Abstract
We evaluated the commonly prescribed analgesic buprenorphine in a postoperative pain model in rats, assessing acute postoperative pain relief, rebound hyperalgesia, and the long-term effects of postoperative opioid treatment on subsequent opioid exposure. Rats received surgery (paw incision under isoflurane anesthesia), sham surgery (anesthesia only), or neither and were treated postoperatively with 1 of several doses of subcutaneous buprenorphine. Pain sensitivity to noxious and nonnoxious mechanical stimuli at the site of injury (primary pain) was assessed at 1, 4, 24, and 72 h after surgery. Pain sensitivity at a site distal to the injury (secondary pain) was assessed at 24 and 72 h after surgery. Rats were tested for their sensitivity to the analgesic and locomotor effects of morphine 9 to 10 d after surgery. Buprenorphine at 0.05 mg/kg SC was determined to be the most effective; this dose induced isoalgesia during the acute postoperative period and the longest period of pain relief, and it did not induce long-term changes in opioid sensitivity in 2 functional measures of the opioid system. A lower dose of buprenorphine (0.01 mg/kg SC) did not meet the criterion for isoalgesia, and a higher dose (0.1 mg/kg SC) was less effective in pain relief at later recovery periods and induced a long-lasting opioid tolerance, indicating greater neural adaptations. These results support the use of 0.05 mg/kg SC buprenorphine as the upper dose limit for effective treatment of postoperative pain in rats and suggest that higher doses produce long-term effects on opioid sensitivity.
Relief of postoperative pain is mandated in the Guide for the Care and Use of Animals18 and the Public Health Service Policy17 and is a major objective of laboratory animal medicine. Buprenorphine is one of the most commonly used opioid analgesics for postoperative pain in laboratory animals, mainly because of its long duration of action.10 The typical recommended dose range of buprenorphine in rats is 0.02 to 0.05 mg/kg SC.10 The upper end of this range, although effective at relieving acute postoperative pain in rats, is associated with side effects such as enhanced postoperative pain after the drug has worn off (rebound hyperalgesia),23 respiratory depression,21 nausea or gastrointestinal distress and pica,25 and neural adaptations (for example, sensitization) that may lead to long-term changes in neural function in the central nervous system and consequent changes in behavior.14 Central sensitization is a well-studied neural adaptation expressed in the brain and spinal cord and induced by nociceptive stimulation (that is, pain-induced by surgical manipulation) that manifests as hyperalgesia (decreased pain threshold to noxious stimuli) and allodynia (appearance of pain-like responses to nonnoxious tactile stimuli) during the recovery period.16,29 Central sensitization contributes to persistent pain during the postoperative recovery period (that is, maintenance of increased pain sensitivity during tissue recovery) and chronic pain in some pathologic conditions (that is, persistent pain sensitivity after full tissue recovery). Central sensitization also accounts for the spread of hyperalgesia and allodynia to noninjured areas of the body distal to the injury.31 This phenomenon is referred to as ‘secondary pain’ (secondary hyperalgesia and allodynia), because it is not directly associated with the primary injury site.
Opioid analgesics inhibit pain by acting on the nervous system to block transduction of pain signals traveling in sensory neurons toward the central nervous system and by facilitating activity of the descending pain inhibition neural pathway.16 Opioid analgesics also induce neural adaptations in the nervous system, phenomena that underlie the pronounced changes in behavior associated with addiction to narcotics.2 Notably, opioid analgesics have been shown to enhance central sensitization initiated by pain transmission.6,8,14,20 This property means that opiate analgesics facilitate both the inhibition of pain and central sensitization that leads to the enhancement of pain. Because central sensitization is a neural adaptation, the interaction of opiates on this pain mechanism outlasts the presence of the drug; in contrast, opiate effects on pain inhibition are limited to the presence of the drug. This arrangement is thought to account for rebound pain, that is, increased pain sensitivity after the opiate analgesic has worn off. Opiate side effects can compromise the success of recovery by increasing the level of distress experienced during recovery (for example, inducing nausea) and possibly increasing the duration of distress during recovery (for example, allowing for rebound pain). Moreover, and of importance specifically to laboratory animal medicine, the general neural adaptations induced by even a single dose of an opiate analgesic26 may induce changes in the nervous system that alter and therefore compromise the validity of the animal model under study (for example, opioid mechanisms involved in behavioral control).
We previously evaluated the feasibility of oral administration of buprenorphine.15,25 As a basis for comparison, we used the ‘gold-standard’ postoperative buprenorphine dose of 0.05 mg/kg SC. The results of those studies showed that oral administration of buprenorphine was not feasible because the dose necessary to produce analgesia comparable to the standard dose of 0.05 mg/kg SC was 10 times the oral dose recommended in the literature and because the resulting concentration of oral buprenorphine was too bitter for rats to ingest voluntarily in a volume of flavored foodstuff that they could eat in a single meal.15,25 We also observed that both subcutaneous and oral buprenorphine caused conditioned aversion to flavors,25 suggestive of gastrointestinal distress5, with a greater effect for the oral route. Our conclusions and the associated clinical recommendation were limited by our presumption that buprenorphine at 0.05 mg/kg SC was the ideal postsurgical dose.
An assessment of the literature that established this dose identified 2 problems. First, little or no research had directly assessed the effect of buprenorphine on pain sensitivity in animals in the hyperalgesic state that characterized the postoperative period,23 and to our knowledge, no study has directly assessed the dose–response function of postsurgical buprenorphine on hyperalgesia. We hypothesized that endogenous opioids activated during the postoperative period24 might act synergistically with buprenorphine to allow adequate relief of postoperative pain with a lower dose of buprenorphine than is necessary in an algesiometric test, thereby making predictions and extrapolations from algesiometric tests inaccurate. Second, we found that little consideration had been given to the consequences of other physiologic effects of buprenorphine on the recovery process (for example, gastrointestinal distress5, rebound hyperalgesia, and allodynia). As stated earlier, recent research on central sensitization has determined that although opioid analgesics inhibit pain sensation acutely, they also enhance neural adaptations that account for rebound pain and other long-term chronic pain conditions.16,28,29,31 We hypothesized secondarily that a lower dose of buprenorphine, if effective acutely, would result in reduced side effects and be less likely to initiate or enhance neural adaptations, such as rebound hyperalgesia and allodynia.
The current study had 2 goals. The first was to establish the minimum dose of buprenorphine needed to relieve acute postoperative pain effectively in rats. As a starting point, we defined effective relief of acute pain as the induction of isoalgesia during the postoperative period; isoalgesia is the normal level of pain sensation, in contrast to analgesia (absence of pain sensation) or hypoalgesia (lower-than-normal pain sensation). The second goal was to evaluate the effect of postoperative buprenorphine on factors that slow recovery (that is, rebound hyperalgesia and allodynia) or create long-term changes (that is, sensitization or tolerance to opiates). We tested our hypothesis by using various doses of buprenorphine in a rat model of incisional pain.3,4,31 This model was selected because it induces cutaneous and muscular pain common to most surgery and generates mild to moderate persistent pain so that both the acute inhibitory effects of the buprenorphine (that is, pain relief) and the lasting effects of buprenorphine (that is, rebound hyperalgesia) could be studied.
Materials and Methods
Subjects.
The study used 206 male Long Evans (hooded) rats weighing 305 to 511 g. Rats were acquired from a commercial vendor (Harlan Sprague Dawley, Indianapolis, IN) or were first- or second-generation outbred offspring from an inhouse breeding colony that was stocked from Harlan. Rats were housed and cared for in accordance with the Guide for the Care and Use of Animals18 in clear, standing, polycarbonate cages (46 × 25 × 21 cm) on aspen hardwood shavings (Northeastern Products, New York, NY) and had ad libitum access to tap water and rodent chow (Teklad Rodent Diet 2018, Harlan Teklad, Madison, WI). Rats were maintained under a 14:10-h light:dark cycle (lights on at 0700 h Eastern Standard Time), at 22° ± 2°C, 30% to 70% relative humidity, and 10 to 15 air changes per hour. Semiannual health surveillance tests were performed on all rats in the facility by using sentinel rats placed on dirty bedding. Study rats were free of cilia-associated respiratory bacillus, Mycoplasma pulmonis, Kilham rat virus, H1 virus, rat parvovirus, pneumonia virus of mice, rat coronavirus, Sendai virus, lymphocytic choriomeningitis virus, reovirus, and fur mites. Syphacia muris was detected in some study rats during the course of the experiment; infected rats were quarantined but not treated for this infection. All procedures were done in accordance with federal, state, and institutional guidelines and were approved by the University at Buffalo Institutional Animal Care and Use Committee in an AAALAC-accredited facility. The rats had not participated in any previous study and at the conclusion of this study were either euthanized or transferred to another investigator for use in another study.
