Abstract
Objective
Inwardly-rectifying K+ (Kir) channels are responsible for maintaining membrane potentials in a variety of cell types including endothelial cells where they modulate endothelium-dependent vasorelaxation. The goal of this study is to determine the functional expression of Kir channels in porcine bone marrow-derived side population (BM-SP) cells that demonstrate phenotypes of endothelial progenitor cells (EPCs). We further asses the hypercholesterolemia sensitivity of Kir channels in BM-SP cells, which may play a key role in hypercholesterolemia-mediated regulation of EPCs.
Methods
To assess the effect of hypercholesterolemia on Kir channels in BM-SP, Kir currents were recorded in SP cells sorted from the bone marrow of healthy or hypercholesterolemic animals.
Results
We found Kir channels constitute the major conductance in porcine bone marrow-derived side population (BM-SP) cells. These cells are defined by their efficiency of Hoechst dye efflux and have been reported to differentiate into multiple cell lineages including endothelium in vivo. We demonstrate here that porcine BM-SP cells differentiate to an endothelial lineage (CD31+, vWF+) supporting the hypothesis that these cells are endothelial progenitor cells. Also, BM-SP cells express Kir with biophysical properties recapitulating those in mature endothelial cells, but with a much higher current density. Flow cytometric (FACS) analysis indicated that the number of SP cells was unaffected by hypercholesterolemia. However, hypercholesterolemia significantly inhibited Kir channels in BM-SP cells.
Conclusions
We successfully demonstrate that BM side population cells represent an origin of endothelial progenitor cells. This study further shows, for the fist time, that the functional expression of Kir channels in bone marrow (BM) -derived SP. Moreover, we demonstrate that hypercholesterolemia condition significantly suppresses the Kir channels in BM-SP cells, suggesting that hypercholesterolemia-mediated regulation of Kir channels may be an important factor not only in dysfunction of mature endothelium but also in dysfunction of BM-SP cells.
Keywords: progenitor cells, endothelium, potassium channels, cholesterol, lipoproteins, flow, vasodilatation
INTRODUCTION
Endothelial dysfunction occurs early and throughout the pathogenic course of atherosclerosis[1, 2]. A growing number of studies suggest that maintenance of the endothelium and repair involves recruitment of bone marrow-derived endothelial progenitor cells (EPCs). Supporting this concept is the finding that different subsets of CD34+ hematopoietic progenitor cells are capable of differentiating into endothelial cells in vitro [3–5] and incorporating into the sites of neovascularization in vivo[3, 6, 7]. Also, the number of circulating EPCs is reduced in patients with coronary artery disease (CAD) [8]and in patients with elevated serum cholesterol, hypertension, and diabetes [9] indicating a dynamic interaction between to bone marrow and endothelium. It is important to note that when these three cardiovascular risk factors were adjusted for the age of subjects, hypercholesterolemia was the most highly associated factor[9]. Conversely, a decrease in serum cholesterol by HMG-CoA Reductase inhibitors (statins) increased the number of circulating EPCs [8, 10] and facilitated re-endothelization of balloon-injured arterial segments in rats[10].
Atherosclerosis is associated not only with a reduction in number of EPCs, but also with impairment of EPC function. For example, Vasa et al showed that EPCs isolated from CAD patients display an impaired migratory response[8]. Furthermore, Rauscher and colleagues demonstrated that transfusion of bone marrow stem cells from young non-atherosclerotic ApoE−/− mice prevents lesion progression in Apo E−/− recipients, while cells derived from older mice with manifest atherosclerosis did not[11]. Atherosclerosis progression is associated with a loss of the EPC repair capacity[12]. Little is known, however, about the mechanisms responsible for the functional impairment of EPCs in atherosclerosis. Our previous studies showed that hypercholesterolemia strongly suppresses inwardly-rectifying K+ channels (Kir) in aortic endothelial cells in vitro [13, 14 and in vivo[14]. Other reports indicate that suppression of Kir channels in human hematopoietic progenitor cells impairs expansion and differentiation of these cells[15, 16]. These observations are consistent with the general role of K+ channels in control of cellular proliferation and differentiation (reviewed by[17, 18]).
The goal of this study is to determine whether K+ channels are expressed in bone marrow-derived EPCs and whether the activity of K+ channels in these cells is suppressed by hypercholesterolemia. We utilize a porcine model of atherosclerosis because porcine lipoprotein profiles are similar to those in humans [19] and because hypercholesterolemic pigs develop atherosclerotic lesion within a few months after the initiation of a high-cholesterol diet[20]. However, there are limited surface markers for porcine progenitor cells. Therefore, we isolated side population (SP) cells which include a subpopulation of EPCs [21]. These SP cells are lineage marker-negative[22], [21]. In vitro culture studies have shown that rhesus SP cells are highly enriched for long-term culture-initiating cells (LTC-ICs), an indicator of primitive hematopoietic cells. Also, SP cells can incorporate into the arterial wall where they display the characteristics of endothelial cells[21].
In this study, we demonstrate that similar to mature aortic endothelial cells, potassium membrane conductance of SP cells freshly derived from porcine bone marrow (BM) is dominated by strongly-rectifying Kir channels. We also show that the functional expression of Kir channels in bone marrow (BM) -derived SP cells is significantly higher than that in mature endothelial cells isolated from the aorta. Furthermore, differentiation of BM-SPs into EC-like cells in vitro is accompanied by the partial loss of the Kir current. Finally, we show that Kir current in BM-SPs is markedly suppressed in cells isolated from animals with diet-induced hypercholesterolemia. These data demonstrate that diet-induced hypercholesterolemia impairs the functional expression of Kir channels in SPs while they still reside within the bone marrow. We suggest that suppression of BM-SPs Kir contributes to the functional deficiency of EPCs in atherosclerosis.
