Abstract
Young rats treated daily with intraperitoneal 4-vinylcyclohexene diepoxide (VCD) undergo selective destruction of primordial follicles, resulting in gradual ovarian failure resembling the menopausal transition in women. To determine whether VCD has similar effects on ovaries of older rats, adult and peripubertal Sprague–Dawley rats were injected intraperitoneally daily for 30 d with vehicle or VCD at 40 or 80 mg/kg. Body weight, food intake, complete blood counts, and markers of liver injury and renal function were measured during VCD treatment. Complete gross necropsy and microscopic observations were performed on day 31, and ovarian follicles were counted. At 80 mg/kg, VCD destroyed primordial and primary follicles to a similar extent in both adult and peripubertal animals, although adult rats likely started with fewer follicles and therefore approached follicle depletion. Treatment with VCD did not affect body weight, but food intake was reduced in both adult and peripubertal rats treated with 80 mg/kg VCD. Adult rats treated with 80 mg/kg VCD had neutrophilia and increased BUN and creatinine; in addition, 4 of these rats were euthanized on days 25 or 26 due to peritonitis. VCD treatment did not increase alanine aminotransferase levels, a marker of liver injury, although the 80-mg/kg dose increased liver weights. In conclusion, VCD effectively destroys small preantral follicles in adult Sprague–Dawley rats, making them a suitable model of the menopausal transition of women. However, because adult rats were more sensitive to the irritant properties of VCD, the use of a lower dose should be considered.
Abbreviations: VCD, 4-vinylcyclohexene diepoxide
Studies attempting to model the human menopause have relied heavily on using animals from which the ovaries have been removed surgically (ovariectomy). This approach has important limitations because women who enter natural menopause still have ovaries, which continue to produce hormones. Therefore, studies using ovariectomized animals cannot model the hormonal changes associated with the menopausal transition and postmenopausal period. However, rodent models of the menopausal transition and menopause that more closely mimic those of women have recently been developed.32,33,36 Mice or rats treated with daily intraperitoneal injections of the chemical 4-vinylcyclohexene diepoxide (VCD) undergo selective destruction of primordial and primary follicles.25 This treatment results in a gradual onset of ovarian failure because remaining larger follicles continue to develop and then ovulate or undergo atresia until they are depleted.36 These studies also demonstrate that the length of time to ovarian failure is dependent on VCD dose and duration of treatment.33,37 Moreover, in VCD-treated mice, the resulting follicle-depleted, stroma-intact ovary retains the ability to produce androgens.36 Therefore, taken together, these characteristics indicate that VCD-treated animals could be used to model the menopausal transition of women and enable research on diseases affecting women postmenopausally.
The ability of VCD to destroy preantral follicles in rats by repeated dosing has been well documented.16,23,24,37 However, to our knowledge, all of the VCD studies using rats that have been published to date have used peripubertal or young (28 to 58 d) Fisher 344 rats. Although younger animals have been useful in separating the effect of age from the effect of hormonal changes associated with VCD-induced ovarian failure,22,27,32,37 the use of older rodents may provide a more appropriate model for studying the combined effects of aging and hormonal aspects of menopause (for example, osteoporosis, cognitive decline, ovarian cancer).
Both young and adult Sprague–Dawley rats have been used extensively to model menopausal effects on osteoporosis,3,4,13,38,49 brain and cognitive functioning,2,14,15,29,34 lipids and cardiovascular health,30,35,53 bladder health and incontinence,6,21,31 and breast cancer.8,18,43,44 These studies used ovariectomized Sprague–Dawley rats ranging in age from 42 to 210 d. The use of this chemically induced model of menopause would be enhanced by determining whether VCD affects Sprague–Dawley rats differently and whether VCD has deleterious effects on nonovarian tissues. Furthermore, although more than a dozen publications have reported that repeated VCD dosing does not adversely affect young rodents,19,32,33,36,56 similar data have not been reported for adult Sprague–Dawley rats. The purpose of this study was to determine whether VCD affects the ovaries of peripubertal (28 d) and adult Sprague–Dawley rats differently.
Materials and Methods
Animal subjects, drugs, and diets.
