of chromosomal aberrations were observed and, following treatment with a DNA interstrand cross-linking agent, mitomycin C, cells showed decreased cell growth and survival and decreased formation of damage induced αIISp and XPF nuclear foci. Thus depletion of αIISp in normal cells leads to a number of defects observed in FA cells, such as chromosome instability and a deficiency in cross-link repair.
Keywords: α-spectrin, siRNA knockdown, Chromosomal aberrations, DNA interstrand crosslinks, Damage-induced nuclear foci, XPF, DNA repair, Fanconi anemia
Spectrin is a structural protein, which in the cytoplasm of nonerythroid cells plays an important role in providing mechanical support for the cell membrane and is also involved in protein sorting, organelle and vesicle trafficking, neurite outgrowth and neurotransmitter release, as well as cell growth and differentiation [1-4]. We have demonstrated that nonerythroid α-spectrin (αIISp) is present in mammalian cell nuclei and is involved in repair of DNA interstrand cross-links [5-7]. It preferentially binds to purified DNA containing an interstrand cross-link; it co-localizes with the cross-link repair protein, XPF, and the Fanconi anemia protein, FANCA, in cross-link induced nuclear foci, and it is needed for the production of incisions produced by XPF at the site of an interstrand cross-link [5-7].
We have shown that there is a deficiency in αIISp in cells from patients with Fanconi anemia (FA), a genetic disorder characterized by bone marrow failure, diverse congenital abnormalities, genomic instability and a marked predisposition to develop cancer [5,7-10]. Cells from patients with FA have a defect in ability to repair DNA interstrand cross-links [10-16] and this defect correlates with reduced levels of αIISp in these cells [5,14,16]. Based on these results, we have proposed a model for the role of αIISp in DNA repair. In this model, αIISp binds to DNA at sites of damage and acts as a scaffold to aid in the recruitment of repair proteins to these sites, thus enhancing the efficiency of the repair process [5-7,17]. In FA cells, decreased levels of αIISp leads to reduced binding of αIISp to damaged DNA and decreased recruitment of repair proteins to sites of damage, thus leading to decreased levels of DNA repair.
In order to further demonstrate the importance of the role that αIISp plays in normal human cells and in the repair defect in FA cells, studies were carried out to determine the effects of knocking down αIISp in normal cells by siRNA. The results showed that depletion of αIISp in normal cells by siRNA led to increased chromosomal instability, as ascertained by an increase in chromosomal aberrations/breaks. Damaging these cells with two different DNA interstrand cross-linking agents, mitomycin C (MMC) or 8-methoxypsoralen (8-MOP) plus UVA light, resulted in decreased cell survival and decreased formation of damage induced nuclear foci of proteins involved in DNA interstrand cross-link repair. Thus knocking down of αIISp in normal human cells leads to a number of the defects observed in FA cells, including chromosome instability and a deficiency in cross-link repair.
Materials and methods
Cell culture
Normal human lymphoblastoid cells (GM3299) (Coriell Institute for Medical Research) and FA complementation group A (FA-A) lymphoblastoid cells (HSC 72) (a gift from Dr. Manuel Buchwald, Toronto, Canada) were grown in RPMI 1640 medium (Hyclone) as previously described [5,7]. HeLa S3 cells (ATCC) were grown in F12K medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (Hyclone).
siRNA analysis
siRNA against the N-terminal of αIISp, nucleotides 224-252, (AAGATTCCTATCGATTCCAGTCCTGTCTC) was purchased from Dharmacon and a control non-silencing siRNA was from Qiagen. Normal human lymphoblastoid cells were transfected with αIISp siRNA or non-silencing siRNA using Lipofectamine 2000 Transfection Reagent (Invitrogen) following the manufacturer's instructions. The siRNAs and Lipofectamine were combined in serum-free RPMI 1640 media and the serum levels were adjusted 4 hours post transfection to normal culture levels. HeLa cell transfections were carried out as above using Oligofectamine Transfection Reagent (Invitrogen). Cell survival was assessed using trypan blue exclusion. Cells were harvested at 24 and 48 hours after transfection and whole cell protein lysates prepared by resuspending the cells in Laemmli Sample Buffer plus β-mecaptoethanol (BioRad). Ciells were viewed using a Leitz DMRB microscope (Leica) equipped with a DEI-750 analog camera (Optronics). Images were obtained using Image Pro-Plus 6.0 software (Media Cybernetics).