MATERIALS and METHODS
Isolation of Bone Marrow Cells
Castrated male Yorkshire pigs 27–32 kg in weight were randomized into two groups of hypercholesterolemia (n=4) and control (n=6). Hypercholesterolemia was induced by administration of atherogenic diet (0.5% cholesterol, 10% lard, and 1.5% sodium cholate) for 3–6 months. Control group received standard chow diet. A lipid profile was assessed at baseline, monthly thereafter, and prior to euthanasia on all animals. The study was approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. A sternal bone marrow aspirate was performed under sterile conditions on a series of two groups of pigs (hypercholesterolemic or control); aspirate (5–25 mL) was collected in heparinized tubes and immediately processed for flow cytometric analysis and cell sorting. The aspirate was washed with Hanks Balanced Salt Solution (HBSS) containing 2% fetal bovine serum and 10 mM HEPES buffer solution and then filtered through a 70 μm mesh filter. The aspirate was then centrifuged for 10 minutes at 1000 RPM and the supernatant aspirated. Ammonium chloride lysis was performed on the remaining pellet to remove red blood cells. Once completely lysed, the pellet was resuspended in HBSS and washed twice. The final (white) pellet was then resuspended in 10–30 mL of HBSS and the cells counted.
Hoechst Staining of Bone Marrow Cells
Cells were aliquoted into tubes of 2E6 cells and incubated in Hoechst solution (5 ug/mL) for two hours at 37°C, following a protocol similar to that used by Goodell et al[22]. As a control, select tubes of cells were incubated in a 200 μM solution of Verapamil for 7–10 minutes prior to incubation with Hoechst. After the two hour incubation with Hoechst, cells were centrifuged, the pellets combined and resuspended in 2 μg/mL propidium iodide (PI) solution at a concentration of 20E6 cells/mL and kept on ice. Typically 2–6 samples of 40E6 cells were analyzed and sorted.
Sorting of BM-SP Cells
Using a Becton-Dickinson FACSDiVA cell sorter, viable (as assessed by PI exclusion), low to medium side scatter singlets were analyzed for BM-SP cells, or efflux of the Hoechst dye, and sorted for in vitro culture studies. Singlets were gated as the prominent cluster of cells identified from a plot of side scatter width versus forward scatter width to ensure that cell aggregates were excluded from analysis. Cells incubated with Verapamil prior to Hoechst staining were used as controls, to confirm the location and presence of SP cells.
Cell Culture and Immunostaining
Side population cells were plated and cultured in M-199 media containing 20% calf serum, 1% L-glutamine, 0.5% pen/strep, and 0.1mg/ml heparin for one week before electrophysiological recordings or in vitro expansion. After a week of culture in growth factor-free media, adherent cells were recultured in M-199 media containing 0.03 mg/ml lyophilized endothelial cell growth supplement (Sigma) for 2 days before electrophysiological recordings or immunostainings. Expanded cells were immuostained with EC-specific anti-platelet endothelial cell adhesion molecule 1 (anti-PECAM-1) and anti-von Willebrand factor (anti-vWF) antibodies as well as smooth muscle cell-specific anti-α-actin antibody (α-SMA) [23, 24].
Electrophysiology
Ionic currents of cells were measured using standard voltage-clamp techniques, as previously described[24]. Whole-cell currents were recorded during 500-ms linear voltage ramps from −160 to +60 mV at an interpulse interval of 3s. The external solution for voltage-clamp experiments contained (in mM) 156 KCl, 10 HEPES, 1.5 CaCl2, 1 MgCl2 and 1 EGTA, pH 7.3. The low K+ external solution contained (in mM) 150 NaCl, 6 KCl, 10 HEPES, 1.5 CaCl2, 1 MgCl2 and 1 EGTA, pH 7.3. The pipette contained (in mM) 145 KCl, 10 HEPES, 1 MgCl2, 1 EGTA, and 4 ATP, pH 7.3 in all experiments. The recordings were performed at high (156 mM) extracellular K+ to increase the amplitude of the currents[25]; as expected under this condition, all currents had a reversal potential at ~ −2mV. We showed earlier that cholesterol sensitivity of Kir is not affected by changes in the extracellular K+[14].
RESULTS
In Vitro Expansion and Differentiation of Porcine SP Cells
To identify SP cells in the porcine bone marrow, whole bone marrow specimens extracted from castrated male Yorkshire pigs were incubated with Hoechst 33342 dye. Hoechst 33342 staining, when observed simultaneously at red and blue emission wavelengths, demonstrated a complex fluorescence-emission pattern indicative of cycling cell populations. A distinct population of cells with dim Hoechst staining (SP cells) was consistently observed in all experiments (data not shown). We further demonstrated that the SP population signal was specifically eliminated in the presence of the drug verapamil as previously published by Goodell et al[26]. Verapamil-mediated ablation of porcine SP cells suggested that the low Hoechst staining was due to high activity of membrane efflux pumps of the ATP-binding cassette (ABC) transporter superfamily, a common feature across SP cells derived from hematopoietic and non-hematopoietic tissues[22, 27]. The verapamil-sensitive SP subpopulation represented ~0.05% of the total viable cell content of porcine bone marrow, consistent with previous observations in the murine model[22, 27].
