Abstract
The purpose of this study was to evaluate the potential efficacy of 3-dimensional time-of-flight magnetic resonance angiography (TOF-MRA) to validate a canine ischemic stroke model. Ischemic stroke was induced through permanent middle cerebral artery occlusion (MCAO) in 5 healthy Beagle dogs. T2-turbo spin echo images and TOF-MRA were obtained with a 1.5-T magnetic resonance system before and 3 and 10 d after MCAO. In 3 dogs, angiograms of the brain obtained at 3 d after MCAO showed complete occlusion of the MCA; in addition, T2 hyperintensities were present unilaterally in the striatocapsular and cerebral cortex lesions. Partial occlusion of the proximal part of the MCA was identified in the 2 remaining dogs, with T2 hyperintensities present only in the striatocapsular lesions. The occluded sites were confirmed at necropsy. The results of this study demonstrate the potential of TOF-MRA to provide a detailed description of intracranial arteries and aid in the evaluation of flow impairment in a canine MCAO model.
Abbreviations: CE-MRA, contrast-enhanced magnetic resonance angiography; CVA, cerebral vascular accident; MCAO, middle cerebral artery occlusion; TOF-MRA, time-of-flight magnetic resonance angiography
Cerebral vascular accidents (CVAs) are the most common clinical presentation of cerebrovascular disease; they are the leading cause of death and long-term disability in humans.17 Preclinical and translational research into the causes, pathogenesis, and therapeutic management of stroke has used many animal models to improve our understanding of CVAs.7 Among them, nonrodent models (for example, cats, dogs, pigs, sheep, and monkeys) have several distinct advantages over rodent models (for example, mice, rats, and gerbils). First, nonrodent models accommodate the acquisition of concurrent and multiple blood samples in the same animal as well as complex physiologic monitoring. Second, higher mammals have gyrencephalic (convoluted) brains, which are structurally and functionally similar to the human brain. Finally, optimal resolution for magnetic resonance imaging techniques can be achieved due to the large brain size.7,15,17 Among nonrodent models, dogs are readily available, easy to care for, and have predictable intercurrent diseases. Therefore, canine models can be useful in the study of human neurologic disease.
CVAs can occur due to hemorrhage (hemorrhagic stroke) or infarction (ischemic stroke).6,12 Most animal stroke models are based on ischemic stroke.7 Experimental cerebral ischemia can be global, hemispheric, multifocal, or focal. Middle cerebral artery occlusion (MCAO) with an intraluminal filament is a useful method for inducing focal ischemia. Advantages of intravascular occlusion of a single major cerebral blood vessel by intraarterial blockade include avoiding the need for a craniotomy and the possibility of reperfusion.1,9 Many factors, including variable thread diameter and vessel size and biologic (interindividual or strain-related) differences, can lead to variability in the outcomes of vascular occlusion.1,21 Therefore, the MCAO procedure must be validated in the species used to obtain a reproducible model of focal ischemia. Because the canine vasculature is similar to that in humans in terms of vessel size and variability, using both dogs and humans to assess new diagnostic or endovascular techniques may be reasonable.
Until recently, the intracranial vessel status in dogs has been assessed by conventional angiography, including X-ray and digital subtraction angiography.5,6,15 However, the technical difficulties and disadvantages of conventional angiography, including the toxicity of ionizing radiation and contrast agents, inability to obtain cross-sectional imaging, invasiveness of the surgery needed for catheterization, and superimposition of images of the intra- and extracranial vessels, have led to investigation of alternative methods.13
Magnetic resonance angiography (MRA) is a useful diagnostic and experimental procedure because it can be used for rapid (that is, within 1 min) noninvasive assessment of the intracranial vascular status. Two techniques used for MRA are time-of-flight (TOF) and contrast-enhanced (CE) MRA.6 TOF-MRA is a technique based on the macroscopic motion of water protons, which results in intrinsic contrast between the stationary tissue and flowing blood, whereas CE-MRA is a technique based on the reduction of the T1 relaxation that occurs with contrast agents.1,2 Several preliminary studies have evaluated the canine vasculature. 4,13,14
MRA is a noninvasive screening tool for assessing the intracranial status of vessels in ischemic stroke.16 TOF-MRA can be performed with unlimited repetition to obtain a temporal profile or to evaluate the response to vascular therapy. In addition, because no contrast material is needed for TOF-MRA, the potential for decreased perfusion due to an impaired blood–brain barrier and subsequent poor resolution is eliminated, as is the concern regarding overlying contrast-enhanced cerebral veins that obscure the region of interest, thus complicating interpretation of resulting angiograms.2,18 We therefore hypothesized that the TOF-MRA might be useful for evaluation of a canine MCAO model because of the imaging method's ability to assess the vascular status of the main cerebral arteries.