Female Sprague–Dawley rats (Taconic Farms, Albany, NY) were used for all 3 studies. Animals were pair-housed in clear polycarbonate conventional housing cages with woodchip bedding, in a controlled environment [21.1 ºC (70 °F), 12:12-h light:dark cycle] and had ad libitum access to water and rat chow (LabDiet, Richmond, IN). For all studies, the occupational chemical VCD (96% purity, Sigma-Aldrich, St Louis, MO) was used, and all mixing of this chemical was done under a fume hood. Once opened, VCD was stored at −20 °C with a dessicant to prevent water condensation and potential reaction with the epoxides. All animal procedures were done in accordance with federal, state and institutional guidelines, and all studies were approved by the Institutional Animal Care and Use Committee of Wake Forest University.
Experimental design: route and dose determination.
Two preliminary dose finding studies were conducted, 1 using intramuscular dosing, and the other using intraperitoneal dosing. The main study used intraperitoneal dosing.
Intramuscular dosing study.
In the first preliminary study, a potential alternative to intraperitoneal administration of VCD was investigated. Ten adult female rats (mean age 129 ± 4.8 d, range 107 to 148) were assigned randomly to the following treatment groups: vehicle only (n = 5); VCD at 80 mg/kg body weight (n = 2); and VCD 160 mg/kg body weight (n = 3). The vehicle was cottonseed oil and was mixed with VCD at a dilution of either 1:1 (160 mg/kg) or 1:5 (80 mg/kg). All treatments were administered via daily intramuscular injections (total volume, 0.08 to 0.10 mL) into the hindleg muscles (semimembranosus–biceps femoris) for as long as 15 d. To ensure that the injection site was consistent and could be identified at necropsy, the skin over the muscle was tattooed. Rats were anesthetized with isoflurane (3% to effect) for all injections to ensure accurate placement of the needle and to avoid direct injection into the sciatic nerve. The total anesthetic time was 3 to 5 min per injection. Although the study was designed to include 15 daily injections, the animals treated with 160 mg/kg VCD (n = 3) became ill (exhibited lethargy and difficulty ambulating) after 12 to 13 d of dosing and, along with 3 vehicle-treated animals to serve as controls, were euthanized and necropsied at that time. Animals treated with 80 mg/kg VCD (n = 2), along with the remaining vehicle-treated animals (n = 2), completed the 15-d treatment period without adverse clinical signs and were necropsied.
IP dosing study.
In the second preliminary study, the effect of 2 previously published doses23,36 of VCD were administered by the traditional intraperitoneal route. Eighteen female adult rats (mean age, 154 ± 4.1 d) were assigned randomly to the following treatment groups: vehicle only (n = 6); VCD at 80 mg/kg (n = 6); and VCD at 160 mg/kg (n = 6). For both VCD groups, sesame oil (20 mL) was mixed with VCD (1.2 mL) for a dilution of approximately 17:1. Daily injections were administered through daily intraperitoneal injection (total volume, 0.4 to 0.8 mL) for as long as 30 d. Rats were anesthetized as described above for the intramuscular study to prevent movement and potential injection of VCD into visceral organs. As in the intramuscular study, rats treated with 160 mg/kg VCD became ill (exhibited lethargy and dyspnea) after 10 to 13 d of dosing and were euthanized. Rats treated with vehicle alone or 80 mg/kg VCD IP completed the 30 daily injections and were necropsied at day 31 (that is, after 30 full days of dosing).
Main study: adult compared with peripubertal rats.
For the main study, 60 female rats were allocated into one of three treatment groups [vehicle (sesame oil), VCD at 40 mg/kg mixed with sesame oil, or VCD at 80 mg/kg mixed with sesame oil]. Each treatment group consisted of 10 adult and 10 peripubertal rats, but 1 adult rat in the VCD80 group died of nontreatment-related illness shortly into the study; therefore, the adult VCD80 group only contained 9 subjects. Rats were dosed at 40 or 80 mg/kg VCD IP for 30 d. This dosing period was chosen because in most studies using 80 mg/kg VCD IP in Fisher 344 rats, the dosing period did not exceed 30 d. We elected to use the same dosing regimen so that we could compare the previous results with ours.
Peripubertal rats were 28 d of age at the beginning of the experiment. Treatment groups were balanced by initial body weight. Because Sprague–Dawley rats enter puberty at about 30 d of age,21 the term ‘peripubertal’ is used here as a descriptive term for rats that are near the beginning of puberty, rather than as a term of classification. For adult rats, treatment groups were balanced prior to the experimental period by both baseline body weight and age (range, 205 to 319 d; mean, 269 d). As in the preliminary studies, rats were anesthetized for dosing to ensure accurate needle placement to avoid inadvertent injection of VCD into visceral organs. VCD was formulated and administered as described for the preliminary IP study. To account for their rapid rate of growth, peripubertal rats were weighed each day to determine the treatment dose, whereas adult rats were weighed every 5 d to determine treatment dose.