As shown in Figure 1Ai, after cultured in basal media without growth factors for 7 days, adherent porcine SP cells assume a sphere-shaped morphology; however, cell type-specific phenotypes such as endothelial cells, smooth muscle cells or neurons were not observed in culture. After 7 days of culture in growth factor-free media, non-adherent cells were removed and adherent SP cells were recultured in media supplemented with Endothelial Cell Growth Factor (ECGF). After 2 days of culture in growth factor-containing media, adherent SP cells demonstrated an elongated spindle-like shape, as shown in Figure 1Aii. The cell lineage of the spindle-like cells was defined by staining these cells with antibodies for the endothelial cell markers platelet-endothelial cell adhesion molecule (PECAM) and von Willebrand factor (vWF) (Figure 1B), as well as the smooth muscle cell-specific marker, smooth muscle α-actin (Figure 1C). Positive staining for PECAM and wWF was observed along with the absence of smooth muscle α-actin staining indicating that the SP cells differentiated into cells with endothelial characteristics. These data are consistent with a previous study showing SP cells displayed the characteristics of differentiated endothelial cells in vivo[21]. Indeed, we believe this is the first study to demonstrate in vitro differentiation of BM-SP cells into endothelial cells or endothelial-like cells.
Figure 1.
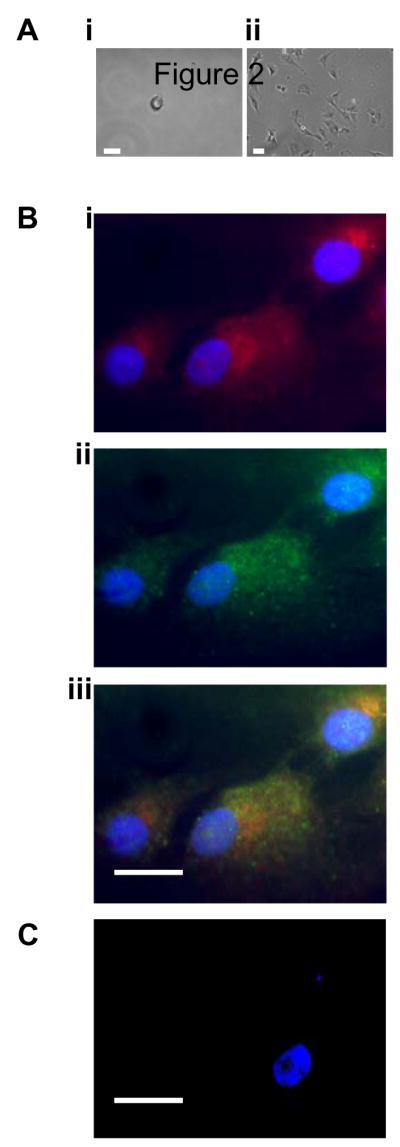
In vitro expansion and differentiation of porcine SP cells. (A) Bright-field microscopic picture of porcine bone marrow SP cells cultured in basal media for 7 days (i) or SP cells with extended culture in growth factor-containing media (ii). (B) Immunofluorescence imaging of expanded SP cells (cells shown in Figure 2Aii) stained with vWF (i), CD31 (ii), or vWF/CD31 (iii). (C) Immunofluorescence imaging of expanded SP cells stained with smooth muscle α-actin. The nuclei of the cells were stained purple by Hoechst throughout the immunostained images. Bar = 30 μm.
Functional Expression of Kir Channels in Porcine SP Cells
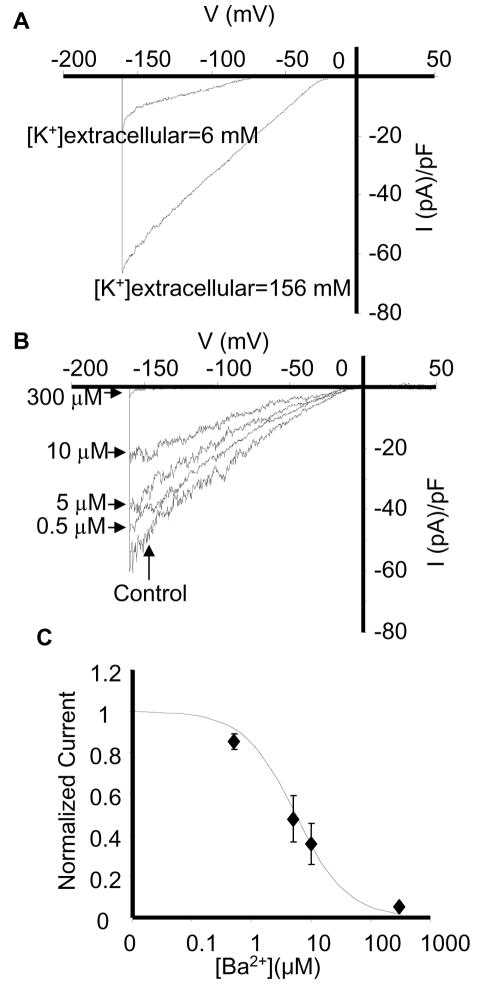
To examine the functional expression of potassium channels in porcine SP cells, the cells were isolated form the bone marrow, sorted and then maintained for 7–14 days in M-199 medium supplemented with 20% fetal bovine serum and the currents were recorded on days 7 and 14 (a minimum of 7 days was required for the cells to attach preventing the recordings to be made at earlier time points, no difference was observed between the days 7 and 14). Under resting conditions, all SP cells showed a pronounced inwardly-rectifying K+ current (Figure 2A) with the current-voltage (I-V) relationship similar to the I-V relationship of currents carried by Kir2.x subunits in porcine aortic endothelial cells, human aortic endothelial cells[24], and CHO cells over-expressing Kir2.x channels[28]. The reversal potential of the current was −79 ± 2 mV and −2 ± 1 mV for low and high extracellular K+ recording solutions respectively, close to the theoretical reversal potentials of a K+-specific current under these recording conditions (−80 and −2 mV). Thus, shift in the reversal potential of the current was in agreement with the reversal potential for potassium ions (EK), theoretically predicted by the Nernst equation[29]. Furthermore, we demonstrated that BM-SP K+ currents were sensitive to extracellular Ba2+ with a calculated IC50 of 5.4 μM Ba2+, which is virtually identical to the IC50 of the Ba2+ block (3.42 μM) of Kir2 channels underlying K+ conductance in mature aortic endothelial cells (Figure 2B&C)[24]. Taken together, these data suggest that the current is carried by the channels belonging to the Kir2 family of inward rectifiers. This is the first study to describe functional expression of Kir channels in SP stem cells.