αIISp mRNA levels
Total RNA was extracted, as previously described [18], from siRNA transfected normal lymphoblastoid cells using the RNeasy Mini Kit (Qiagen). RT-PCR was performed and the RT-PCR products were resolved by electrophoresis on 8% polyacrylamide gels, which were stained with Syber Green (Sigma-Aldrich) and scanned using a FluorImager (Molecular Dynamics).
Immunoblotting
For analysis of levels of αIISp in the siRNA transfected cells, whole cell lysates were subjected to SDS-PAGE and western blot analysis carried out as previously described [5,6]. Immunoblots were probed with anti-α spectrin (mAb 1622, Chemicon) or anti-tubulin (Santa Cruz Biotechnology). Immunoblots were developed using Pierce Ultra chemiluminescent substrate and exposed to X-ray film. Images were scanned and quantitation of band intensity was done using ImageQuant software (Molecular Dynamics).
Chromosome analysis
Normal lymphoblastoid cells transfected with either αIISp or nontarget siRNA were incubated for 46 hours at 37°C, 5% CO2. Colcemid (Sigma-Aldrich) (0.1 μg per ml) was added and incubation continued for 2 hours. The cells were harvested, swollen in hypotonic solution (0.075 M KCl) and fixed in 3:1 methanol/acetic acid. Slides were prepared and stained with either Wright's or Giemsa stain. At least 100 metaphases from either αIISp or nontarget siRNA transfected cells were scored for chromosomal abnormalities. Metaphases were viewed and images obtained as above.
Treatment of cells with DNA interstrand cross-linking agents and cell growth analysis
Normal lymphoblastoid cells transfected with either αIISp or nontarget siRNA were treated with MMC (0-500 nM) 24 hours after transfection. Cell growth was assessed at 24 hours after MMC treatment using trypan blue exclusion by comparing the number of live cells for each siRNA treatment to the original number of undamaged nontarget siRNA treated cells. In other experiments, these cells were treated with 3.5 μM 8-MOP (Sigma-Aldrich) plus two dosages of UVA light, as previously described [7], and cell survival assessed 16 hours post damage.
Indirect immunofluorescence
Normal lymphoblastoid cells transfected with either αIISp or nontarget siRNA were treated with 400 nM MMC or 3.5 μM 8-MOP plus UVA light 24 hours after transfection. The cells were harvested 16 hours post damage and examined for nuclear localization of αIISp and XPF using indirect immunofluorescence as previously described [7]. Primary antibodies used were anti-α spectrin or anti-XPF (sc-10161, Santa Cruz Biotech). Secondary antibodies were Alexafluor 488 goat anti-mouse IgG conjugate or Alexafluor 594 rabbit anti-goat IgG conjugate (Molecular Probes). Stained cells were viewed with a Leitz DMRB microscope at 440× and images were captured using a DEI-750 analog camera [6]. Images were imported into a computerized imaging system using Image Pro-Plus 6.0 software and Adobe Photoshop CS.
Results
Transfection of cells with αIISp siRNA leads to reduction in expression of αIISp and decreased cell survival
Transient transfection of normal lymphoblastoid cells with αIISp siRNA led to a reduction in levels of αIISp. Immunoblot analysis showed that levels of αIISp were slightly decreased 24 hours after transfection with 25 pmol of siRNA and by 48 hours levels of αIISp were 40% of those in cells transfected with a nontarget siRNA (Figs. 1A and B). A similar reduction in levels of αIISp (36% of controls) was observed in HeLa cells 48 hours after transfection with 30 pmol of αIISp siRNA (Fig. 1C); cell survival in these cells was approximately 85% (Fig. 1D). Increasing the levels of αIISp siRNA above 30 pmol led to a dramatic decrease in cell survival 48 hours after transfection (Fig. 1D), which correlated with decreasing levels of αIISp in these cells (Fig. 1E). Reducing levels of αIISp in the cells had no effect on the levels of FANCA (Fig. 1E). At 90 pmol αIISp siRNA, levels of αIISp in HeLa cells were totally depleted (data not shown) and there was no cell survival (Fig. 1D). In contrast, when HeLa cells were transfected with 90 pmol of nontarget siRNA, 84% of the cells survived (Fig. 1D). A similar correlation between decreasing levels of αIISp and cell survival was seen in normal lymphoblastoid cells (data not shown). Thus αIISp is essential for cell survival. For this reason, additional studies on these transfected cells examined cells 48 hours post transfection with 25-30 pmol of αIISp siRNA.