The purpose of this study was to evaluate the efficacy of TOF-MRA to validate the results of the MCAO procedure. We therefore investigated the cerebral vasculature of dogs before and after MCAO by using TOF-MRA
Materials and Methods
Animals.
Five healthy laboratory Beagle dogs (2 male and 3 female; age, 2 to 3 y; weight, 10 to 12 kg; Harlan Interfauna, Huntingdon, UK) were used. All of the dogs were healthy with no history of neurologic disease; they had no signs of neurologic problems on physical examination. The dogs were screened for metabolic diseases by complete blood count and serum chemistry analysis. After arrival to our facility, dogs were allowed to acclimate for 2 wk, during which they were assessed daily for neurobehavioral abnormalities and general health. Each dog was housed in a single cage and fed twice daily with commercial dry food (Science Diet, Hill's Pet Nutrition, Topeka, KS); fresh water was supplied continuously by automatic dispenser under controlled environmental conditions [12:12-h light:dark cycles (light on from 8:00 to 20:00)]; temperature, 18 to 24 °C; relative humidity, 55% ± 10%; 8 air changes hourly]. The holding and care units for dogs were accredited by the Institutional Animal Care and Use Committee of Konkuk University (Seoul, Korea); the IACUC also approved the surgical procedures and experimental protocol. The approved study endpoint was at 10 d after MCAO. After the imaging studies (10 d after stroke), all dogs were euthanized with sodium pentobarbital (80 mg/kg IV; Entobar, Hanrim Pharm, Gyeonggi, Korea). At the end of the study, brains were removed carefully and the site of occlusion identified. Criteria for early euthanasia included: serious neurologic or clinical compromise and the inability of the animal to care for itself; inability to self-feed after the initial recovery period, and inactivity and lack of alertness for a continuous 24-h period.
Animal preparation, monitoring and surgery.
Feeding was restricted to 12 h before the induction of anesthesia. The dogs were premedicated with atropine (0.02 mg/kg SC; Atropine sulfate, Je-Il Pharm, Daegu, Korea) and acepromazine (0.2 mg/kg IM; Sedaject, Samu Median, Chungnam, Korea) and anesthetized 30 min later by using propofol (5 mg/kg IV; Anepol, Hana Pharm, Gyeonggi, Korea), orally intubated, and then mechanically ventilated. Anesthesia was maintained with isoflurane (Attane, Minrad, Orchard Park, NY) at 2% to 3% of the inspired volume during surgery. The oxygen delivery and ventilation rates were continuously monitored and adjusted to maintain the heart rate, blood oxygen saturation, and blood pH within normal limits. The fluid balance was maintained by intravenous administration of 0.9% sodium chloride. Rectal temperature was monitored continuously and maintained at 37 to 38 °C throughout the surgery.
Cerebral ischemia was induced by MCAO as previously described.9 Briefly, animals were positioned in lateral the recumbent position. Under sterile conditions, a cervical incision was made, and then the carotid sheath, at the level of bifurcation under the sternomastoideus muscle, was exposed by using blunt dissection and palpating the carotid pulse. After the internal and external carotid arteries were identified, a 16-gauge venous catheter was inserted into the internal carotid artery through the carotid bulb. An 18-gauge catheter connected to a 20-mL syringe, which was loaded with 12 mL physiologic saline and an embolus made by attaching a 7-mm length of silicone (Dow Corning, Midland, MI) to the end of 4-0 silk suture (B Braun Medical Industries, Penang, Malaysia), was inserted through the 16-gauge venous catheter. The embolus was flushed into the internal carotid artery to the origin of the MCA by using moderate force on the syringe plunger. The injection rate of saline was 2 mL/s. The catheters were removed, and the neck incision was sutured. In all dogs, MCAO was maintained until euthanasia.
Recovery.