Figure 2.
Basic properties and Ba2+ sensitivity of endogenous Kir current in porcine BM-SP cells. (A) Comparison of Kir currents recorded at low (6 mM) and high (156 mM) extracellular [K+] solutions in the same cell. Note a shift in the reversal potential from −79 to −2 mV. (B) Kir current recorded in a single SP cell at 0, 0.5, 5, 10, and 300μM extracellular Ba2+. (C) Concentration dependence of Ba2+ block. Fractional block was determined at −100 mV and calculated as (Icontrol − IBa2+)/Icontrol, where Icontrol is the current recorded before application of Ba2+ and IBa2+ is the current recorded at a specific Ba2+ concentration. Each point is the mean ± SE (n = 5). The data were fitted with the function IBa2+/Icontrol = 1/(1 + [Ba2+]/Kd) where Kd is the dissociation constant.
Decline of Kir Channel Activities in Differentiated Porcine BM-SP Cells
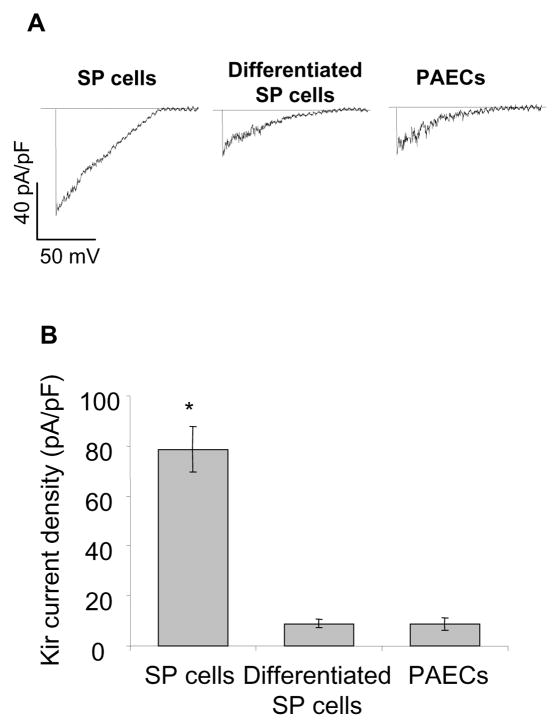
It is important to note that the current density of Kir in BM-SP cells observed in this study is several-fold higher than the currents described previously in low-passage or freshly isolated mature aortic endothelial cells[14, 24]. The average Kir density in BM-SP at −155 mV, 156 mM extracellular K+ was ~80 pA/pF, whereas the currents in mature aortic ECs ranged between 10 and 20 pA/pF under the same experimental conditions. We therefore examined whether inducing the differentiation of the SP cells by exposing them to the endothelial growth factors, as described above, is sufficient to decrease Kir current density. As shown in Figure 3, upon the differentiation, Kir current density dropped from 79±9 pA/pF to 9±2 pA/pF. Furthermore, we observed that Kir density in BM-SPs that underwent differentiation into endothelial-like spindle-shaped cells was nearly identical to Kir currents recorded from mature aortic endothelial isolated from the same animals.
Figure 3.
Descent of Kir current in differentiated porcine BM-SP cells. (A) Representative recordings of Kir currents in porcine BM-SP cells, expanded SP cells, and freshly-isolated porcine aortic endothelial cells. (B) Average Kir peak current densities (−155 mV) recorded from the same populations (BM-SP cells, n=35; expanded SP cells, n=7; porcine aortic endothelial cells, n=25).