Fig. 1.

Knocking down of αIISp in normal lymphoblastoid and HeLa cells by siRNA. (A) Immunoblot analysis of normal lymphoblastoid cells 24 and 48 hr after transfection with 25 pmol of αIISp siRNA or nontarget (Nt) siRNA. Blots were probed with anti-α spectrin. Tubulin was used as a loading control. (B) Immunoblots were scanned and levels of αIISp quantitated. (C) Immunoblot analysis of HeLa cells 24 and 48 hr after transfection with 30 pmol of αIISp or Nt siRNA. (D) HeLa cell survival 48 hours after transfection with increasing concentrations of αIISp or Nt siRNA. (E) Immunoblot of effect in HeLa cells of increasing concentrations of αIISp siRNA on levels of αIISp 48 hours post transfection. FANCA was used as a loading control. (F) RT-PCR analysis of total RNA extracted from normal lymphoblastoid cells 48 hours after transfection with 25 pmole αIISp or Nt siRNA. Vertical lines represent ± SEM.
Levels of αIISp mRNA in the αIISp siRNA transfected cells were also decreased. RT-PCR analysis of total RNA from normal lymphoblastoid cells 48 hours post transfection with 25 pmol of αIISp siRNA showed that levels of αIISp mRNA were 38% of those of the nontarget siRNA transfected cells (Fig. 1F).
Transfection of cells with αIISp siRNA leads to changes in cell morphology
Morphological changes were observed in the αIISp siRNA transfected normal lymphoblastoid cells. Normal lymphoblastoid cells in culture are very pleiomorphic, with numerous pseudopodia (Fig. 2A). However, 48 hours after αIISp siRNA transfection, these cells appeared rounder and many were smaller with few if any pseudopodia (Fig. 2A). This change correlated with decreased levels of αIISp in these cells (Fig. 2B). These αIISp siRNA transfected normal cells morphologically resembled FA-A lymphoblastoid cells, which are typically round and smaller than normal cells (Fig. 2A). The reduction of levels of αIISp in FA-A cells (35% of normal) (Fig. 2C) are similar to those observed in the αIISp siRNA transfected normal cells (Fig. 2B).
Fig. 2.

Cellular morphology of αIISp siRNA transfected normal lymphoblastoid cells compared to FA-A lymphoblastoid cells. (A) Microscopic examination of normal cells 48 hours after transfection with 25 pmoles αIISp or Nt siRNA. This is compared to FA-A cells in culture. (B) Western blot analysis of levels of αIISp in the Nt and αIISp siRNA transfected normal cells shown in A. (C) Western blot analysis of the levels of αIISp in the FA cells shown in A compared to normal cells.
Chromosomal abnormalities occur in normal cells transfected with αIISp siRNA
Metaphase spreads from normal lymphoblastoid cells were examined for chromosomal aberrations 48 hours after transfection with αIISp siRNA. The number of metaphases in these cells showing intrachromatid exchanges, radials/fusions and breaks was significantly greater than in the nontarget siRNA transfected cells (Fig. 3A and B). When the chromatid interchange figures (exchanges and radials/fusions) were converted into breaks, where one interchange was counted as one break [19], the total number of chromatid breaks was approximately 5 fold greater than in the nontarget siRNA transfected cells (Fig. 3B), which was a significant difference (P<0.01). After damage with MMC (120 nM), the αIISp siRNA transfected cells showed a small (10%) increase in chromosomal aberrations, compared to nontarget siRNA transfected cells (data not shown).
Fig. 3.