After surgery and recovery from isoflurane anesthesia, dogs were extubated and returned to cages in the animal recovery room. A heating lamp was placed on the floor in front of the cage and the radiant heat directed to one side of the cage (not directly at the animal). Recirculating warm-water blankets were placed beneath a portion of the cages. The animals were watched continuously until they had recovered completely (about 6 h). The next day, dogs were transported to the holding area and checked every 3 h until euthanasia. The feeding behavior, hydration status, body temperature, and body weight were examined at least once daily. Softened food pallets were provided to facilitate food intake. Ampicillin (20 mg/kg PO every 12 h; Unibiotech, Gyeonggi, Korea) was administered for 1 wk to prevent bacterial infection. The incision site was examined and cleaned on follow-up until healed. To control pain, butorphanol (0.2 mg/kg, IM; Myungmoon Pharm, Seoul, Korea) was given as needed, usually twice a day for the first 3 to 5 d after surgery.
Imaging protocol and analysis.
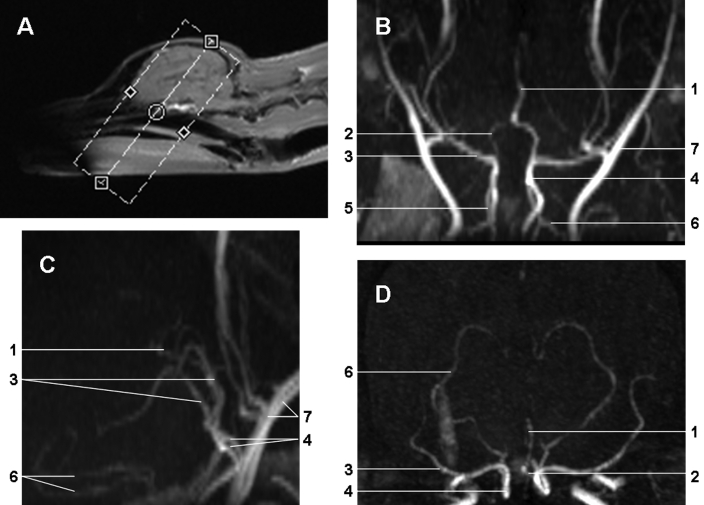
Imaging was performed with a 1.5-T MR system (Magnetom Avanto, Siemens AG, Berlin, Germany). The scans were performed serially before and 3 and 10 d after MCAO surgery. All dogs were fasted the night before MRA as a precaution for anesthesia. Dogs were premedicated and anesthetized by using the same procedures described in the animal preparation and monitoring section. Anesthesia was adjusted to maintain immobility during the scan. T2-weighted images were acquired by using a turbo spin echo sequence with a repetition time of 6980 ms, echo time of 86 ms, 30 sections, slice thickness of 3 mm, field of view of 15 × 15 cm, and a 512 × 512 matrix, for a total duration of 4 min 30 s. MRA was performed around the circle of Willis (Figure 1 A) with a 3-dimensional TOF sequence of a repetition time of 30 ms, echo time of 4.9 ms, a flip angle of 35º, field of view of 8 × 8 cm, a 160 × 256 matrix, 72 slices, and slice thickness of 0.69 mm. The acquisition time was 4 min 30 s.
Figure 1.
(A) Single-slab acquisition used for imaging of the intracranial circulation. Maximal intensity projection of 3D TOF-MRA, presented in the (B) dorsal, (C) sagittal, and (D) transverse orientations to depict the major cerebral arteries of the dog brain before MCAO. 1, anterior cerebral artery; 2, anterior communicating artery; 3, middle cerebral artery; 4, internal carotid artery; 5, caudal communicating artery; 6, caudal cerebral artery; and 7, maxillary artery
Angiograms were obtained by generating maximal intensity projection 3D reconstruction of the raw data. T2-weighted images were examined independently by both a neurologist and a neuroradiologist, neither of whom had any knowledge of the dog's symptoms. Ischemic lesions were measured on a separate workstation. The areas of abnormal hyperintensity were traced on each slice of the T2-weighted images, and then summed and multiplied with the slice thickness and the interslice gap to calculate the volume of the cerebral lesion. Results (in cm3) are expressed as mean ± SD unless otherwise stated.