Design of experiment 1.
To test all of the hypotheses about the effects of buprenorphine on pain sensitivity during postoperative recovery, we used a 3 × 4 factorial design [surgical condition (surgery, anesthesia only, no manipulation) × buprenorphine dose (0.00, 0.005, 0.01, 0.05 mg/kg SC) × test time (1, 4, 24, 72 h after surgery)], with repeated measures on the test time variable. A paw pressure algesiometric test19 was used to measure pain threshold at 1, 4, 24, and 72 h after surgery. Buprenorphine was administered at the end of surgery, immediately after the incision was closed and before the rat regained consciousness. Nylon filaments were used to measure primary (proximal) tactile allodynia4 at 1, 4, 24, and 72 h after surgery and secondary (distal) allodynia at 24 and 72 h after surgery. Algesiometric and allodynia testing at the 1 and 4 h time points evaluated relief of immediate postoperative pain (primary pain) by buprenorphine, whereas testing at the 24 and 72 h time points evaluated persistent hyperalgesia and allodynia, including rebound hyperalgesia and allodynia (primary and secondary pain), after the drug had worn off. The dependent variables at each time point were paw-withdrawal latency to a noxious stimulus (a measure of pain threshold) and paw-withdrawal threshold to a nonnoxious stimulus applied proximal to the site of injury (a measure of primary allodynia) or distal to the site of injury (a measure of secondary allodynia). A goal in this study was to secure a measure of pain that was mediated solely by central nervous system changes so that we could more precisely measure secondary (rebound) pain, which develops slowly after the acute effects of buprenorphine have dissipated.23 Therefore we began the measurement of secondary pain at 24 h, a time point that, based on our earlier research, is beyond the acute effects of buprenorphine and is slightly before the initiation of secondary pain according to other published reports.11,13,22
Nine to 10 d after surgery, in these same rats, we assessed their sensitivity to the analgesic and locomotor effects of morphine to determine whether any long-term neural adaptations had been induced by exposure to the single postoperative dose of buprenorphine. A diminished or an enhanced response to the morphine injection in rats previously injected with buprenorphine would indicate tolerance or sensitization, respectively.27 Because tolerance and sensitization phenomena have been shown to be more or less likely to occur depending on the particular effect under study,11,13,22 we assessed the effect of morphine on 2 different behaviors: pain threshold and locomotor activity.
Rats were tested in sets of 3, and 2 to 4 sets were tested weekly for 12 wk. Within each set, rats were randomly assigned to a surgical condition and within each week rats were randomly assigned to a buprenorphine dose. By using this strategy, each experimental group was represented equally over the 12 wk of testing.
Nine to 10 d after surgery (6 to 7 d after the last test for pain and allodynia threshold), changes in sensitivity to the analgesic and the locomotor-stimulating effects of morphine were assessed in all rats. The pain-threshold measure was tail-withdrawal latency to a hot water stimulus before (baseline) and after morphine exposure. Locomotor activity was measured as distance traveled in an open field during five 20-min periods. Injections were given before the baseline measurement (saline); at 20-min intervals thereafter, the three morphine injections were given at the doses of 1.0, 2.0, and 2.0 mg/kg, which produced cumulative doses of 1.0, 3.0, and 5.0 mg/kg. The final tail-withdrawal test (morphine tail-withdrawal test) was conducted at the end of the last 20-min measurement of locomotion (20 min after the last morphine injection). The pattern of morphine injections was designed to induce a cumulative dose-response measure for the effects of a low dose of morphine (total cumulative exposure was 5 mg/kg) that has previously been shown to cause sensitization.27 Therefore, the overall design used to capture both measures was a 3 × 4 × 5 factorial design {surgical condition [surgery, anesthesia only, no manipulation] × postoperative buprenorphine dose [0.00, 0.005, 0.01, 0.05 mg/kg] × test after exposure to a cumulative dosing regimen of morphine [no injection (habituation), 0, 1, 2, and 2 mg/kg morphine at 20-min intervals]}, with repeated measures on the test variable. As described earlier, the dependent variables were pain threshold and locomotor activity.
Design of experiment 2.
A second experiment was necessary to evaluate rebound hyperalgesia23 in greater detail. The design of this study was intended to accomplish 2 things: to test a higher dose of buprenorphine and to reduce the number of repeated measures. In this way, the possible confound introduced by repeatedly testing paw-pressure withdrawal, which could produce irritation or inflammation, would be eliminated. Allodynia was tested only at the later time points. To evaluate the effect of postoperative buprenorphine on rebound hyperalgesia, we used a 2 × 4 × 2 factorial design [surgical condition (surgery, anesthesia only) × buprenorphine dose (0.00, 0.01, 0.05, 0.1 mg/kg) × test time (24, 72 h after surgery)], with repeated measures on the test time variable. The dependent variable at each time point was limited to paw-withdrawal threshold to a nonnoxious stimulus applied proximal to the site of injury (a measure of primary allodynia) or distal to the site of injury (a measure of secondary allodynia). Eight sets of 8 rats were tested, and group assignments were made as described earlier.
In addition, all rats were assessed for sensitivity to the analgesic and locomotor-stimulating effects of morphine 9 to 10 d after the surgery because the dose range for postoperative buprenorphine was wider than that used in the first experiment. This additional assessment provided another opportunity to test our hypothesis about the long-term consequences of postoperative buprenorphine on behavioral state. The testing procedure was modified somewhat from that used in experiment 1. First, baseline tail-withdrawal latency was assessed before the start of locomotor testing, after saline injection (new to experiment 2), and after morphine treatment to ensure that exposure to the open-field boxes did not change baseline pain threshold (a possible confound in experiment 1). Second, a single injection of morphine was used instead of the cumulative dosing regimen described in experiment 1. Rats were placed in the open field immediately after the baseline tail-withdrawal test and locomotor activity was monitored for a 20-min habituation period. The rats then were injected with saline, and locomotor activity was measured for another 20 min, after which the saline tail-withdrawal test was conducted; then the rats were injected with a single 5-mg/kg dose of morphine and locomotor activity was measured for a final 20-min period. Therefore the final design to test this secondary hypothesis was a 2 × 4 × 3 factorial {surgical condition [surgery, anesthesia only] × postoperative buprenorphine dose [0.00, 0.01, 0.05, 0.1 mg/kg] × test [no injection (habituation), 0, and 5 mg/kg morphine at 20-min intervals]} with repeated measures on the test variable.
Surgery.
A rat model of incisional postoperative pain3,4,31 was used as an experimental variable and involved surgical manipulation of the left hindfoot of one third of the rats. Briefly, rats in the surgery group were anesthetized with isoflurane and placed in dorsal recumbency, and the plantar surface of the left foot was prepared aseptically and draped. A 1-cm incision was made in the plantar aspect of the foot, starting 0.5 cm from the proximal edge of the heel and extending distally. The plantaris muscle was bluntly dissected and elevated and then incised longitudinally, preserving its origin and insertion. The skin was closed with 2 interrupted mattress sutures by using 5-0 polydioxanone suture. Rats received 5 mL sterile saline subcutaneously postoperatively for hydration. Surgery rats were monitored carefully during the first postoperative week for signs of severe pain, such as self-destructive behavior (chewing the incision), tachypnea, lethargy, vocalizations, or hunched body posture so that they could immediately be removed from the study and euthanized with CO2. However, it was not necessary to remove any rat because of severe pain during the course of the study.
Control rats received either isoflurane anesthesia for the same duration as surgery rats, sterile saline subcutaneously, and no surgical manipulations (anesthesia-only controls) or no anesthesia and no surgery (no-manipulation controls). Rats were tested in groups of 3, each containing 1 surgery rat, 1 anesthesia-only rat, and 1 no-manipulation rat. No-manipulation controls remained in their home cage in the colony room while the other 2 rats underwent their surgical procedures. Two control groups were needed in experiment 1 to control for an interaction between isoflurane anesthesia, surgery, and opioid treatment on pain threshold and allodynia. Because no differences between these 2 control groups were detected in experiment 1, only a single control group (anesthesia only) was used in experiment 2.
Drugs.