Hypercholesterolemia has no Effect on the Number of BM-SP Cells but Induces Suppression of Kir Channels in BM-SP Cells
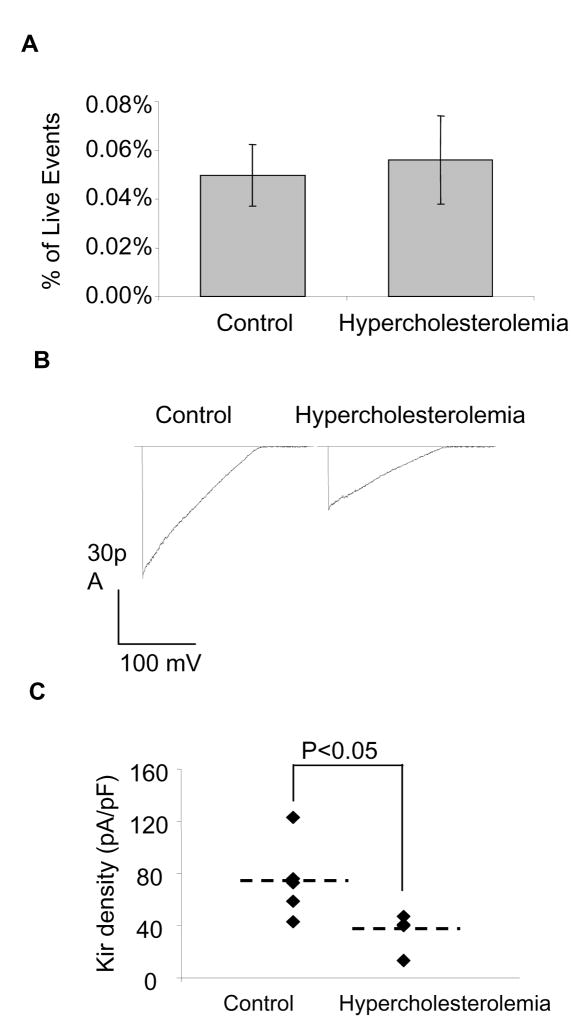
Since the association between risk factors for atherosclerosis and low circulating levels of BM-derived EPCs has been established[9], we examined the hypothesis that hypercholesterolemic animals exhibit lower levels of BM-derived side population cells, which in turn, leads to lower numbers of circulating EPC. However, no significant differences were observed in the number of SP cells (0.02–0.1% of viable cells) collected for hypercholesterolemic and control pigs (Figure 4A) suggesting that hypercholesterolemia did not affect the number of bone marrow-derived SP cells. Our earlier studies have shown that endothelial Kir current is suppressed by an increase in cellular cholesterol concentration [13] and diet-induced hypercholesterolemia suppressed Kir currents in porcine aortic endothelial cells in a model of atherosclerosis[14]. To determine whether diet-induced hypercholesteromia modulated Kir currents in BM-SP cells, pigs were maintained on a high-cholesterol diet resulting in significant elevations in total plasma and LDL cholesterol and evidence of pre-clinical endothelial dysfunction accessed by loss of flow-induced vasodilatation[14]. BM-SP cells were isolated from control and hypercholesteromic pigs as described above and maintained in media. As shown in Figure 4, hypercholestemia inhibited Kir currents in BM-SPs isolated from both control and hypercholesterolemic pigs. Thus, hypercholesterolemia-induced suppression of the Kir current in BM-derived SP cells develops in vivo. Furthermore, these observations indicate that hypercholesterolemia-induced impairment of endothelial function is not limited to vascular or even circulating endothelial cells, but may occur directly within the bone marrow.
Figure 4.
Suppression of Kir channels in porcine BM-SP cells by hypercholesterolemia. (A) The percentage of side population cells in porcine bone marrow cells in control (n=3) and hypercholesterolemia (n=4) animals. (B) Representative recordings of Kir currents in BM-SP cells from control and hypercholesterolemic pigs. (C) Kir peak current densities (−155 mV) recorded from the same populations (control: 6 animals, 7–10 cells per animal; hypercholesterolemia: 4 animals, 7 to 10 cells per animal). Mean values of each group are indicated by dashed lines.
DISCUSSION
Identification of BM-SP Cell Derived Endothelial Cells
Side population cells are a rare cell population characterized by their typical low-stained fluorescence profiles of Hoechst red and Hoechst blue [22, 27, 30]. Goodell et al first described the capacity of BM-SP cells to repopulate the bone marrow in a lethally-irradiated murine model[22]. They further described the cardiomyogenic potential of BM-SP cells, showing that these cells displayed the characteristics of differentiated cardiomyocytes and endothelial cells in cardiac muscle and vessel structures[21]. However, the in vitro differentiation of SP cells into cells with cardiomyocyte and endothelial characteristics was not described in their studies. The current study describes a rare cell population (~0.05% viable ells) with a typical SP phenotype from porcine bone marrow aspirations demonstrating, low Hoechst fluorescence intensity and verapamil sensitivity. Furthermore, we demonstrated that in endothelial growth factor-containing media, porcine BM-SP cells expanded and more importantly, began to express endothelial-specific markers (vWF/CD31), but not smooth muscle α-actin. The in vitro differentiation of BM-SP cells into a cell with an endothelial phenotype indicates that SP cells include a population of cells with the capacity to differentiate into bone marrow-derived EPCs. Thus, we conclude that BM-SP cells studied here include cells capable of differentiating into endothelial cells. It is important to note, however, that this cell population likely also includes progenitor cells capable of differentiating into other types of cells.