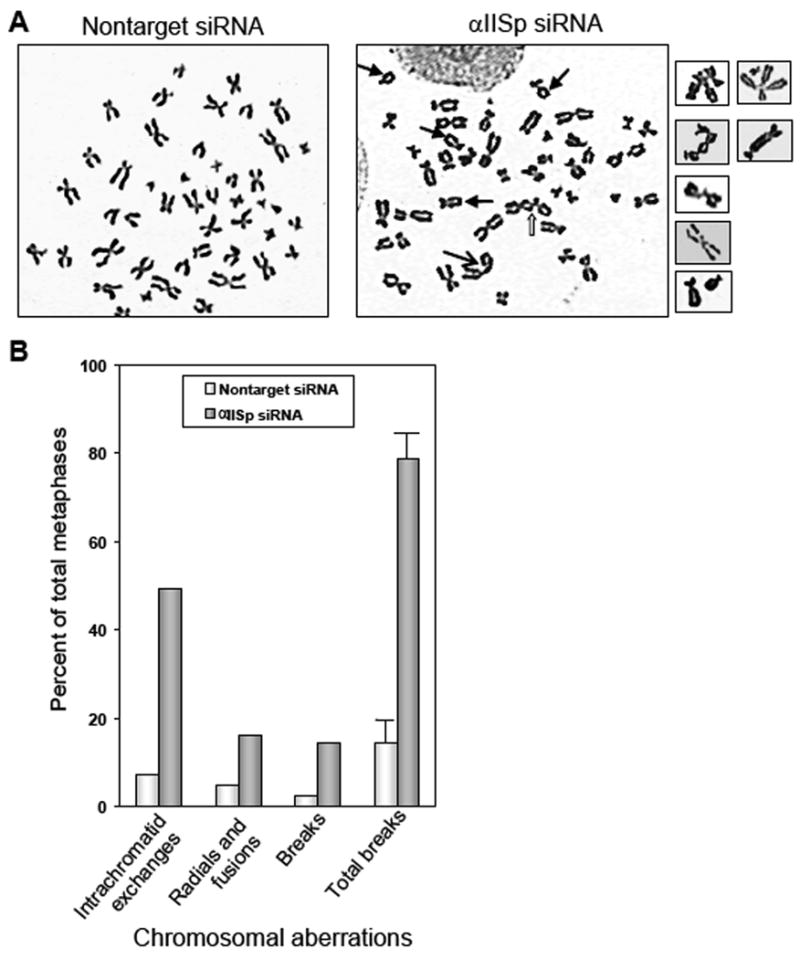
Analysis of chromosomal aberrations in normal lymphoblastoid cells transfected with αIISp siRNA. (A) Metaphase spreads from normal cells were examined for chromosomal aberrations 48 hours after transfection with 25 pmoles of αIISp or nontarget siRNA. Arrows indicate interchromatid exchanges (
 ), radials (
), radials (
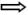 ) and breaks (→). (B) 100 metaphase spreads were scored for intrachromatid exchanges, radials/fusions and breaks. Chromatid interchange figures were converted into breaks, where one interchange was counted as one break, to give the total number of chromatid breaks. Vertical lines represent ± SEM.
) and breaks (→). (B) 100 metaphase spreads were scored for intrachromatid exchanges, radials/fusions and breaks. Chromatid interchange figures were converted into breaks, where one interchange was counted as one break, to give the total number of chromatid breaks. Vertical lines represent ± SEM.
Knockdown of αSpII in normal cells leads to increased sensitivity to DNA interstrand cross-linking agents
Knockdown of αIISp in normal cells led to a distinct hypersensitivity of these cells to DNA interstrand cross-linking agents. A marked decrease in cell growth was observed 24 hours after addition of MMC which was dependent upon the concentration of MMC used (Fig. 4A). At 500 nM MMC, the growth of the αIISp siRNA transfected cells was only approximately 50% of that observed for the nontarget siRNA transfected cells.
Fig. 4.

Effects of mitomycin C on normal lymphoblastoid cells transfected with αIISp siRNA. (A) Cell growth was examined in normal cells 24 hours after treatment with varying concentrations of MMC (48 hours post transfection with αIISp or nontarget siRNA). (B) Normal cells were examined for αIISp or XPF nuclear foci 16 hours treatment with 400 nM MMC (40 hours after transfection with αIISp (+) or nontarget (-) siRNA) using indirect immunoflourescence and staining with anti-α-spectrin or anti-XPF. Vertical lines represent ± SEM.