Buprenorphine HCl (Buprenex, Bedford Laboratories, Bedford, OH) was diluted in sterile saline, by using a serial dilution method, to concentrations ranging from 0.005 to 0.1 mg/mL and administered subcutaneously in a volume of 0.1 ml/kg body weight. Buprenorphine dilutions were prepared weekly and stored in covered glass vials in a dark cabinet until use within 72 h of dilution. Morphine sulfate (Mallinckrodt, St Louis, MO) was mixed in sterile saline and prepared as 5 mg/mL stock solution each week and stored in a dark refrigerated cabinet until use within 8 d of preparation. In experiment 1, morphine was diluted with sterile saline to 1 and 2 mg/mL concentrations; in experiment 2, morphine was not diluted further. All drugs were injected subcutaneously by using 1-mL plastic syringes and 26-gauge needles (BD Tuberculin syringes, VWR, Rochester, NY); buprenorphine was delivered just above the hip on the dorsal posterior surface of the rat; morphine was delivered at the hip.
Postsurgical pain threshold tests.
Pain threshold was assessed at 1, 4, 24, and 72 h after surgery by using the hindpaw-withdrawal response to noxious paw pressure.19 Rats were restrained in a black cotton sock so that only the left rear leg and tail of the rat were exposed. The left hindpaw then was placed on a force gauge (Compact Force Gauge, Mecmesin, Sterling, VA), and the gauge was zeroed. Increasing mechanical pressure was applied to the dorsal surface of the foot between the 3rd and 4th metacarpals with a blunt probe until the rat withdrew its foot. The dependent variable was the amount of pressure, in grams, that provoked the rat to remove its foot from the force-gauge stage. To prevent tissue damage to the rat's foot, the test was terminated at 400 g if no response occurred. This test was performed 4 times, with trials separated by 1 min. Paw-withdrawal thresholds were defined as the average of the last 3 of 4 paw-withdrawal latencies. The first trial was eliminated because of the high variability among rats in this response. Rats were habituated to the procedure and equipment, with no pressure applied to their foot, by 5 daily exposures to the restraint and procedure during the week before the experiment.
Postsurgical allodynia tests.
Nineteen nylon filaments were constructed to be used as nonnoxious mechanical stimuli (tactile filaments). The tactile filaments were constructed from monofilament fishing line (Berkley Big Game, Pure Fishing, Spirit Lake, IA) that was cut to various lengths and glued to wood handles (Jumbo Woodcrafts Craft Sticks, Gurnee, IL). The nylon fishing line ranged in test weight from 2 to 40 lbs. The tactile filaments were constructed so as to apply the following forces before bending: 0.01, 0.05, 0.1, 0.15, 0.25, 0.3, 0.6, 0.8, 1.0, 1.7, 2, 3, 4, 5, 8, 10, 15, 22, and 28 g. The filaments were calibrated on a weekly basis. Laboratory humidity on the test days varied from 26% to 46%. Weekly calibration controlled for measurement error induced by the fluctuation in humidity.
Tests for primary tactile allodynia were performed at 1, 4, 24, and 72 h after surgery by using the tactile filaments at a site on the foot near the paw incision (proximal allodynia test), as previously described.3,4,31 Rats were placed in 24 × 19 × 18-cm stainless steel, wire-mesh cages suspended over a 45° mirror, and allowed to acclimate for 15 min prior to each test. The filament was applied to a site medial to the incision near the base of the heel, until it bowed (negative response) or the rat withdrew its foot (positive response). A modified version of an ‘up and down’ testing paradigm9 was used. Briefly, testing was started with a medium-force filament (filament 9; 1.0 g), and if a negative response was obtained, the next higher force filament was applied. If a positive response was obtained, the next lower force filament was applied. Application of the filaments in this manner was continued, with a 10-s period between filament applications, until a repeatable withdrawal response with a specific filament was obtained. The test then was repeated 5 min later. If no response occurred (ceiling response), rats were assigned a response value of the highest filament. The dependent variable was the median filament force, in grams, that caused a repeatable withdrawal response on both tests or a ceiling score of the force, in grams, of the highest filament. Rats were habituated to the testing cages, with no filaments applied to their feet, by a daily 15-min exposure for 3 d during the week before the experiment. Primary tactile allodynia provides a measure of pain sensitivity (a pain response to a nonnoxious stimulus). Postsurgery changes in this test reflect pain mediated by physiologic changes in both the tissue surrounding the injury and the central sensitization that occurs in response to atypical pain stimulation (that is, surgery and postoperative activity that stimulates pain).
A test for secondary tactile allodynia was conducted at 24 and 72 h by using the same set of nylon filaments described earlier at a site on the left hindpaw approximately 10 mm distal to the distal end of the paw incision.4,31 Postsurgery changes in this test reflect pain mediated by physiologic changes in central sensitization alone.
Morphine-induced hypoalgesia test.
Pain threshold was measured to assess morphine-induced hypoalgesia by using a standard hot-water tail-withdrawal assay.12 The water was maintained at 52°C in a constant-temperature water bath and was monitored by use of a thermometer. The distal third of the rat's tail was immersed in the bath, and the time required for the rat to remove its tail was measured by use of a stopwatch (upper limit of 30 s). Rats were allowed to crawl into a black cotton sock, and the tail was then immersed in the bath. The tail-withdrawal latency score was calculated as the mean of the last 3 of 4 trials, separated by 30-s intervals. Our procedure has been described previously;15 2 or 3 pain threshold tests were conducted on each rat. Rats were habituated to the procedure and equipment (but not the hot water) used in this assay by daily exposure to the procedure for 3 d during the week before the start of this study.
Morphine-induced locomotor activity.
Rats were tested for the locomotor effects of morphine by measuring the amount of forward locomotor activity exhibited in an open-field apparatus. The apparatus consisted of a 40.6 × 40.6-cm clear acrylic box. Rats were placed into the center of the box after the baseline hot-water tail-withdrawal assay and allowed to habituate for 20 min. After habituation, rats were removed from their locomotor box, injected with saline, and then placed immediately back into the open field for another 20 min. After the saline injection, rats were removed from the open field, given a second tail-withdrawal test (experiment 2 only), injected with morphine, and then placed immediately back into the open field for another 20 min. In experiment 1, several doses of morphine were administered in consecutive 20-min blocks to construct a cumulative morphine dose-response curve; in experiment 2, a single dose of morphine (5 mg/kg) was administered, and locomotor activity was measured for 20 min. The dependent variable was distance traveled in each 20-min block. Rat behavior in the open field was recorded by a video camera (PM61760 Black and White Home Cameras, Phillips Magnavox, Andover, MA) that was suspended over the locomotor boxes. Distance traveled was calculated as millimeters of forward locomotion; the calculation was accomplished with the aid of a computer software program designed to capture and track the rat's movement during the test (TopScan version 2.00, Behavioral Recognition Software, Clever Sys, Reston, VA). Forward locomotion was defined as forward movement of at least 100 mm at 60 mm/0.5 s.
Data analysis.
Parametric tests were used (SPSS for Windows, release 15.0.1.1., SPSS, Chicago IL) to evaluate the effects of surgical condition and buprenorphine dose on each experimental variable, except for the overall analysis of proximal allodynia. The majority of rats in the control groups for this measure failed to show any reliable response to the tactile filaments and were assigned a score equal to the pressure induce by strongest filament. Therefore, proximal allodynia data from the control groups were at ‘ceiling’ and therefore lacked the necessary distribution for parametric analysis. Nonparametric statistics (Kruskal–Wallis, χ2 tests) were used to test the initial hypothesis that surgery induced proximal allodynia. After this result was established, data analysis on proximal allodynia measures was limited to the surgery group and assessed by using traditional parametric statistics. In all analyses, significance was defined as a P value of less than 0.05 and, where appropriate, Greenhouse–Geisser corrections for multiple repeated measures were used; in simple effect probes, the mean-square error was adjusted to reflect the most reliable estimate of error.
Results
Experiment 1
Subjects.
A total of 138 rats were tested, and 137 rats were included in the statistical analysis of the hypotheses. One rat was excluded because of experimenter error in the injection of the buprenorphine dose.
Postoperative analgesia.