Ovarian follicles.
For all studies, both ovaries were removed at necropsy, trimmed of fat, weighed, and fixed in Bouin's solution [75 mL picric acid solution (1.3%), 25 mL formaldehyde (37%), and 5 mL glacial acetic acid] for 24 h and then transferred to 70% ethanol. One (right) ovary from each rat was processed for standard histologic interpretation. The second (left) ovary was transferred to the University of Arizona for follicle counting (PBH, PJC). The ovaries were sectioned serially (4 to 5 µm) and stained with hematoxylin and eosin. Follicles were classified and counted in every 40th section to avoid double counting of small preantral follicles, and only follicles with an oocyte nucleus were counted. The total number of sections per ovary varied from 15 to 19, depending on differences in ovarian size and the presence (or absence) of corpora lutea. Data are reported as total follicles counted per ovary. Follicles were classified as primordial (oocytes surrounded by a single layer of flattened granulosa cells), primary (oocyte surrounded by a single layer of cuboidal granulosa cells), and secondary (oocytes surrounded by 2 or more layers of cuboidal granulosa cells).41
Body weight.
In the rats given VCD intramuscularly, body weight was recorded at baseline; on day 8, 9, or 10; and at necropsy (after 12 to 13 d for animals treated with VCD 160 mg/kg and 3 control animals; on day 15 for all other animals). In the intraperitoneal dosing study using adult rats only, body weight was recorded at baseline; days 9, 17, and 23; and at necropsy (after 10 to 13 d for animals treated with VCD 160 mg/kg; on day 31 for all other animals). For the main study, adult animals were weighed at baseline and then every 5 d until necropsy (day 25 or 26 for 4 animals treated with 80 mg/kg VCD and day 31 for all other animals). Peripubertal animals were weighed every day beginning at baseline and ending at necropsy (day 31).
Food intake.
For the main study only, food intake was measured daily. A digital scale was used to weigh 250 g of fresh chow twice weekly, which then was placed in each cage. Daily combined food intake for the 2 rats in each cage was measured by weighing all uneaten chow in the cage each day and subtracting that amount from the previous day's amount. Twice each week, the cage was emptied of all remaining food, and 250 g of fresh rodent chow was offered. Because rats were pair-housed, individual food intake could not be determined.
Complete blood counts and clinical chemistry measures.
In the main study only (that is, comparing peripubertal and adult rats with 40 and 80 mg/kg VCD), blood was collected from each animal at baseline and on days 10, 20, and 31 (necropsy) by using 5.5-mm lancets (Golden Rod Animal Lancets, Medipoint, Mineola, NY), heparinized 125-µL mini-capillary blood collection tubes (Safe-T-Fill, Ram Scientific, Yonkers, NY), and 0.5-mL microcentrifuge tubes (Sarstedt, Newton, NC). The area surrounding the lateral or medial saphenous vein was cleaned with alcohol. The vessel of interest was visualized and punctured, and the sample was collected into the microcentrifuge tubes. A minimum of 100 µL blood was collected in the anticoagulant tube for hematology and 200 to 250 µL in the microcentrifuge tube for clinical chemistry analysis of serum. Hematology measures analyzed (Hemavet model 950, Drew Scientific, Oxford, CT) included total white blood cell count, platelets, hemoglobin, hematocrit, segmented neutrophils, and lymphocytes. Chemistry measures analyzed (ACE Alera Clinical Chemistry System, Alfa Wassermann, West Caldwell, NJ) included: BUN, creatinine, and alanine aminotransferase.
Necropsy.
A complete necropsy was done on the day of the final dose (day 15) in the intramuscular study and 24 h after the final VCD or vehicle injection for the intraperitoneal studies, unless adverse clinical signs required euthanasia of the animal prior to the end of the study. For any such animals, a complete diagnostic necropsy was performed by a board-certified pathologist (JMC, NDK). In the intramuscular and intraperitoneal preliminary dosing studies, animals were euthanized by first being anesthetized with isoflurane and then injected with pentobarbital (100 mg/kg IP). In the main IP study, animals were euthanized with CO2 followed by thoracotomy.