Results
No dog met the criteria for early euthanasia, and anesthesia and physiologic parameters were well maintained throughout surgery in all dogs. Body weight and temperature remained unchanged throughout the study. No dog exhibited behavior suggestive of pain such as stiff body movements, reduced appetite, shivering, increased respirations with panting, vocalization, and biting or scratching at the incision sites.
Maximal intensity projection of TOF-MRA data in the dorsal, transverse, and sagittal orientations revealed not only the main cerebral arteries but also the branches of the middle and caudal cerebral arteries (Figure 1 B through D). Venous structures were not detectable due to their low flow velocity. The origin and branching of both MCAs were most apparent in the transverse view (Figure 1 D).
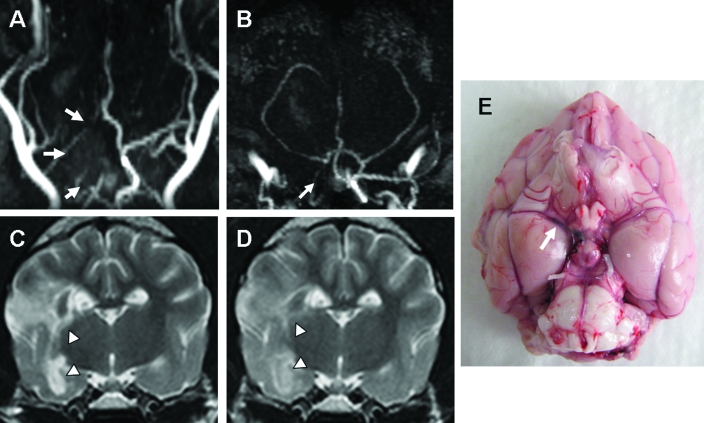
In 3 dogs, the angiograms of the brain obtained at 3 d after MCAO demonstrated the absence of flow at the occluded MCA, ipsilateral part of the circle of Willis, and internal carotid artery (Figure 2 A, B). T2 hyperintensities were noted unilaterally at the striatocapsular and cerebral cortex lesions (lesion volume, 6.08 ± 0.68 cm3; Figure 2 C). Neurologic signs related to these lesions, including altered mentation, hemianopsia, hemiparesis, head turning, circling, and perceptual deficits (menace response and facial sensation), were present. After 7 d, the volume of the T2 lesions was 5.94 ± 1.67 cm3, which is not significantly different from the day 3 values (Figure 2 D). In addition, altered mentation, motor functions, head turning, and circling were partially improved. TOF-MRA images did not differ at 3 and 10 d after MCAO (data not shown). Obstruction of the MCA in these 3 dogs was confirmed at necropsy (Figure 2 E).
Figure 2.
(A) Dorsal and (B) transverse TOF-MRA of the brain of a dog at 3 d after MCAO verified the absence of blood flow at the occluded MCA, ipsilateral part of the circle of Willis, and the internal cerebral artery (arrows). (C) Hyperintensities of the striatocapsular and cerebral cortex lesions were present unilaterally on T2-weighted images (arrowheads). (D) At 7 d after MCAO, these lesions were decreased on T2-weighted images (arrowheads). (E) Obstruction of the MCA (arrow) was confirmed at necropsy.
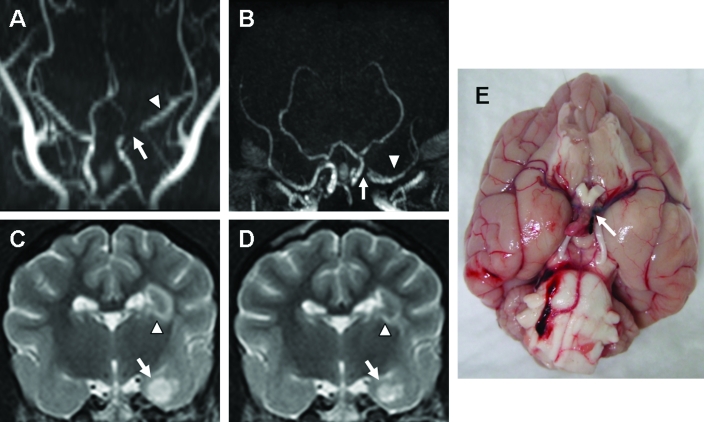
In 2 dogs, TOF-MRA at 3 d after MCAO revealed signal attenuation from the proximal branch of the MCA, anterior communicating artery, and internal carotid artery (Figure 3 A, B). T2 hyperintensities were noted in the striatocapsular lesions (volume, 1.12 ± 0.61 cm3; Figure 3 C); the volume of the T2 lesions was 0.76 ± 0.13 cm3 after 7 d (Figure 3 D). These dogs showed head turning, circling, and reduced responsiveness at 3 d after MCAO, but these signs had disappeared by 10 d after surgery. However, TOF-MRA at 10 d after MCAO did not show improvement of the impaired blood flow (data not shown). Even though located at the proximal part of the MCA, the suture embolus did not completely occlude the vessel (Figure 3 E).