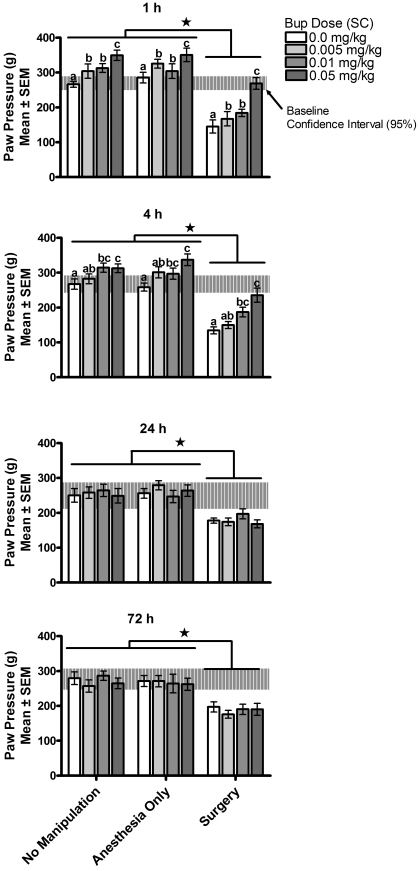
Results from the analysis of pain threshold using the paw-withdrawal method are illustrated in Figure 1. A 3-way ANOVA comparing paw-withdrawal latencies (pain threshold test) by surgical condition (surgery, anesthesia only, no manipulation), buprenorphine dose (0, 0.005, 0.01, 0.05 mg/kg), and postoperative test time (1, 4, 24, and 72 h after surgery and buprenorphine treatment) with repeated measures on test time revealed a significant surgical condition × test time interaction [F(6,355) = 4.37, P < 0.001] and a significant buprenorphine dose × test time interaction [F(9,355) = 8.44, P < 0.001]. The 3-way interaction and the 2-way interaction between surgical condition and buprenorphine dose were not significant [F(18,355) < 1 and F(6, 125) = 1.04, P > 0.5, respectively]. Simple-effect probes of the surgical condition × test time interaction found significant differences in paw-withdrawal latencies by surgical condition at each postoperative test time; specifically, significantly shorter paw-withdrawal latencies (lower pain threshold, greater sensitivity) were found among rats that had surgery than among rats that were exposed to anesthesia only or no manipulation. Simple-effect probes of the buprenorphine dose × test time interaction found significant differences in paw-withdrawal latencies among doses of buprenorphine at 1 and 4 h only [1h, F(3, 378) = 14.07, P < 0.001; 4h, F(3,378) = 9.60, P < 0.001; 24h, F(3,378) < 1; 72 h, F(3,378) < 1]. At 1 h, rats receiving no buprenorphine (0 mg/kg) had significantly shorter paw-withdrawal latencies (lower pain thresholds, more pain sensitivity) than did rats receiving buprenorphine at any dose; rats receiving the highest dose of buprenorphine (0.05 mg/kg) had significantly longer latencies (higher pain thresholds, less pain sensitivity) than did rats treated with all other doses of buprenorphine. At 4 h, rats receiving no buprenorphine had significantly shorter paw-withdrawal latencies (more pain sensitivity) than did rats receiving buprenorphine at any dose; rats receiving the highest dose of buprenorphine (0.05 mg/kg) had significantly longer paw-withdrawal latencies (less pain sensitivity) than did rats receiving 0.005 and 0 mg/kg buprenorphine. Paw-withdrawal latencies in rats receiving the intermediate dose of buprenorphine (0.01 mg/kg) were significantly longer (less pain sensitivity) than those of rats receiving no buprenorphine and were intermediate to, but not significantly different from, the latencies shown by rats in the highest and lowest buprenorphine doses. At 24 and 72 h, no differences in paw-withdrawal latencies were observed as a consequence of prior buprenorphine dose. Overall, these data suggest that, as expected, surgery induces hyperalgesia (decrease in pain threshold, increase in sensitivity to pain) in the affected paw and that treatment with buprenorphine after surgery induces a dose-dependent decrease in pain sensitivity (increase in pain threshold, decrease in hyperalgesia) in rats in the surgery group and hypoalgesia in rats in the control groups.
Figure 1.
Experiment 1—primary hyperalgesia (pain threshold). Primary hyperalgesia was assessed at 1, 4, 24, and 72 h after buprenorphine (Bup) administration. Only rats that received 0.05 mg/kg SC experienced isoalgesia during the immediate postoperative period (1 and 4 h). Surgery increased pain sensitivity and buprenorphine reduced pain sensitivity in a dose-dependent manner. Different symbols within groups and * indicate significant (P < 0.05) differences.
The effect of buprenorphine did not depend on surgical condition: buprenorphine increased pain threshold acutely and to a similar magnitude in all rats regardless of surgical condition. In rats in the surgery group, buprenorphine reduced hyperalgesia (all doses of buprenorphine) and induced isoalgesia at the 0.05-mg/kg dose. No effect of buprenorphine treatment on pain threshold was observed at 24 and 72 h after surgery (postinjection). Rats in the surgery group still showed hyperalgesia at 24 and 72 h and all buprenorphine groups had similar pain thresholds at 24 and 72 h. For rats in the 0 mg/kg buprenorphine group, paw-withdrawal latencies at 72 h were significantly longer (lower pain sensitivity) than at 1 h [F(3,355) = 7.3, P < 0.001] and this effect was due to changes in pain sensitivity among rats in the surgery group (72 h paw-withdrawal latency was 179% ± 39% of the 1-h level). Control rats in the 0-mg/kg buprenorphine groups, in contrast, showed shorter paw-withdrawal latencies (72-h paw-withdrawal latency was 85% ± 6.0% and 92% ± 16% of the 1-h test for anesthesia-only and no-manipulation groups, respectively). For rats in the 0.05-mg/kg buprenorphine groups, all rats showed shorter paw-withdrawal latencies at later times (higher pain sensitivity) [F(3,355) = 4.32, P = 0.005], and this effect was greatest in rats in the surgery group (72 h paw-withdrawal latency was 64% ± 9.2% of the 1-h test for rats in the surgery group and 79% ± 11.3% and 71% ± 7.6% for anesthesia-only and no-manipulation control groups, respectively). Rats in the 0.01-mg/kg buprenorphine groups showed only a small but time-limited change in paw-withdrawal latencies (tests at 1 h = 4 h = 72 h < 24 h) [F(3,355) = 4.03, P = 0.007]. Rats given the lowest dose of buprenorphine, 0.005 mg/kg, showed significantly longer latencies (hypoalgesia) only at 1 h [F(3,355) = 3.98, P = 0.008].
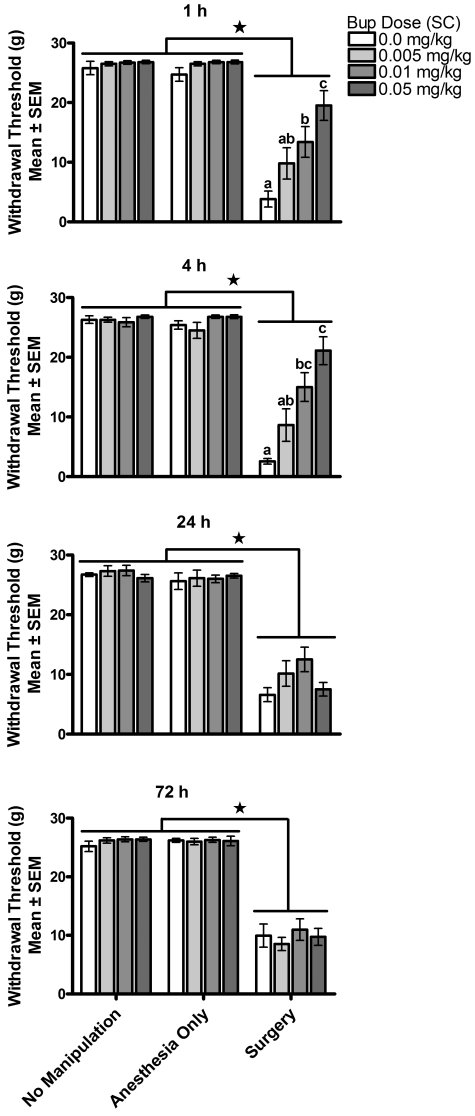
Results of the analysis of postoperative proximal allodynia using the nylon filament test are illustrated in Figure 2. Rats in the anesthesia-only and no-manipulation control groups showed no significant response to the nylon filaments regardless of dose of buprenorphine (including vehicle only). In this test, the filaments were applied to the heel of the left paw, and the failure to induce a response is in keeping with the nonnoxious property of the filaments. In contrast, all rats in the surgery group showed a response to the nylon filaments at all time points [surgery group differed significantly from controls at 1 h [χ2(2) = 54.735, P < 0.001]; 4 h [χ2(2) = 45.798, P < 0.001]; 24 h [χ2(2) = 90.277, P < 0.001]; and 72 h [χ2(2) = 84.83, P < 0.001]. The sensitivity of the response in the surgery group differed significantly by test time and buprenorphine dose. The nature of these differences was analyzed by using parametric statistical analyses on the effect of buprenorphine on proximal allodynia in the surgery condition only. A 2-way ANOVA, buprenorphine dose × test time with repeated measures on test time, was conducted on proximal allodynia threshold and revealed a significant interaction [F(1,126) = 5.68, P < 0.01 using a Greenhouse - Geisser correction]. Simple-effect probes revealed significant effects of buprenorphine dose at 1 and 4 h only [1h, F(3,135) = 9.57, P < 0.01: 4h, F(3,135) = 13.17, P < 0.01; 24 h, F(3,135) = 1.53, P > 0.05; 72 h, F(3,135) < 1]. At 1 h, a significant linear dose-dependent decrease in sensitivity to the tactile filaments was observed, in which 0.05 mg/kg buprenorphine induced a significantly greater decrease in allodynia than did 0.01 mg/kg buprenorphine, and induced a significant decrease in allodynia relative to controls. The responses of rats receiving 0.005 mg/kg buprenorphine fell intermediate to, but were not significantly different from, those receiving 0 and 0.01 mg/kg buprenorphine. At 4 h, the effect of buprenorphine on proximal allodynia was similar to the 1 h results, except that the magnitude of the difference between 0.05 and 0.01 mg/kg was not significant (4 h: 0.05 = 0.01 > 0; 1 h: 0.01 = 0.005 = 0.0 mg/kg buprenorphine). Proximal allodynia among rats in the group receiving surgery plus 0 mg/kg buprenorphine diminished significantly over time [F(2, 99) = 4.5, P =0.0135; 1 h = 4 h > 24 = 72 h], demonstrating the expected decrease in pain sensitivity with recovery. In contrast, proximal allodynia in rats receiving surgery plus 0.005 mg/kg buprenorphine and surgery plus 0.01 mg/kg buprenorphine remained the same over these test times [F(2,99) < 1 for each], and proximal allodynia actually increased in the group receiving surgery plus 0.05 mg/kg buprenorphine [F(2,99) = 21.0, P < 0.001; 1 h = 4 h < 24 h and 72 h].