Expression of Kir channels in progenitor cells
Inwardly-rectifying K+ channels are known to be ubiquitously expressed in a variety of mammalian cells and to play critical roles in the maintenance of the membrane potential and K+ homeostasis[25, 29, 31]. However, expression of Kir channels in different populations of progenitor cells appears to be less universal. Significant Kir currents, identified as Kir1.1 and Kir4.3, were observed in primitive human hematopoietic progenitor cells isolated from the cord blood and characterized as CD34+/CD33− or CD34+/CD38-[15, 16]. Furthermore, treatment of these cells with stem cell factor and interleukin-3 enhanced the expression of Kir channels and promoted cell expansion into lineage-restricted precursors[15, 16]. Kir currents were also found in non-hemapotopoietic fraction of human umbilical cord blood that was expanded as a non-immortalized cell line and then further differentiated into neuron-like cells[32]. In contrast, several studies have shown that mesenchymal stem cells isolated form the bone marrow express multiple types of voltage-gated and Ca2+-sensitive K+ channels, but do not express functional Kir channels[33–35]. No Kir channels were also detected in pluripotent embryonic stem cells[36]. Our study is first to analyze functional expression of Kir channels in BM-SP cells. We show here that in contrast to BM-derived mesenchymal stem cells, BM-SP cells have strong Kir currents that clearly belong to the family of strong inward-rectifiers and dominate K+ conductance in BM-SP cells. More specifically, Ba2+ sensitivity of the BM-SP Kir currents was found to be virtually identical to that in mature endothelial cells, whose K+ conductance is regulated via Kir2.1 and Kir2.2 channels[24]. Interestingly, Kir 2.2 channel mRNA is expressed in mesenchymal stem cells, but, as described above, the channels appear to be non-functional[33]. We have not observed any voltage-gated K+ channels in the BM-SP cell population. Thus, the pattern of K+ channel expression observed in BM-SP cells appears to differ significantly from the patterns described previously in different types of progenitor cells. We suggest, therefore, that expression of Kir channels may be one of the functional identifiers of BM-SP cell population.
Transient Nature of Kir Expression in BM-SP cells
In this study, we show that not only do BM-SP cells have pronounced Kir currents, but these currents are ~8 fold higher than the currents recorded in mature endothelial cells isolated from the same animals. Furthermore, inducing differentiation of BM-SP cells into endothelial-like cells down regulates Kir current to a level identical to that observed in mature endothelial cells. Interestingly, several earlier studies have demonstrated transient appearance of different types of K+ channels, including Kir, in differentiating cells during early embryonic development implicating K+ channels in the control of cell differentiation[37–40], as well as in adult differentiating cells[41, 42]. An increase in Kir expression was shown to be essential for stem cell factor (SCF)-dependent expansion of primitive human hematopoietic progenitor cells CD34+33− [15] and in the differentiation of an earlier and more quiescent population of primitive hematopoietic progenitor cells CD34+38− [16]. In this regard it is noteworthy that cell cycle progression is accompanied by changes in the expression of different types of K+ channels and that K+ channel blockers inhibit cell proliferation in a variety of cell types (reviewed by[17, 43]). However, the role of Kir channels in the regulation of cell proliferation is still controversial. While inhibition of Kir was shown to inhibit cell proliferation in human melanoma cell line[44], it was also shown to enhance proliferation of glial cells[41]. Indeed, dynamic regulation of Kir2 expression has been demonstrated to play a key role in myoblast differentiation and fusion by determining the cellular membrane potential and controlling the intracellular Ca2+ concentration[45]. We suggest, therefore, that the transient nature of Kir channel expression in BM-SP cells may play a critical role in regulating the differentiation of these cells. We also suggest that high levels of Kir expression may be important for the control of BM-SP proliferation.
KIR Channels and Vascular Function
Numerous studies have shown that endothelial K+ channels play an important role in endothelium-mediated regulation of vascular tone[46–51]. K+ channels set the negative resting membrane potential of endothelial cells, providing the driving force for Ca2+ influx and regulating Ca2+ dependent intracellular signaling[52]. Specifically, blocking K+ channels in aortic endothelial cells results in a decrease of Ca2+ influx, inhibition of bradykinin- and acetylcholine-induced release of nitric oxide (NO), a major vasorelaxing, anti-thrombotic, and anti-inflammatory factor, and a decrease in the release of the anticoagulant prostaglandin I2[46]. Our earlier studies have shown that hypercholesterolemia suppresses Kir channels in mature endothelial cells suggesting that inhibition of Kir may contribute to the endothelial dysfunction in early atherosclerosis[13, 14]. Our current study results indicate that hypercholesterolemia impairs Kir channel function of BM-SP cells suggesting that endothelial cell function may be impaired already in endothelial progenitor cells that still reside in the bone marrow before maturation and mobilization occurs. Given the importance of Kir channels in overall vascular function, these results suggest a new paradigm whereby cardiovascular risk factors may concomitantly affect a bone marrow insult contributing to systemic endothelial dysfunction.
Limitations and Future Directions
The current study is the initial description of functional expression and hypercholesterolemia sensitivity of inwardly-rectifying potassium channels in porcine BM side population cells. The significance and biological utility of Kir channels in bone marrow progenitor cells is unclear, but deserves more study given the questions raised from the current study. For example, how does hypercholesterolemia result in a decrease in the functional expression of Kir channels and do statin drugs reverse this effect? How does differential expression of Kir channels affect mobilization, differentiation, and homing of BM side population cells? The study of bone marrow progenitor cell Kir channels in normal and proatherosclerotic conditions will most likely result in a better understanding of the evolving importance of the bone marrow-systemic interaction on vascular function. The bone marrow appears an important sentinel for cardiovascular health and drug and lifestyle interventions that favorably affect bone marrow health may also reduce cardiovascular morbidity and mortality.