Knockdown of αIISp in normal cells also influenced cross-link induced nuclear foci formation. In nondamaged, nontarget transfected cells, both αIISp and XPF were present in the nucleus in a diffuse pattern (Fig. 4B). 16 hours after treatment of the nontarget siRNA transfected cells with MMC (400 nM), damage-induced αIISp and XPF nuclear foci were observed (Fig. 4B). These results are similar to those we described in non siRNA transfected lymphoblastoid cells in which we showed that 16 hours after treatment with a DNA interstrand cross-linking agent, there is a peak in formation of damage-induced αIISp and XPF nuclear foci [7]. In contrast, in undamaged αIISp siRNA transfected cells the levels of αIISp but not XPF were significantly reduced (Fig. 4B), indicating that knockdown of αIISp does not affect the levels of XPF in these cells. 16 hours after treatment of the αIISp siRNA transfected cells with MMC (400 nM) there was a marked reduction in damage-induced αIISp and XPF nuclear foci with only a few foci observed (Fig. 4B). However, levels of XPF were not reduced in these cells (Fig. 4B).
Similar results were obtained for normal lymphoblastoid cells transfected with αIISp siRNA and treated with 8-MOP plus UVA light. 16 hours after treatment of the αIISp siRNA transfected cells with 8-MOP plus UVA, levels of αIISp were markedly reduced (Supplementary Fig. 1A). Survival of the αIISp siRNA transfected cells after damage was approximately 45% of that observed for the nontarget transfected cells (Supplementary Fig.1B). A marked reduction in αIISp nuclear foci was also observed in αIISp siRNA transfected cells 16 hours after treatment with 8-MOP plus UVA light (Supplementary Fig 1C).
Discussion
We have previously shown that αIISp is present in the nucleus of normal human cells where it plays an important role in repair of DNA interstrand cross-links and that there is a deficiency in αIISp in FA cells which correlates with their cross-link repair defect [5,7,14,16]. The present studies showed that complete knockdown of αIISp is lethal, in keeping with reports that αIISp is an essential protein in the cell [20,21]. Therefore, in the present study levels of siRNA knockdown of αIISp were adjusted so that they were approximately 35-40% of normal, similar to those found in FA cells [5,14,16].
The present results showed that decreased expression of αIISp in normal cells by αIISp siRNA led to a number of changes in cellular morphology, chromosomal stability and response to DNA damage. Morphologically the cells, normally very pleomorphic with numerous pseudopodia, became rounder with few pseudopodia and more closely resembled FA-A cells in culture. This could indicate that αIISp, which is also present in the cytoplasm, is important for structural support of the cell membrane and contributes to the normal morphology of the cell. Spectrin has been shown in nonerythroid mammalian cells to be important in membrane structure and integrity and cell shape [1,2,22].
The present results also show that αIISp is important for chromosomal stability. Reduction in levels of αIISp to 35-40% of normal by siRNA led to a marked (5 fold) increase in chromosomal aberrations, which included intrachromatid exchanges, radials, fusions and breaks. This increased level is similar to or slightly higher than the spontaneous level of chromosomal aberrations reported for FA-A and other FA cell lines [19,23]. Spontaneous chromosomal aberrations/breaks are characteristic of FA cells and thought to be an important determinant in the associated chromosomal instability associated with this disorder [19,23,24]. Based on the present results, it is possible to speculate that the spontaneous chromosomal aberrations/breaks that occur in FA cells could in part be due to their deficiency in αIISp. In normal cells in which αIISp has been knocked down, these chromosomal aberrations increased after exposure to MMC, just as they do in FA cells [19,23]. Though this increase was not as great as in FA cells, this may be attributable to the increased sensitivity of the αIISp siRNA transfected cells to MMC.
We have previously shown that reduced levels of αIISp in FA cells are not due to reduced expression of this protein but rather to decreased stability [18]. We have proposed that one or more of the FA proteins is important in maintaining the stability of αIISp in cells [17,18]. In support of this, we have shown that the FA protein, FANCG, binds directly to αIISp, specifically to the SH3 domain of αIISp [18]. This binding could be important for maintaining the stability of αIISp [17]. Since FA genes are proposed to be involved in maintenance of genomic stability [8,23,24], it is possible that this may in part be through the potential role of one or more of the FA proteins in maintaining the stability of αIISp in the cell, which in turn is important for chromosomal stability.