Figure 2.
Experiment 1—primary allodynia (allodynia threshold). Primary allodynia was assessed at 1, 4, 24, and 72 h after buprenorphine (Bup) administration. Buprenorphine reduced allodynia in a dose-dependent manner at 1 and 4 h, but had no effect at 24 and 72 h. Surgery induced allodynia and buprenorphine reduced allodynia in a dose-dependent manner. Different symbols within groups and * indicate significant (P < 0.05) differences.
Results of the analysis of postoperative distal allodynia assessed with the tactile-filament test are illustrated in Figure 3. Most rats moved their foot in response to the pressure produced by the nylon filament placement on the site 10 mm distal to the surgical incision (toward the toes at the tori). A 3-way ANOVA (surgical condition × buprenorphine dose × test time) with repeated measures on test time revealed a significant 2-way interaction between surgical condition and test time but no significant interaction involving buprenorphine dose. Probes of this significant interaction revealed a significant difference in response at 72 h [F(2,239) = 9.20, P < 0.01] but not 24 h [F(2, 239) = 1.32, P > 0.05]; rats receiving surgery showed a greater response (allodynia) than did rats that did not receive surgery. This result is consistent with the appearance of secondary pain associated with the paw injury. Buprenorphine treatment at the end of surgery had no effect on distal allodynia at 24 and 72 h in this experiment.
Figure 3.
Experiment 1—secondary allodynia (allodynia threshold). Secondary allodynia was assessed at 24 and 72 h after buprenorphine (Bup) administration. Allodynia thresholds in the surgery group were similar to thresholds in the control groups, regardless of buprenorphine dose, at 24 h. All rats that had surgery showed secondary allodynia at 72 h, although buprenorphine dose had no effect on the magnitude of allodynia. *, Significantly (P < 0.05) different.
Long-term changes in opioid responsiveness.
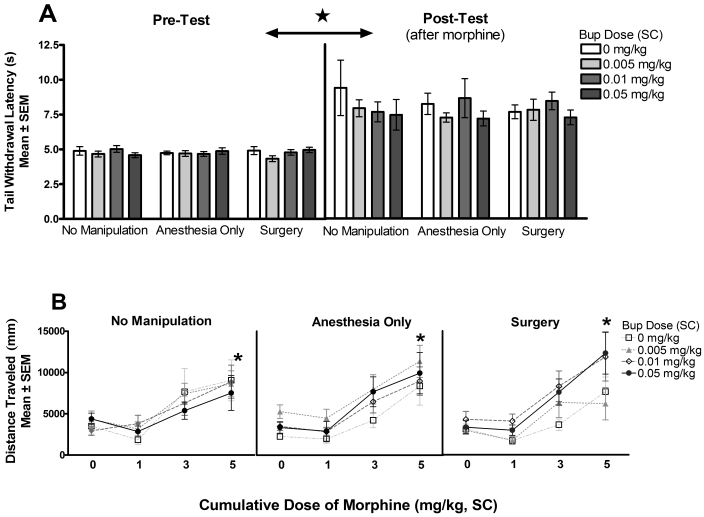
Nine to 10 days after surgery and buprenorphine treatment (6 to 7 d after the last postoperative analgesia test), rats were tested for their sensitivity to the analgesic and locomotor effects of a general opioid agonist—morphine sulfate—to address the question of long-term consequences of postoperative opioid treatment on subsequent sensitivity to experimental manipulations. The results of this challenge are depicted in Figure 4 A, B. A 3-way ANOVA analyzing original surgical condition × (postoperative) buprenorphine dose × test (before and after exposure to morphine) found no effect of surgical condition or buprenorphine dose on the analgesic effect of morphine. All interactions were nonsignificant [3-way interaction, F(6,124) < 1, using a Greenhouse-Geisser correction; condition × buprenorphine dose F(6, 124) < 1; surgical condition × time, F(2,124) < 1; buprenorphine dose × time, F(3,124) < 1], as were the main effects of surgical condition [F(2,124) < 1] and buprenorphine dose [F(3,124) = 1.32, P > 0.05]. As expected, the main effect of test was significant [F(1,124) = 146.22, P < 0.001], reflecting the analgesic action of the cumulative dose of 5 mg/kg morphine.
Figure 4.
(A) Experiment 1—analgesic effects of morphine (tail-withdrawal latency). Sensitivity to the analgesic effects of morphine was tested 9 to 10 d after postoperative buprenorphine (Bup) administration. Neither surgery nor any of the postoperative doses of buprenorphine induced lasting changes in subsequent exposure to the analgesic effects of morphine. *Morphine induced a significant (P < 0.05) increase in tail-withdrawal latency. (B) Experiment 1—locomotor effects of morphine (locomotor testing). Sensitivity to the locomotor effects of morphine was tested 9 to 10 d after postoperative buprenorphine administration. Neither surgery nor any of the postoperative doses of buprenorphine induced lasting changes in subsequent exposure to the locomotor-stimulating effects of morphine. *, Morphine induced a significant (P < 0.05) increase in locomotor activity.
Similar results were observed in the measure of morphine-induced locomotor activity. First, no effect of original surgical condition or postoperative buprenorphine dose on locomotor activity was observed during habituation to the testing arena. Two-way ANOVA analyzing surgical condition × buprenorphine dose on distance traveled in the open field during the habituation test yielded no significant effects of any variable [2-way interaction F(6,124) < 1; main effect of surgical condition F(2,124) = 1.22, P > 0.05; main effect of buprenorphine dose F(3,124) < 1]. Second, no effect of original surgical condition or postoperative buprenorphine dose on locomotor activity was observed in response to vehicle or morphine exposure. Three-way ANOVA analyzing distance traveled for surgical condition × buprenorphine dose × test (cumulative morphine doses) with repeated measures on test yielded a significant main effect of test only [3-way interaction F(11,372) < 1; buprenorphine dose × morphine dose F(9,372) < 1; surgical condition × morphine dose F(95,372) < 1; surgical condition × buprenorphine dose F(6,124) = 1.28, P > 0.05; main effect of surgical condition F(2,124) < 1; main effect of buprenorphine dose F(3,124) = 1.17, P > 0.05; main effect of morphine dose F(2,372) = 71.49, P < 0.01]. As depicted in Figure 4 B, the main effect of test reflects the increase in locomotor activity, in all groups, induced by the increasing dose of morphine, as expected.27
Experiment 2
Subjects.
A total of 68 rats were tested, and data from 64 rats were included in the statistical analysis. Four rats were excluded because of experimenter error in the administration of postsurgical testing procedures.