External observations at necropsy included: general body condition (for example overweight, underweight, emaciation); condition of hair coat and underlying skin; eyes, mouth and lips; nose (for example, smooth and glistening, dry, crusty or inflamed, fresh or dried exudate or serous fluid); anus and external genitalia; and any other external lesions. Body weight was recorded at the time of necropsy, and blood was collected for complete blood counts and analysis (main study). Ovaries, uterus, liver, spleen, kidneys, adrenal glands, heart, lungs, brain, small and large intestines, stomach, and urinary bladder from rats in all treatment groups were examined grossly, and tissue samples from each organ were collected and fixed in 10% neutral buffered formalin (all studies). After removal, organs were trimmed of fat and adhesions prior to being weighed. Ovaries were weighed separately from the uterus, with the uterus defined as the tissue from the uterine horns to the cervix.
In the preliminary intramuscular study, a section of hip muscle (approximately 1.5 × 1.5 × 1 cm), including the semimembranosus and biceps femoris muscles, and a section of the sciatic nerve were collected and fixed in 10% neutral buffered formalin. In the main study, the following tissues were collected but not weighed: vagina and cervix, sternum (bone marrow), eyes, thyroid glands, parathyroid glands, trachea and esophagus, cervical salivary glands, skin, abdominal wall muscle, mammary glands, mesenteric lymph nodes, cecum, and aorta. All tissues were embedded in paraffin, sectioned at 4 to 6 µm, stained with hematoxylin and eosin, and examined by light microscopy. Histopathologic evaluation of all tissues was done by a board-certified pathologist (NDK, JMC) who was blinded to treatment.
Data analysis.
For all 3 studies, data were analyzed by using SAS software (version 9.1.3, SAS Institute, Cary, NC) with 2-tailed significance. The threshold for statistical significance was a P value of less than 0.05.
Intramuscular dosing study.
Rats were assigned randomly to 1 of 3 treatment groups (vehicle, 80 mg/kg VCD, 160 mg/kg VCD).The Shapiro–Wilk test was used to determine that the assumption of normality was not violated for any of the variables of interest (primordial, primary, and secondary follicles and body weight). Variables then underwent 1-way ANOVA and posthoc analyses using pairwise (2-tailed) t tests to identify significant treatment group differences.
Intraperitoneal dosing study.
The goal of the statistical analyses was to determine differences in follicle counts, body weight and organ weights among the treatment groups (vehicle, VCD at 80 mg/kg, VCD at 160 mg/kg). For the analysis of follicle counts and organ weights, the vehicle treatment group was compared with both the VCD80 and the VCD160 groups. Differences between the VCD80 and VCD160 groups were not examined because the VCD160 group was removed on days 16 and 17. Poisson regression (genmod procedure) was used to look for differences in follicle counts, and the model used age and treatment assignment as independent variables and the number of observed follicles as the dependent variable. Organ weights were analyzed (mixed procedure), and the model included baseline body weight and treatment assignment as independent variables. Residual diagnostics were performed to investigate model fit. Ovary and uterine weights required log transformations to stabilize the variance. The change in body weight from baseline to day 9 was investigated between all treatment groups. Differences in body weight between the vehicle and VCD80 groups were investigated by using a repeated-measures analysis at study end.
Main study: adult compared with peripubertal rats.
The intent of the statistical analyses in the main study was to determine the effect of age and treatment (vehicle, VCD at 40 mg/kg, and VCD at 80 mg/kg) on ovarian follicle counts, body weight, food intake, complete blood counts and clinical chemistry measures, and organ weights. The genmod procedure was used to do Poisson regression with a log-link to determine differences in follicle counts. The model used age and treatment condition as independent variables and the number of observed follicles as the dependent variable. The 2 age groups were analyzed separately for food intake, body weight, complete blood count values, and clinical chemistry measures because these 2 populations (peripubertal and adults) have their own norms for these measures (for example, peripubertal rats are expected to eat increasingly more food each day and gain weight, whereas adult rats are expected to eat the same amount of food each day and weigh the same each day). Food intake, body weight, complete blood counts, and chemistry analyses were modeled by using repeated measures with an autoregressive correlation structure. Because some biochemistry values were outliers (creatinine, BUN, and alanine aminotransferase), the log of those individual variables was used in analysis. To investigate the influence of the extreme values on the analysis, these values were removed, and the analysis was rerun, providing similar results. Organ weights were analyzed by using the mixed procedure. Left and right organs were added together for total organ weight, and organ weights were normalized by body weight to be expressed as grams per 100 g body weight. Using the body weight at baseline or at necropsy for normalization of organ weight gave similar results for adult rats, and so necropsy body weights were used for normalization, both to account for young rats’ growth and because organs were collected at necropsy. The independent variables in the model included body weight at necropsy, treatment condition, age group, and 2-way interactions that were significant. Residual diagnostics were done to determine model fit. Some of the organs (heart, uterus, lung, kidney) required log transformation to stabilize the variance. Differences in least square means were generated for meaningful comparisons. For looking at differences of simple effects, Bonferroni-adjusted P values were used to determine significance. Raw P values were used to determine significance for the comparison of main effects.