We have also shown that αIISp plays a role in repair of DNA interstrand cross-links where it co-localizes in damage-induced nuclear foci with the cross-link repair protein, XPF, along with FANCA [5-7]. The present study shows that knockdown of αIISp in normal cells leads to decreased formation of αIISp and XPF nuclear foci after cross-link damage, even though levels of XPF in these cells are normal. This finding is similar to our demonstration that in FA-A cells, where levels of αIISp are similar to those in αIISp knocked-down cells, there is a reduction in damage-induced nuclear foci containing both αIISp and XPF, even though levels of XPF in these cells are normal [7]. The present results also show that the reduced formation of XPF foci in the damaged αIISp siRNA treated cells is not due to decreased levels of FANCA. Thus, in both FA-A cells and αIISp siRNA transfected normal cells, reduced formation of damage-induced XPF foci is due to a deficiency in αIISp in these cells. These results are in agreement with our model that αIISp acts as a scaffold in the nucleus to aid in the recruitment of repair proteins, such as XPF/ERCC1, to sites of damage, thus enhancing the efficiency of the repair process [6,7,17]. In FA cells [5,14,16], or normal cells in which αIISp has been knocked down, the reduced levels of αIISp lead to decreased levels of DNA repair.
Based on the present findings of development of chromosomal aberrations/breaks in cells in which αIISp has been knocked down, this model can now be extended to include a role for αIISp in maintaining genomic stability. Thus in FA cells, a deficiency in αIISp may not only lead to a defect in DNA repair but also contribute to the marked chromosomal instability associated with this disorder. Maintaining the stability of αIISp in the cell could thus be a critical factor in maintance of chromosomal stability and, as our recent studies suggest, FA proteins may play an important role in this process [17].
Supplementary Material
Acknowledgments
This research was supported by NIH Grant R01 HL054860 (to M.W.L.). J.A.L. is a recipient of a New Jersey Commission on Cancer Predoctoral Fellowship
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the on line version, at.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodman SR, Zimmer WE, Clark MB, Zagon IS, Barker JE, Bloom ML. Brain spectrin: of mice and men. Brain Res Bull. 1995;36:593–606. doi: 10.1016/0361-9230(94)00264-2. [DOI] [PubMed] [Google Scholar]
- 2.De Matteis MA, Morrow JS. Spectrin tethers and mesh in the biosynthetic pathway. J Cell Sci. 2000;113:2331–2343. doi: 10.1242/jcs.113.13.2331. [DOI] [PubMed] [Google Scholar]
- 3.Gascard P, Mohandas N. New insights into functions of erythroid proteins in nonerythroid cells. Current Opin Hematol. 2000;7:123–129. doi: 10.1097/00062752-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Bennet V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 5.McMahon LM, Walsh CE, Lambert MW. Human alpha spectrin II and the Fanconi anemia proteins FANCA and FANCC interact to form a nuclear complex. J Biol Chem. 1999;274:32904–32908. doi: 10.1074/jbc.274.46.32904. [DOI] [PubMed] [Google Scholar]
- 6.McMahon LW, Sangerman J, Goodman SR, Kumaresan K, Lambert MW. Human alpha spectrin II and the FANCA, FANCC and FANCG proteins bind to DNA containing psoralen interstrand cross-links. Biochemistry. 2001;40:7025–7034. doi: 10.1021/bi002917g. [DOI] [PubMed] [Google Scholar]
- 7.Sridharan D, Brown M, Lambert WC, McMahon LW, Lambert MW. Nonerythroid αII spectrin is required for recruitment of FANCA and XPF to nuclear foci induced by DNA interstrand cross-links. J Cell Sci. 2003;116:823–835. doi: 10.1242/jcs.00294. [DOI] [PubMed] [Google Scholar]