Postoperative analgesia.
As in experiment 1, rats in the anesthesia-only group showed no significant response to the nylon filaments placed on the heel of the left paw at a site equivalent to the site proximal to the injury in the surgery rats. The results of this test are shown in Figure 5. In contrast, almost all rats in the surgery group showed a response to the placement of the nylon filaments proximal to the injury. Nonparametric statistical comparison showed that the responses to nylon filaments at the proximal site of incision in the surgery group were significantly greater than those of the anesthesia-only group at 24 and 72 h [χ2(1) = 38.64, P < 0.001 and χ2(1) = 31.94, P < 0.001, respectively]. Two-way ANOVA analyzing the threshold for withdrawal from filaments among rats in the surgery group for each buprenorphine dose at 24 and 72 h was not significant [buprenorphine dose × test time, F(3,28) < 1; buprenorphine dose, F(3,28) = 1.27, P > 0.05; test time F(1,28) < 1]. As seen in experiment 1, postoperative buprenorphine treatment had no long-lasting effect on measures of proximal allodynia. Increasing the dose of buprenorphine to 0.1 mg/kg did not change this; rebound allodynia was not observed.
Figure 5.
Experiment 2—primary allodynia (allodynia threshold). Primary allodynia was assessed at 24 and 72 h after buprenorphine (Bup) administration. *, Surgery-induced primary allodynia (P < 0.05). Postoperative buprenorphine treatment had no long-lasting effect on measures of proximal allodynia at later time points (24 and 72 h).
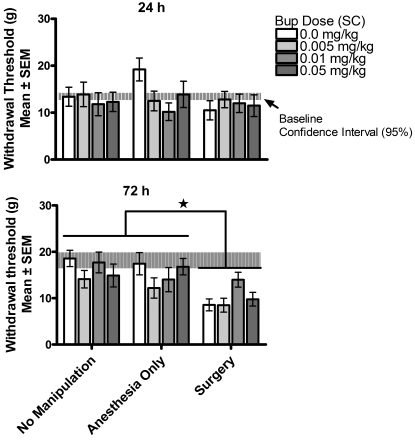
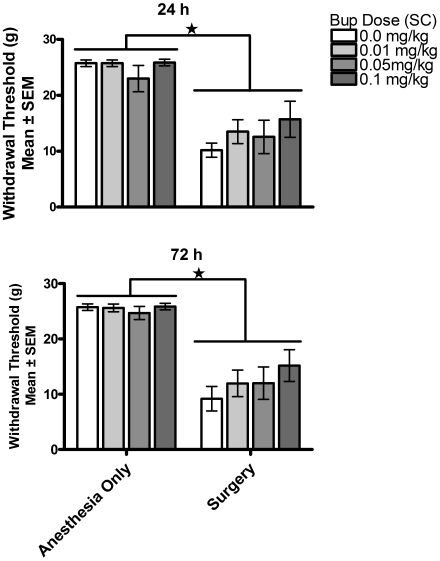
In contrast, the analysis of secondary pain (distal allodynia measure), shown in Figure 6, yielded a significant effect of both surgical condition and buprenorphine dose. A 3-way ANOVA (surgical condition × buprenorphine dose × test time), with repeated measures on test time, found a significant interaction between surgical condition and buprenorphine dose [F(3,56) = 3.47, P = 0.022]. The 3-way interaction [F(3,56) = 1.31, P > 0.05], 2-way interactions between surgical condition and test time [F(1,56) = 3.55, P > 0.05] and between buprenorphine dose and test time [F(3,56) < 1], and the main effect of test time [F(1,56) < 1] were not significant. Simple-effect probes of the significant surgical condition × buprenorphine dose interaction indicated that rats in the surgery group showed greater responses (allodynia) at all doses of buprenorphine except 0.05 mg/kg, which did not differ significantly from controls [0 mg/kg, F(1,56) = 35.40, P < 0.01; 0.01 mg/kg, F(1,56) = 30.08, P < 0.01; 0.05 mg/kg, F(1,56) < 1; 0.1 mg/kg, F(1,56) = 5.01, P < 0.01]. Buprenorphine treatment had no effect on measures of distal allodynia in anesthesia-only controls, but buprenorphine treatment, specifically 0.05 mg/kg, significantly reduced the distal allodynia in rats in the surgery condition [F(3,56) = 5.01, P < 0.01]; this effect was notable at 24 h, at which time 75% of the rats showed a response within the 95% confidence interval defined by controls.
Figure 6.
Experiment 2—secondary allodynia (allodynia threshold). Secondary allodynia was assessed at 24 and 72 h after buprenorphine (Bup) administration. Surgery induced secondary allodynia at both 24 and 72 h; buprenorphine (0.05 mg/kg SC) treatment induced a significant (*, P < 0.05) delay in the onset of secondary allodynia.
Long-term changes in analgesic sensitivity to morphine.
As in experiment 1, rats were tested for the appearance of tolerance and sensitization to the analgesic effects of morphine, to address the question of long-term effects of postoperative opioid treatment on subsequent sensitivity to experimental manipulations. The results of this challenge are depicted in Figure 7. A 3-way ANOVA analyzing original surgical condition × (postoperative) buprenorphine dose × test time (baseline, after saline, and after morphine exposure) revealed a significant effect of buprenorphine dose on the subsequent analgesic effect of morphine [buprenorphine dose × test time, F(6,104) = 3.03, P < 0.01] and no effect of surgical condition [surgical condition × test time, F(2,104) < 1; main effect of surgical condition, F(1,52) < 1] or the 3-way interaction among these variables [F(6,104) < 1]. Statistical probes of the significant interaction between buprenorphine dose and test time found no group differences at baseline [F(3,122) < 1], or after the saline injection [F(3,122) < 1], but revealed significant group differences after the morphine injection [F(34,122) = 8.62, P < 0.01]. Rats that had received 0.1 mg/kg buprenorphine postoperatively showed a significantly diminished response to morphine subsequently (tolerance).
Figure 7.
Experiment 2—analgesic effects of morphine (tail-withdrawal latency). Sensitivity to the analgesic effects of morphine was tested 9 to 10 d after postoperative buprenorphine (Bup) administration. Morphine induced a significant increase in tail-withdrawal latency in all groups. *, Prior postoperative buprenorphine (highest dose), but not surgery per se, induced a lasting and significant (P < 0.05) decrease in sensitivity to the analgesic effects of morphine.
An analysis of the locomotor data revealed, as in experiment 1, no effect of surgery condition or postoperative buprenorphine dose on baseline locomotor activity during the habituation period [surgical condition × buprenorphine dose, F(3,44) < 1] and no effect of these variables on the rats’ locomotor response to 5 mg/kg morphine [surgical condition × buprenorphine dose × test (saline versus morphine), F(3,44) < 1]. In addition, and unlike experiment 1, a bolus injection of 5 mg/kg morphine did not induce a significant increase in locomotor activity that was detectable 20 min later.
Discussion
The results of experiments 1 and 2 suggest that buprenorphine affected multiple mechanisms of postoperative pain. A single treatment with buprenorphine induced a dose-dependent decrease in pain sensitivity in all rats: the net effect on rats in the surgery group was reduced hyperalgesia, and the net effect on rats in the control groups was hypoalgesia. The effect of buprenorphine on surgery-induced pain was apparent for both hyperalgesia and allodynia; buprenorphine reduced pain sensitivity to both noxious and nonnoxious stimuli. In experiment 1, results of pain-threshold testing showed that the 0.05-mg/kg dose of buprenorphine was the only dose that produced isoalgesia in the immediate postoperative period [again, isoalgesia is defined as a mean pain threshold lying within the 95% confidence interval of the no-manipulation (0.0 mg/kg buprenorphine) control group]. However, even doses as low as 0.005 mg/kg produced some pain relief, as shown by significantly higher pain thresholds at 1 h in those rats than in rats receiving surgery with no buprenorphine. As expected, the 0.01-mg/kg dose of buprenorphine produced results intermediate to those of the 0.005- and 0.05-mg/kg doses; the linear relationship between buprenorphine dose and pain relief is well established.1,7 These results support the use of the 0.05-mg/kg dose of buprenorphine for postoperative pain management. Buprenorphine effectively and in a dose-dependent fashion also reduced proximal allodynia (a pain response to a nonnoxious stimulus applied to the area of the surgery) during the immediate postoperative period (1 and 4 h). Consistent with the pain threshold results, the 0.05-mg/kg dose of buprenorphine produced the greatest decrease in allodynia, although the 0.01-mg/kg dose was statistically effective at reducing proximal allodynia in that period. However, none of the doses tested (0.005 to 0.05 mg/kg) abolished allodynia.