Figure 3.
In 1 dog, signal attenuation from the proximal branch of the MCA (arrow), anterior communicating artery, and internal carotid artery was found on the (A) dorsal and (B) transverse TOF-MRA acquired 3 d after MCAO. The flow remaining at the ipsilateral MCA (arrowhead) was apparent on both angiograms. (C) T2 hyperintensities were found in striatocapsular lesions, including the internal capsule (arrowhead), piriform lobe, and amygdala (arrow), and (D) decreased after 7 d. (E) The suture was located at the proximal part of MCA (arrow) and not completely inserted into the MCA.
Discussion
The TOF-MRA of 3 of the dogs studied showed the main cerebral arteries and their branches. The development of vascular obstruction and changes in blood flow in these arteries in canine ischemic stroke was documented by TOF-MRA. The site and the severity of occlusion were related to disrupted blood flow and ischemia.
In the 3 dogs with complete occlusion of the distal internal and middle cerebral arteries, TOF-MRA confirmed lack of blood flow at the ipsilateral section of the circle of Willis, including the anterior and caudal communicating and middle and internal cerebral arteries. These dogs had striatocapsular and cerebral cortex lesions on T2-weighted imaging. The MCA has tree-like branches that provide blood to the entire lateral cortex of each hemisphere. The rostral choroid artery most often originates from the MCA in dogs3 and supplies blood to the internal capsule, lateral thalamus, amygdaloid body, caudal caudate nucleus, optic tract, and lentiform nucleus. The central branches of the MCA are the medial and lateral striatal arteries, which supply the basal ganglia, internal capsule, and thalamus.6 Because the MCAs of these 3 dogs likely were occluded completely, from the circle of Willis to the distal branches, ischemic lesions were apparent in the cerebral cortex and the striatocapsular regions.
Two dogs had incomplete MCA occlusion. Because the reduced flow signal in the proximal part of the MCA was accompanied by opacification of the distal branches and delayed flow signals on the TOF-MRA, we suspected partial occlusion. The T2-weighted images showed only a lacunar infarct, without abnormalities in the cerebral cortex. These findings suggest partial occlusion of the proximal branches of the MCA and maintenance of the blood supply from the distal MCA branches to the cortex.
In all dogs, the severity of the neurologic signs abated between 3 and 10 d after MCAO surgery. Because the ipsilateral MCA remained occluded and the occluded vessels did not regain patency, recruitment of blood flow through collateral vessels appeared to have developed but these collateral vessels were not visible on TOF-MRA at 10 d after the MCAO. TOF-MRA is sensitive to fast-flowing blood; only unsaturated blood entering the imaging volume between subsequent radiofrequency pulses produces a high signal.1 The technique overestimates the degree of vascular obstruction caused by saturation of slow flow and therefore can prompt a false diagnosis of vascular occlusion.11 However, because CE-MRA uses paramagnetic contrast agents, such as gadopentetate dimeglumine, to provide information on blood flow dynamics and the venous vascular anatomy, it can reveal arterial segments with low flow and avoid overestimation of vascular occlusion.1,11
Because we used only TOF-MRA, our study was subject to inaccurate estimation of the degree of vascular obstruction and did not detect collateral vasculature. However, in a prior rodent study19 normalization of the T2 relaxation time in ischemic lesions was not closely related to tissue recovery. This finding suggests that an ischemic injury can continue even though the volume of the T2 hyperintense lesions is decreased. Therefore the neurologic signs in our dogs might have improved due to reduced vasogenic edema and intracranial pressure without tissue recovery and reperfusion of the occluded vessels. For humans, many studies have been performed to determine the usefulness of CE-MRA in ischemic stroke.10,11,20 TOF-MRA can be repeated without limitation and has a short acquisition time; for these reasons, TOF-MRA has been used in clinical settings as well as in animal studies on ischemic stroke.1,2,6,11 A single preliminary study13 has assessed the CE-MRA features of the cranial vessels in normal dogs. Comparisons of the usefulness of the TOF-MRA and CE-MRA techniques require additional studies in dogs.