- 8.Joenje H, Patel KJ. The emerging genetic and molecular basis of Fanconi anaemia. Nat Rev Genet. 2001;2:446–457. doi: 10.1038/35076590. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy RD, D'Andrea AD. The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes Dev. 2005;19:2925–2940. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi T, D'Andrea AD. Molecular pathogenesis of Fanconi anemia: recent progress. Blood. 2006;107:4223–4233. doi: 10.1182/blood-2005-10-4240. [DOI] [PubMed] [Google Scholar]
- 11.Papadopoulo D, Averbeck D, Moustacchi E. The fate of 8-methoxypsoralen-photoinduced DNA interstrand crosslinks in Fanconi's anemia cells of defined genetic complementation groups. Mutat Res. 1987;184:271–280. doi: 10.1016/0167-8817(87)90026-5. [DOI] [PubMed] [Google Scholar]
- 12.Lambert MW, Tsongalis GJ, Lambert WC, Hang B, Parrish DD. Defective DNA endonuclease activities in Fanconi's anemia cells, complementation groups A and B. Mutat Res. 1992;273:57–71. doi: 10.1016/0921-8777(92)90050-d. [DOI] [PubMed] [Google Scholar]
- 13.Lambert MW, Tsongalis GJ, Lambert WC, Parrish DD. Correction of the DNA repair defect in Fanconi anemia complementation groups A and D cells. Biochem Biophys Res Commun. 1997;230:587–591. doi: 10.1006/bbrc.1996.6008. [DOI] [PubMed] [Google Scholar]
- 14.Kumaresan KR, Lambert MW. Fanconi anemia, complementation group A, cells are defective in ability to produce incisions at sites of psoralen interstrand cross-links. Carcinogenesis. 2000;21:741–751. doi: 10.1093/carcin/21.4.741. [DOI] [PubMed] [Google Scholar]
- 15.Niedernhofer LJ, Lalai AS, Hoeijmakers JH. Fanconi anemia (cross)linked to DNA repair. Cell. 2005;123:1191–1198. doi: 10.1016/j.cell.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Kumaresan KR, Sridharan DM, McMahon LW, Lambert MW. Deficiency in incisions produced by XPF at the site of a DNA interstrand cross-link in Fanconi anemia cells. Biochemistry. 2007;46:14359–14368. doi: 10.1021/bi7015958. [DOI] [PubMed] [Google Scholar]
- 17.Lefferts JA, Wang C, Sridharan D, Baralt M, Lambert MW. The SH3 domain of αII spectrin is a target for the Fanconi anemia protein, FANCG. Biochemistry. 2009;48:254–263. doi: 10.1021/bi801483u. [DOI] [PubMed] [Google Scholar]
- 18.Lefferts JA, Lambert MW. Fanconi anemia cell lines deficient in αII spectrin express normal levels of αII spectrin mRNA. Biochem Biophys Res Commun. 2003;307:510–515. doi: 10.1016/s0006-291x(03)01213-0. [DOI] [PubMed] [Google Scholar]
- 19.Godthelp BC, van Buul PPW, Jaspers NGJ, Elghalbzouri-Maghrini E, van Duijn-Goedhart A, Arwert F, Joenje H, Zdzienicka MZ. Cellular characterization of cells from Fanconi anemia complementation group, FA-D1/BRCA2. Mutat Res. 2006;601:191–201. doi: 10.1016/j.mrfmmm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Lee JK, Coyne RS, Dubreuil RR, Goldstein LS, Branton D. Cell shape and interaction defects in alpha-spectrin mutants of Drosophila melanogaster. J Cell Biol. 1993;123:1797–1809. doi: 10.1083/jcb.123.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norman KR, Moerman DG. α Spectrin is essential for morphogenesis and body wall muscle formation in Caenorhabditis elegans. J Cell Biol. 2002;157:665–677. doi: 10.1083/jcb.200111051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metral S, Machnicka B, Bigot S, Colin Y, Dhermy D, Lecomte MC. αII-Spectrin is critical for cell adhesion and cell cycle. J Biol Chem. 2009;284:2409–2418. doi: 10.1074/jbc.M801324200. [DOI] [PubMed] [Google Scholar]
- 23.Howlett NG, Taniguchi T, Durkin SG, D'Andrea AD, Glover TW. The Fanconi anemia pathway is required for the DNA replication stress response and for the regulation of common fragile site stability. Human Mol Genet. 2005;14:693–701. doi: 10.1093/hmg/ddi065. [DOI] [PubMed] [Google Scholar]
- 24.D'Andrea AD, Grompe M. The Fanconi anaemia BRCA pathway. Nat Rev Cancer. 2003;3:23–34. doi: 10.1038/nrc970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