Results
Route and dose determination studies.
In both preliminary studies (intramuscular dosing for 15 d and intraperitoneal for 30 d), adverse clinical outcomes (weight loss, lethargy, labored respiration, and difficulty ambulating) were seen in adult animals given 160 mg/kg; these rats were euthanized before the end of the study, on days 10 to 13. In addition, rats treated with VCD at 80 mg/kg IM had prominent inflammatory lesions in the muscle at the site of injection (Figure 1). No adverse clinical outcomes were seen in rats treated with 80 mg/kg VCD IP. The results of these preliminary studies informed our decision to use 2 lower doses of VCD (40 and 80 mg/kg) given IP for the investigation of VCD's effects on primordial follicles in adult and peripubertal Sprague–Dawley rats.
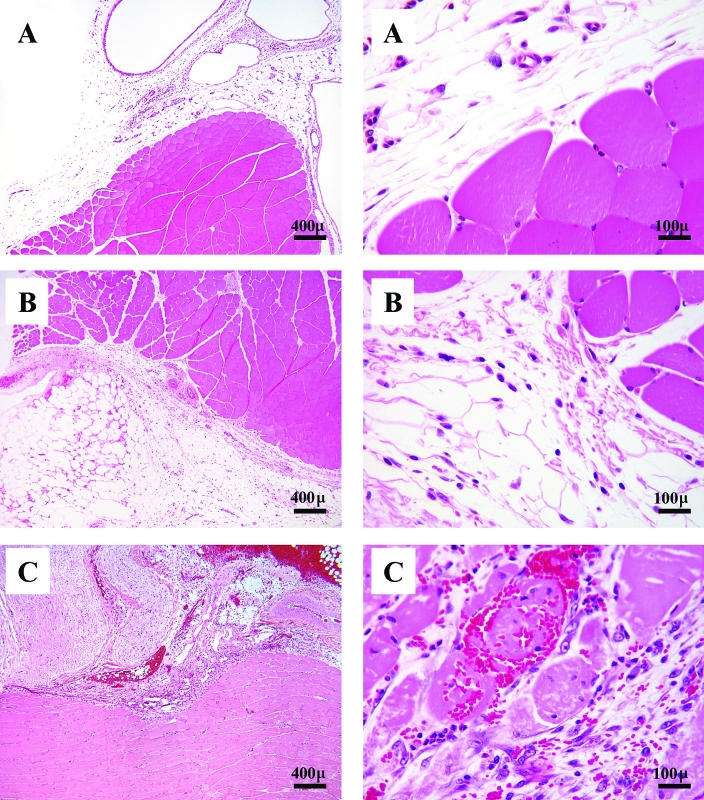
Figure 1.
Photomicrographs of semimembranosus–biceps femoris muscle from adult Sprague–Dawley rats treated with (A) vehicle (showing mild, focal fasciitis); (B) 80 mg/kg VCD (moderate, focal, fasciitis, myositis and steatitis with necrosis; or (C) 160 mg/kg VCD (severe, necrotizing myositis). Hematoxylin and eosin stain; magnification, ×40 (left panel), ×400 (right panel).
Ovarian follicles.
Intramuscular dosing study.