As expected, surgery induced a complex change in pain sensitivity over the 72-h postsurgery observation period. Rats that received no postoperative buprenorphine showed hyperalgesia (increased sensitivity to noxious stimuli) and allodynia (a pain response to nonnoxious stimuli) at the site of injury, and both of these effects improved over the 72-h observation period. These rats also showed an increase in secondary pain, which is characteristic of central sensitization—a neural adaptation induced by the surgical insult. The secondary pain had a delayed onset, apparent at 72 h but not 24 h. This finding means that recovery to isoalgesia is slowed by the later appearance of enhanced pain responses due to central sensitization. These results are consistent with the current understanding that increased pain sensitivity in the postoperative period is a complex phenomenon, involving contributions from diverse and somewhat independent physiologic sources (that is, both peripheral and central nociceptive and tactile somatosensory systems).
Results of experiment 1 also showed that the effect of buprenorphine on hyperalgesia and proximal allodynia was limited to the acute postoperative period; no effect of buprenorphine was apparent at later time points. In other words, all surgery-group rats showed similar amounts of hyperalgesia and proximal allodynia at 24 and 72 h. Judgments about the time course of analgesics are often confounded by the use of repeated-measures testing. However, this time course of the antihyperalgesic effects of buprenorphine is consistent with our previous work,15 which showed that the acute pain-inhibiting effects of buprenorphine last less than 8 h in a nonrepeated-measures design using an algesiometric test. Furthermore, experiment 2, in which testing for proximal allodynia began at 24 h, confirmed that the acute inhibition of primary pain by buprenorphine is limited to a duration of less than 24 h and therefore to the acute action of the drug.
Secondary pain was evaluated by using a distal-allodynia test at 24 and 72 h. Changes in pain sensitivity at this distal site are mediated by changes in neuronal sensory thresholds (central sensitization) rather than by peripheral signals from the site of injury to the sensory neurons.31 We expected that this measurement would be most sensitive to rebound pain induced by buprenorphine treatment. Other investigators23 evaluated buprenorphine by using the same postoperative pain model and found that rats receiving all doses of buprenorphine tested (0.025 to 0.1 mg/kg SC) experienced rebound hyperalgesia in the postoperative period (that is, allodynia scores were higher in rats that had received buprenorphine than in rats that received no analgesic). In our experiment 1, all surgery-group rats showed secondary pain at 72 h, and buprenorphine injection, regardless of dose, had no effect on the magnitude of allodynia. This result was surprising in 2 ways. First, in our test, secondary pain manifested at a much later time than we expected. Previous work31 using the same postoperative pain model showed secondary pain beginning 2 h after surgery and lasting for 24 h after surgery, with tactile thresholds returning to near normal by 48 h after surgery. Second, we did not observe rebound pain at 72 h, as was reported previously.23
Several design differences between our research and earlier studies23,31 could account for the apparent differences in results. We included a pain-threshold test in our study, whereas the other authors limited their testing to allodynia. In our study, perhaps repeated application of the noxious stimulus during pain-threshold testing may have induced inflammation or irritation in the rats’ feet, which then affected both control and surgery groups, making the determination of group differences more difficult. In addition, in 1 earlier study,23 rats received multiple doses of buprenorphine administered once daily on days 0, 1, and 2 after surgery, and postoperative measurements did not begin until 72 h. Rats in our experiment 1 may not have shown the magnitude of rebound hyperalgesia that was seen in the cited experiment because our rats received only 1 dose of postoperative buprenorphine. These differences were addressed in experiment 2 by including a higher dose of buprenorphine (0.1 mg/kg), reducing the number of tests to 2 (24 and 72 h), and eliminating the paw-withdrawal assay.
The results of experiment 2 showed that secondary allodynia is apparent by 24 h. The difference between experiments 1 and 2, on this point, was due mainly to longer latencies among controls in experiment 2 at 24 h [the control value in experiment 1 (Figure 3) was 16.6 ± 1.7 g, compared with 22.9 ± 1.8 g in experiment 2 (Figure 6)], supporting the possibility that either repeated measures prior to 24 h or exposure to the paw-withdrawal assay (a noxious-stimulus assay) confounded the results for distal allodynia in experiment 1. Furthermore, postoperative buprenorphine significantly influenced secondary pain at the 24-h test. Specifically, distal allodynia was absent at 24 h but present at 72 h in rats that received the 0.05-mg/kg dose of buprenorphine, suggesting that secondary pain appeared more slowly for rats in the 0.05-mg/kg dose surgery group. In contrast, all other surgery groups showed significant allodynia relative to controls at both 24 and 72 h. One possible explanation is that the effect of 0.05 mg/kg buprenorphine at 24 h was simply a continuation of an acute pain-inhibitory effect. This effect seems unlikely, however, because primary allodynia at 24 h was not affected by postoperative buprenorphine administration (compare Figure 5 and Figure 6). Moreover, distal allodynia was not absent in the rats receiving the highest dose of buprenorphine (0.1 mg/kg) at 24 h. These rats manifested significant distal allodynia at both 24 and 72 h, and the pain-inhibiting effect of buprenorphine is linear across the dose range tested here. Allodynia should also have been absent in the 0.1 mg/kg group if this observation was due to the acute inhibitory effects of buprenorphine. Another explanation is that the high dose (0.1 mg/kg) enhanced central sensitization and facilitated the emergence of secondary allodynia. In support of this conclusion, rats in the 0.1-mg/kg buprenorphine dose group, but not in lower-dose groups, also showed tolerance to the effects of morphine after 10 d. This phenomenon also relies on opioid-induced neural adaptations and therefore suggests that greater neural adaptations were induced by the 0.1 mg/kg buprenorphine dose than by the lower doses.
We did not find rebound allodynia in the rats receiving 0.1 mg/kg of buprenorphine; their allodynia thresholds were similar to, but not lower than, the 0-mg/kg buprenorphine rats of the surgery group. A limitation of this second experiment is that pain thresholds and allodynia were not assessed beyond 72 h, so our conclusions about rebound pain are restricted to its initiation rather than its duration.
The long-term consequences of postoperative opioid analgesic treatment on subsequent experimental manipulations remain a concern for many investigators. The effect of postoperative buprenorphine on subsequent sensitivity or tolerance to treatment with morphine show that the rats that received 0.1 mg/kg of buprenorphine immediately after surgery experienced increased tolerance to the analgesic effects of morphine administered 9 to 10 d later, suggesting that postoperative buprenorphine dosages of greater than 0.05 mg/kg have the potential to confound subsequent experimental manipulations with morphine. In comparison, neither surgery nor a single dose of 0.05 mg/kg or less of buprenorphine appears to produce a long-lasting change in sensitivity to the locomotor or analgesic effects of subsequently administered morphine.
The results of this study support the use of buprenorphine in the dose range currently prescribed. Our results from using measures of primary and secondary pain are consonant with numerous other reports primarily using indirect measures of pain (for example, body weight). However, this convergence of results does not necessarily mean that the results from all of these studies reflect changes in pain; instead it is more likely that the potency of systemically administered buprenorphine is similar on multiple independent neural circuits. The activation of multiple neural substrates gives rise to multiple behavioral changes that are coincidental and unrelated to pain. For example, opioids can increase feeding independent of their effect on pain;30 therefore, increases in postoperative body weight may be an effect of opioid-induced feeding and not due to pain reduction.
Finally, our results are limited in their application to the evaluation of analgesics used postoperatively and to the evaluation of the impact of a single postoperative dose of buprenorphine. Other investigators23 have reported that multiple injections of buprenorphine at 0.05 mg/kg or lower over the first few days postoperatively induce significant rebound pain. Furthermore, our algesiometric tests included evaluation of only stimulus- or manipulation-induced pain and not resting pain.
In summary, our results suggest that the commonly used therapeutic dose of buprenorphine, 0.05 mg/kg SC, is the minimum dose that produces isoalgesia acutely, and that this isoalgesia lasts for at least 4 h. The results did not support the hypothesis that lower buprenorphine doses could induce isoalgesia. Buprenorphine also inhibited allodynia over the first 24 h; again the 0.05-mg/kg dose seemed to have an advantage over lower and higher doses. The 0.05-mg/kg dose significantly inhibited primary allodynia at 1 and 4 h and, given the results at 24 h, seemed to slow the development of central sensitization, which underlies rebound pain. Doses lower than 0.05 mg/kg had no beneficial effect on the onset of distal allodynia; the 0.05-mg/kg dose adequately blocked allodynia at 24 h, and at 24 h the highest dose (0.1 mg/kg) actually produced distal allodynia (likely the onset of rebound pain mediated by central sensitization). Furthermore, we showed that the 0.05-mg/kg dose of buprenorphine, when given as a single postoperative injection, does not produce a long-term modification of subsequent morphine efficacy, but a higher dose of buprenorphine (0.1 mg/kg) did induce tolerance to the subsequent analgesic effect of morphine.