Signals from a single slab were acquired for imaging the intracranial circulation. The distal extracranial vessels, such as the maxillary and external ophthalmic arteries, were partially visible and interrupted the analysis of the cranial vessel anatomy. Even though the plane of acquisition ideally should be perpendicular to the long axis of the blood vessel,14 it could not be positioned vertically relative to the circle of Willis due to interference from the extracranial arteries. Therefore, further adjustments are needed for the placement of the slab to improve the visualization of the intracranial vessels.
The optimal MR imaging protocol for ischemic stroke includes diffusion-weighted imaging, fluid attenuation inversion recovery, perfusion-weighted imaging, and MRA.11 Perfusion-weighted imaging can estimate brain perfusion and therefore can be used with MRA to obtain comprehensive information on tissue perfusion.11,20 In the present study, we attempted to obtain perfusion-weighted images and related maps, but these attempts were unsuccessful due to technical difficulties. To assess regional impairment of cerebral blood perfusion, further studies with PWI are needed.
This variability of the embolic stroke model fully mimics the heterogeneous nature of human ischemic stroke, thereby facilitating the eventual translation and generalization of the results from this model to clinical practice.8 Because our model had a variable degree of stroke and a reproducible and homogeneous model is desirable when evaluating new therapeutic agents, such as antioxidants and stem cells, obstruction of the cerebral arteries should be verified immediately after MCAO surgery to assess homogeneity in the canine stroke model,. If, for example, inaccurate placement of a filament is confirmed rapidly by the MRA, the filament can be repositioned. In this study, we could not perform the MRA directly after MCAO surgery due to the difficulty of transporting the dogs from the surgical area to the MR facility.
In summary, the results of this study demonstrate the use of TOF-MRA for detailed visualization of intracranial arteries and the evaluation of flow impairment in a canine MCAO model. The use of TOF-MRA together with other MR sequences, including diffusion-weighted imaging, fluid attenuation inversion recovery, and perfusion-weighted imaging, might further increase the utility of the ischemia model in dogs. To overcome the disadvantages of TOF-MRA, the diagnostic and experimental value of CE-MRA should be assessed.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (R11-2002-103) and Bio R&D program through the Korea Science and Engineering Foundation funded by the Ministry of Education, Science and Technology (M10530010001-06N3001-00110).
References
- 1.Beckmann N, Stirnimann R, Bochelen D. 1999. High-resolution magnetic resonance angiography of the mouse brain: application to murine focal cerebral ischemia models. J Magn Reson 140:442–450 [DOI] [PubMed] [Google Scholar]
- 2.Besselmann M, Liu M, Diedenhofen M, Franke C, Hoehn M. 2001. MR angiographic investigation of transient focal cerebral ischemia in rat. NMR Biomed 14:289–296 [DOI] [PubMed] [Google Scholar]
- 3.Bertan V, Wilson CB. 1966. Anatomy of the anterior choroidal artery in the dog. Arch Neurol 14:526–529 [DOI] [PubMed] [Google Scholar]
- 4.Contreras S, Vázquez JM, Miguel AD, Morales M, Gil F, López O, Arencibia A. 2008. Magnetic resonance angiography of the normal canine heart and associated blood vessels. Vet J 178:130–132 [DOI] [PubMed] [Google Scholar]
- 5.Dorn AS. 1972. A standard technique for canine cerebral angiography. J Am Vet Med Assoc 161:1669–1675 [PubMed] [Google Scholar]