Acknowledgments
We thank Drs. Peter Davies and George Rothblat for multiple helpful discussions. This study was supported in part through grant from the National Institutes of Health HL073965 and HD045664 (I.L.) and an American Heart Association (AHA) predoctoral fellowship 0415409U (Y.F.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science. 1997;275:964–966. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 4.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MAS, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 5.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 6.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone Marrow Origin of Endothelial Progenitor Cells Responsible for Postnatal Vasculogenesis in Physiological and Pathological Neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 7.Urbich C, Dimmeler S. Endothelial Progenitor Cells: Functional Characterization. Trends in Cardiovascular Medicine. 2004;14:318. doi: 10.1016/j.tcm.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;6:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 9.Hill JM, Zalos G, Halcox JPJ, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 10.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin Therapy Accelerates Reendothelialization: A Novel Effect Involving Mobilization and Incorporation of Bone Marrow-Derived Endothelial Progenitor Cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 11.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, Progenitor Cell Exhaustion, and Atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 12.Karra R, Vemullapalli S, Dong C, Herderick EE, Song X, Slosek K, Nevins JR, West M, Goldschmidt-Clermont PJ, Seo D. Molecular evidence for arterial repair in atherosclerosis. PNAS. 2005;102:16789–16794. doi: 10.1073/pnas.0507718102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romanenko VG, Rothblat GH, Levitan I. Modulation of endothelial inward rectifier K+ current by optical isomers of cholesterol. Biophys J. 2002;83:3211–3222. doi: 10.1016/S0006-3495(02)75323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Y, Mohler ER, III, Hsieh E, Osman H, Hashemi SM, Davies PF, Rothblat GH, Wilensky RL, Levitan I. Hypercholesterolemia Suppresses Inwardly Rectifying K+ Channels in Aortic Endothelium In Vitro and In Vivo. Circ Res. 2006;98:1064–1071. doi: 10.1161/01.RES.0000218776.87842.43. [DOI] [PubMed] [Google Scholar]
- 15.Shirihai O, Merchav S, Attali B, Dagan D. K+ channel antisense oligodeoxynucleotides inhibit cytokine-induced expansion of human hemopoietic progenitors. Pflugers Arch. 1996;431:632–638. doi: 10.1007/BF02191913. [DOI] [PubMed] [Google Scholar]
- 16.Shirihai O, Attali B, Dagan D, Merchav S. Expression of two inward rectifier potassium channels is essential for differentiation of primitive human hematopoietic progenitor cells. Journal of Cellular Physiology. 1998;177:197–205. doi: 10.1002/(SICI)1097-4652(199811)177:2<197::AID-JCP1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 17.Wang Roles of K + channels in regulating tumour cell proliferation and apoptosis. Pflugers Archiv European Journal of Physiology. 2004;V448:274. doi: 10.1007/s00424-004-1258-5. [DOI] [PubMed] [Google Scholar]
- 18.Lang F, Foller M, Lang KS, Lang PA, Ritter M, Gulbins E, Vereninov A, Huber SM. Ion channels in cell proliferation and apoptotic cell death. J Membr Biol. 2005;205:147–157. doi: 10.1007/s00232-005-0780-5. [DOI] [PubMed] [Google Scholar]
- 19.Swinkels DW, Demacker PN. Comparative studies on the low density lipoprotein subfractions from pig and man. Comp Biochem Physiol B. 1988;90:297–300. doi: 10.1016/0305-0491(88)90076-4. [DOI] [PubMed] [Google Scholar]
- 20.de Smet BJ, van der Zande J, van der Helm YJ, Kuntz RE, Borst C, Post MJ. The atherosclerotic Yucatan animal model to study the arterial response after balloon angioplasty: the natural history of remodeling. Cardiovasc Res. 1998;39:224–232. doi: 10.1016/s0008-6363(98)00085-6. [DOI] [PubMed] [Google Scholar]
- 21.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell lMA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmons CA, Zilberberg J, Davies PF. A rapid, reliable method to isolate high quality endothelial RNA from small spatially-defined locations. Ann Biomed Eng. 2004;32:1453–1459. doi: 10.1114/b:abme.0000042360.57960.2b. [DOI] [PubMed] [Google Scholar]
- 24.Fang Y, Schram G, Romanenko VG, Shi C, Conti L, Vandenberg CA, Davies PF, Nattel S, Levitan I. Functional expression of Kir2.x in human aortic endothelial cells: the dominant role of Kir2.2. Am J Physiol Cell Physiol. 2005;289:C1134–1144. doi: 10.1152/ajpcell.00077.2005. [DOI] [PubMed] [Google Scholar]
- 25.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 26.Goodell MA, Rosenzweig MK, Marks HDF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 27.Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 28.Romanenko VG, Fang Y, Byfield F, Travis AJ, Vandenberg CA, Rothblat GH, Levitan I. Cholesterol sensitivity and lipid raft targeting of Kir2.1 channels. Biophys J. 2004;87:3850–3861. doi: 10.1529/biophysj.104.043273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hille B. Ionic Channels of Excitable Membranes. Sinauer Associates Inc; Sunderland, MA: 1992. [Google Scholar]