The use of opioid analgesics after surgery must be weighed carefully. Opioids such as buprenorphine are not a panacea. There are both positive and negative consequences, depending on dose and route of administration.15,25 Parenteral buprenorphine can relieve pain in the postoperative period but may induce a longer period of postoperative pain by enhancing central sensitization. Finally, an important consideration for some researchers is that intermediate to high doses of parenteral buprenorphine administered postoperatively can produce long-term changes in the response to subsequently administered opioids.
More research into postoperative analgesics is warranted. We suggest further exploration of the efficacy of nonsteroidal antiinflammatory drugs rather than opioids, the efficacy of preemptive (that is, before surgery) rather than postoperative opioid analgesics, the relationship between algesiometric results and those obtained in a pain-test model, and whether isoalgesia rather than hypoalgesia should be the goal of analgesics in the postoperative period. Finally, our results confirm the idea that postoperative pain is a complex phenomenon and that treatment thereof has both peripheral and central consequences. Evaluation of postsurgical analgesics needs to be done more carefully, and decisions need to take into account not only the efficacy of the drug on acute pain but also its effect on rebound hyperalgesia, primary allodynia, rebound allodynia, gastrointestinal distress, and, in experimental contexts, the likelihood that the analgesic will induce sensitization or tolerance to related drugs.
Acknowledgments
The authors would like to thank Seth Hurley and Thomas P Smith for excellent technical assistance during experiment 2. This research was supported by an ACLAM Foundation grant, Developing a metric for comparing analgesic efficacy in algesiometric testing with postsurgical pain relief (to MBK, JMD, and LBEM).
References
- 1.Abbott FV, Bonder M. 1997. Options for management of acute pain in the rat. Vet Rec 140:553–557 [DOI] [PubMed] [Google Scholar]
- 2.Bailey CP, Connor M. 2005. Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol 5:60–68 [DOI] [PubMed] [Google Scholar]
- 3.Brennan TJ. 1999. Post-operative models of nociception. ILAR J 40:129–136 [DOI] [PubMed] [Google Scholar]
- 4.Brennan TJ, Vandermeulen EP, Gebhart GF. 1996. Characterization of a rat model of incisional pain. Pain 64:493–501 [DOI] [PubMed] [Google Scholar]
- 5.Campora E, Merlini L, Pace M, Bruzzone M, Gottlieb A, Rosso R. 1991. The incidence of narcotic-induced emesis. J Pain Symptom Manage 6:428–430 [DOI] [PubMed] [Google Scholar]
- 6.Célérier E, Rivat C, Jun Y, Laulin JP, Larcer A, Reynier P, Simonnet G. 2000. Long-lasting hyperalgesia induced by fentanyl in rats: preventative effect of ketamine. Anesthesiology 92:465–472 [DOI] [PubMed] [Google Scholar]
- 7.Cowen A, Lewis JW, Macfarlane IR. 1977. Agonist and antagonist properties of buprenorphin, a new antinociceptive agent. Br J Pharmacol 60:537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devillers JP, Boisserie F, Laulin JP, Larcher A, Simonnet G. 1995. Simultaneous activation of spinal antiopioid system (neuropeptide FF) and pain facilitatory circuitry by stimulation of opioid receptors in rats. Brain Res 700:173–181 [DOI] [PubMed] [Google Scholar]
- 9.Dixon WJ. 1980. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20:441–462 [DOI] [PubMed] [Google Scholar]
- 10.Flecknell PA. Laboratory animal anesthesia, 2nd ed. San Diego (CA): Academic Press; 1996 [Google Scholar]
- 11.Grecksch G, Bartzsch K, Widera A, Becker A, Höllt V, Koch T. 2006. Development of tolerance and sensitization to different opioid agonists in rats. Psychopharmacology (Berlin) 186:177–184 [DOI] [PubMed] [Google Scholar]
- 12.Hahn EF. 1985. Testing and evaluation of opiate analgesics and antagonists. Methods Find Exp Clin Pharmacol 7:373–381 [PubMed] [Google Scholar]
- 13.Khallouk-Bousselmame R, Costentin J. 1994. Locomotor and analgesic effects of morphine and acetorphan in rats chronically treated with morphine or thiorphan. Eur Neuropsychopharmacol 4:137–143 [DOI] [PubMed] [Google Scholar]
- 14.Larcher A, Laulin JP, Célérier E, Le Moal M, Simonnet G. 1998. Acute tolerance associated with a single opiate administration: involvement of N-methyl-D-aspartate-dependent pain facilitatory systems. Neuroscience 84:583–589 [DOI] [PubMed] [Google Scholar]
- 15.Martin LBE, Thompson AC, Martin T, Kristal MB. 2001. Efficacy of orally administered buprenorphine in rats. Comp Med 51:43–48 [PubMed] [Google Scholar]
- 16.Melzack R, Coderre TJ, Katz J, Vaccarino AL. 2001. Central neuroplasticity and pathological pain. Ann N Y Acad Sci 933:157–174 [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health Public Health Service Policy on Humane Care and Use of Laboratory Animals. Bethesda (MD): Office of Laboratory Animal Welfare, National Institutes of Health; 2002 [Google Scholar]
- 18.National Research Council Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academy Press; 1996 [Google Scholar]
- 19.Randall LO, Sellitto JJ. 1957. A method for the measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther 111:409–419 [PubMed] [Google Scholar]
- 20.Richebé P, Rivat C, Laulin JP, Maurette P, Simonnet G. 2005. Ketamine improves the management of exaggerated postoperative pain observed in perioperative fentanyl-treated rats. Anesthesiology 102:421–428 [DOI] [PubMed] [Google Scholar]
- 21.Schug SA, Zech D, Grond S. 1992. Adverse effects of systemic opioid analgesics. Drug Saf 7:200–213 [DOI] [PubMed] [Google Scholar]
- 22.Spanagel R. 1995. Modulation of drug-induced sensitization processes by endogenous opioid systems. Behav Brain Res 70:37–49 [DOI] [PubMed] [Google Scholar]
- 23.Stewart L, Martin WJ. 2003. Evaluation of postoperative analgesia in a rat model of incisional pain. Contemp Top Lab Anim Sci 42:28–34 [PubMed] [Google Scholar]
- 24.Terenius L, Tamsen A. 1982. Endorphins and the modulation of acute pain. Acta Anaesthesiol Scand Suppl 74:21–24 [DOI] [PubMed] [Google Scholar]
- 25.Thompson AC, Kristal MB, Salaj A, Acheson A, Martin LBE, Martin T. 2004. Analgesic efficacy of oral buprenorphine in rats: methodological considerations. Comp Med 54:293–300 [PubMed] [Google Scholar]
- 26.Vanderschuren LJMJ, De Vries TJ, Wardeh G, Hogenboom FACM, Schoffelmeer ANM. 2001. A single exposure to morphine induces long-lasting behavioural and neurochemical sensitization in rats. Eur J Neurosci 14:1533–1538 [DOI] [PubMed] [Google Scholar]
- 27.White DA, Kalinichev M, Holtzman SG. 2004. Individual differences in locomotor reactivity to a novel environment and sensitivity to opioid drugs in the rat. II. Agonist-induced antinociception and antagonist-induced suppression of fluid consumption. Psychopharmacology (Berlin) 177:68–78 [DOI] [PubMed] [Google Scholar]
- 28.Whiteside GT, Harrison J, Bolet J, Mark L, Pearson M, Gottshall S, Walker K. 2004. Pharmacological characterization of a rat model of incisional pain. Br J Pharmacol 141:85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilder-Smith OHG. 2000. Changes in sensory processing after surgical nociception. Curr Rev Pain 4:234–241 [DOI] [PubMed] [Google Scholar]
- 30.Yim GK, Lowy MT. 1984. Opioids, feeding, and anorexias. Fed Proc 43:2893–2897 [PubMed] [Google Scholar]
- 31.Zahn PK, Brennan TJ. 1999. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology 90:863–872 [DOI] [PubMed] [Google Scholar]