- 6.Dreesen RG. 1988. Cerebral angiography of the dog. Radiol Technol 59:513–516 [PubMed] [Google Scholar]
- 7.Graham SM, McCullough LD, Murphy SJ. 2004. Animal models of ischemic stroke: balancing experimental aims and animal care. Comp Med 54:486–496 [PubMed] [Google Scholar]
- 8.Harris AD, Kosior JC, Ryder RC, Andersen LB, Hu WY, Hudon M, Morrish WH, Sevick RJ, Wong J, Frayne R. 2007. MRI of ischemic stroke in canines: applications for monitoring intraarterial thrombolysis. J Magn Reson Imaging 26:1421–1428 [DOI] [PubMed] [Google Scholar]
- 9.Kang BT, Lee JH, Jung DI, Park C, Gu SH, Jeon HW, Jang DP, Lim CY, Quan FS, Kim YB, Cho ZH, Woo EJ, Park HM. 2007. Canine model of ischemic stroke with permanent middle cerebral artery occlusion: clinical and histopathological findings. J Vet Sci 8:369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JH, Shin T, Park JH, Chung SH, Choi NC, Lim BH. 1999. Various patterns of perfusion-weighted MR imaging and MR angiographic findings in hyperacute ischemic stroke. AJNR Am J Neuroradiol 20:613–620 [PMC free article] [PubMed] [Google Scholar]
- 11.Pedraza S, Silva Y, Mendez J, Inaraja L, Vera J, Serena J, Dávalos A. 2004. Comparison of preperfusion and postperfusion magnetic resonance angiography in acute stroke. Stroke 35:2105–2110 [DOI] [PubMed] [Google Scholar]
- 12.Rossmeisl JH, Jr, Rohleder JJ, Pickett JP, Duncan R, Herring IP. 2007. Presumed and confirmed striatocapsular brain infarctions in six dogs. Vet Ophthalmol 10:23–36 [DOI] [PubMed] [Google Scholar]
- 13.Sager M, Assheuer J, Trümmler H, Moormann K. 2007. Contrast-enhanced magnetic resonance angiography (CE-MRA) of intra- and extra-cranial vessels in dogs. Vet J 179:92–100 [DOI] [PubMed] [Google Scholar]
- 14.Seguin B, Tobias KM, Gavin PR, Tucker RL. 1999. Use of magnetic resonance angiography for diagnosis of portosystemic shunts in dogs. Vet Radiol Ultrasound 40:251–258 [DOI] [PubMed] [Google Scholar]
- 15.Shaibani A, Khawar S, Shin W, Cashen TA, Schirf B, Rohany M, Kakodkar S, Carroll TJ. 2006. First results in an MR imaging-compatible canine model of acute stroke. AJNR Am J Neuroradiol 27:1788–1793 [PMC free article] [PubMed] [Google Scholar]
- 16.Tomanek AI, Coutts SB, Demcuk AM, Hudon ME, Morrish WE, Sevick RJ, Simon JE, Frayne R, Buchan AM, Hill MD. 2006. MR angiography compared to conventional selective angiography in acute stroke. Can J Neurol Sci 33:58–62 [DOI] [PubMed] [Google Scholar]
- 17.Traystman RJ. 2003. Animal models of focal and global cerebral ischemia. ILAR J 44:85–95 [DOI] [PubMed] [Google Scholar]
- 18.Weber R, Ramos-Cabrer P, Hoehn M. 2006. Present status of magnetic resonance imaging and spectroscopy in animal stroke models. J Cereb Blood Flow Metab 26:591–604 [DOI] [PubMed] [Google Scholar]
- 19.Wegener S, Weber R, Ramos-Cabrer P, Uhlenkueken U, Sprenger C, Wiedermann D, Villringer A, Hoehn M. 2006. Temporal profile of T2-weighted MRI distinguishes between pannecrosis and selective neuronal death after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab 26:38–47 [DOI] [PubMed] [Google Scholar]
- 20.Yang JJ, Hill MD, Morrish WF, Hudon ME, Barber PA, Demchuk AM, Sevick RJ, Frayne R. 2002. Comparison of pre- and postcontrast 3D time-of-flight MR angiography for the evaluation of distal intracranial branch occlusions in acute ischemic stroke. AJNR Am J Neuroradiol 23:557–567 [PMC free article] [PubMed] [Google Scholar]
- 21.Yang YM, Feng XY, Yao ZW, Tang WJ, Liu HQ, Zhang LL. 2008. Magnetic resonance angiography of carotid and cerebral arterial occlusion in rats using a clinical scanner. J Neurosci Methods 167:176–183 [DOI] [PubMed] [Google Scholar]