- 30.Smalley MJ, Clarke RB. The mammary gland “side population”: a putative stem/progenitor cell marker? J Mammary Gland Biol Neoplasia. 2005;10:37–47. doi: 10.1007/s10911-005-2539-0. [DOI] [PubMed] [Google Scholar]
- 31.Lopatin AN, Nichols CG. Inward rectifiers in the heart: an update on I(K1) J Mol Cell Cardiol. 2001;33:625–638. doi: 10.1006/jmcc.2001.1344. [DOI] [PubMed] [Google Scholar]
- 32.Sun W, Buzanska L, Domanska-Janik K, Salvi RJ, Stachowiak MK. Voltage-Sensitive and Ligand-Gated Channels in Differentiating Neural Stem-Like Cells Derived from the Nonhematopoietic Fraction of Human Umbilical Cord Blood. Stem Cells. 2005;23:931–945. doi: 10.1634/stemcells.2004-0316. [DOI] [PubMed] [Google Scholar]
- 33.Heubach JF, Graf EM, Leutheuser J, Bock M, Balana B, Zahanich I, Christ T, Boxberger S, Wettwer E, Ravens U. Electrophysiological properties of human mesenchymal stem cells. J Physiol (Lond) 2004;554:659–672. doi: 10.1113/jphysiol.2003.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li GR, Sun H, Deng X, Lau CP. Characterization of Ionic Currents in Human Mesenchymal Stem Cells from Bone Marrow. Stem Cells. 2005;23:371–382. doi: 10.1634/stemcells.2004-0213. [DOI] [PubMed] [Google Scholar]
- 35.Li GR, Deng XL, Sun H, Chung SSM, Tse HF, Lau CP. Ion Channels in Mesenchymal Stem Cells from Rat Bone Marrow. Stem Cells. 2006;24:1519–1528. doi: 10.1634/stemcells.2005-0307. [DOI] [PubMed] [Google Scholar]
- 36.Wang K, Xue T, Tsang SY, Van Huizen R, Wong CW, Lai KW, Ye Z, Cheng L, Au KW, Zhang J, Li GR, Lau CP, Tse HF, Li RA. Electrophysiological Properties of Pluripotent Human and Mouse Embryonic Stem Cells. Stem Cells. 2005;23:1526–1534. doi: 10.1634/stemcells.2004-0299. [DOI] [PubMed] [Google Scholar]
- 37.Kubo Y. Comparison of initial stages of muscle differentiation in rat and mouse myoblastic and mouse mesodermal stem cell lines. J Physiol. 1991;442:743–759. doi: 10.1113/jphysiol.1991.sp018817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Day ML, Pickering SJ, Johnson MH, Cook DI. Cell-cycle control of a large-conductance K+ channel in mouse early embryos. Nature. 1993;365:560. doi: 10.1038/365560a0. [DOI] [PubMed] [Google Scholar]
- 39.Okamura Y, Takahashi K. Neural induction suppresses early expression of the inward-rectifier K+ channel in the ascidian blastomere. J Physiol. 1993;463:245–268. doi: 10.1113/jphysiol.1993.sp019593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi K, Okamura Y. Ion Channels and Early Development of Neural Cells. Physiol Rev. 1998;78:307–337. doi: 10.1152/physrev.1998.78.2.307. [DOI] [PubMed] [Google Scholar]
- 41.MacFarlane SN, Sontheimer H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia. 2000;30:39–48. doi: 10.1002/(sici)1098-1136(200003)30:1<39::aid-glia5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 42.Wulff H, Knaus HG, Pennington M, Chandy KG. K+ Channel Expression during B Cell Differentiation: Implications for Immunomodulation and Autoimmunity. J Immunol. 2004;173:776–786. doi: 10.4049/jimmunol.173.2.776. [DOI] [PubMed] [Google Scholar]
- 43.Lang F, Föller M, Lang KS, Lang PA, Ritter M, Gulbins E, Vereninov A, Huber SM. Ion Channels in Cell Proliferation and Apoptotic Cell Death. Journal of Membrane Biology. 2005;V205:147. doi: 10.1007/s00232-005-0780-5. [DOI] [PubMed] [Google Scholar]
- 44.Lepple-Wienhues A, Berweck S, Böhmig M, Leo CP, Meyling B, Garbe C, Wiederholt M. K+ Channels and the Intracellular Calcium Signal in Human Melanoma Cell Proliferation. Journal of Membrane Biology. 1996;V151:149. doi: 10.1007/s002329900066. [DOI] [PubMed] [Google Scholar]
- 45.Konig S, Beguet A, Bader CRBL. The calcineurin pathway links hyperpolarization (Kir2.1)-induced Ca2+ signals to human myoblast differentiation and fusion. Development. 2006;133:3107–3114. doi: 10.1242/dev.02479. [DOI] [PubMed] [Google Scholar]
- 46.Luckhoff A, Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Archiv: European journal of physiology. 1990;416:305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- 47.Ghisdal P, Morel N. Cellular target of voltage and calcium-dependent K(+) channel blockers involved in EDHF-mediated responses in rat superior mesenteric artery. Br J Pharmacol. 2001;134:1021–1028. doi: 10.1038/sj.bjp.0704348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohler R, Brakemeier S, Kuhn M, Behrens C, Real R, Degenhardt C, Orzechowski HD, Pries AR, Hoyer PMJ. Impaired hyperpolarization in regenerated endothelium after balloon catheter injury. Circ Res. 2001;89:174–179. doi: 10.1161/hh1401.093460. [DOI] [PubMed] [Google Scholar]
- 49.Coleman HA, Tare M, Parkington HC. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin Exp Pharmacol Physiol. 2004;31:641–649. doi: 10.1111/j.1440-1681.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- 50.Michelakis ED, Weir EK, Wu X, Nsair A, Waite R, Hashimoto K, Puttagunta L, Knaus HG, Archer SL. Potassium channels regulate tone in rat pulmonary veins. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1138–1147. doi: 10.1152/ajplung.2001.280.6.L1138. [DOI] [PubMed] [Google Scholar]
- 51.Ungvari Z, Csiszar A, Koller A. Increases in endothelial Ca2+ activate KCa channels and elicit EDHF-type arteriolar dilation via gap junctions. Am J Physiol Heart Circ Physiol. 2002;282:H1760–H1767. doi: 10.1152/ajpheart.00676.2001. [DOI] [PubMed] [Google Scholar]
- 52.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]