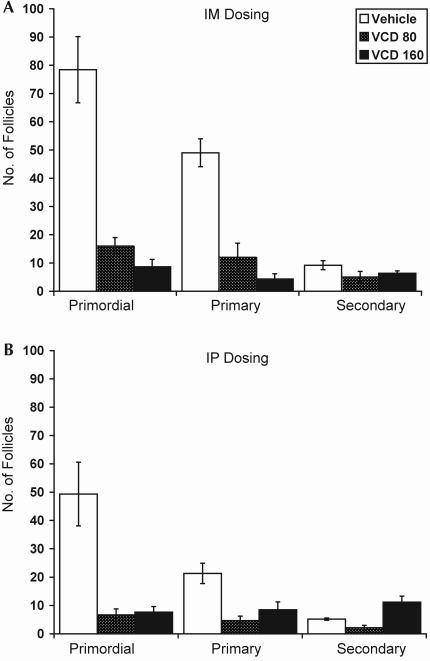
Destruction of primordial and primary follicles due to treatment with 80 mg/kg VCD IM for 15 d in adult Sprague–Dawley rats was statistically indistinguishable from that due to 160 mg/kg IM for 12 to 13 d (Figure 2 A, P < 0.05 for both compared with vehicle; P = 0.7 and P = 0.4, respectively, for 80 and 160 mg/kg). Treatment with 80 mg/kg daily for 15 d decreased the numbers of primordial follicles by 80% (vehicle, 78.4 ± 11.7 follicles per ovary; VCD, 16.0 ± 3.0 follicles per ovary; P < 0.01) and primary follicles by 76% (vehicle, 49.0 ± 1.3 follicles per ovary; VCD, 12.0 ± 5.0 follicles per ovary; P < 0.01). Rats treated with 160 mg/kg VCD for only 12 to 13 d had reductions of approximately 90% in the number of primordial follicles (vehicle, 78.4 ± 11.7 follicles per ovary; VCD, 8.7 ± 2.6 follicles per ovary; P < 0.01) and approximately 99% of primary follicles (vehicle, 49.0 ± 1.3 follicles per ovary; VCD, 4.3 ± 1.9 follicles per ovary; P < 0.001). VCD had no effect on secondary follicles with either dose [F(2,9)= 1.71; P = 0.2483].
Figure 2.
Effect of dose and route of administration of VCD on primordial, primary, and secondary ovarian follicles in Sprague–Dawley rats in preliminary dosing studies. (A) Adult rats (mean age, 129 d) given either vehicle (n = 5) or VCD at 80 mg/kg (n = 2) for 15 d or VCD at 160 mg/kg (n = 3) for 12 or 13 d, delivered intramuscularly. (B) Adult rats (mean age, 154 d) given either vehicle (n = 6) or VCD at 80 mg/kg (n = 6) for 30 d or VCD at 160 mg/kg (n = 6) for 10 to 13 d, delivered intraperitoneally. Data are expressed as number of follicles per ovary (mean ± SE).
Intraperitoneal dosing study.
Daily intraperitoneal injections of VCD for 30 d in adult Sprague–Dawley rats resulted in a similar pattern of decrease in follicular number as was seen with intramuscular administration (Figure 2 B). Compared with control, 80 mg/kg and 160 mg/kg VCD reduced primordial and primary follicle counts by 80% and 60%, respectively (P < 0.001 for both), despite the fact that the treatment duration for rats given 160 mg/kg (10 to 13 d) was less than half that of those given 80 mg/kg (30 d). A reduction in the number of secondary follicles was observed after treatment with 80 mg/kg VCD, whereas rats in the VCD160 group had more secondary follicles compared with controls (vehicle, 5.17 ± 0.4 follicles per ovary; VCD80, 2.1 ± 0.79 follicles per ovary; P = 0.004 compared with controls; VCD160, 8.5 ± 2.75 follicles per ovary; P = 0.0001 compared with controls).
Main study: adult compared with peripubertal rats.
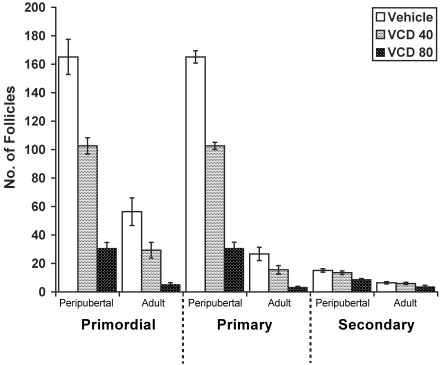
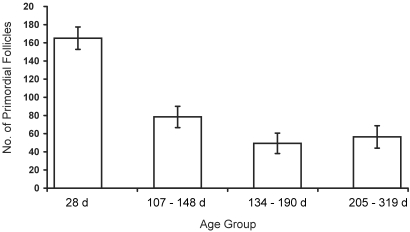
VCD treatment and age each exerted significant, independent effects resulting in a reduction of primordial, primary, and secondary follicles (P < 0.0001 for all comparisons; Figure 3). There was no significant interaction between age and treatment for any follicle type. Comparisons of both age groups showed that, for primordial and primary follicles, the effects of the 3 treatments (vehicle only, VCD40, and VCD80) were significantly different from each other (primordial, P < 0.0001; primary, P = 0.008). For secondary follicles, the effect of VCD80 was significantly different from that of vehicle (P < 0.0001) and VCD40 (P = 0.001), but VCD40 and vehicle did not differ from each other (P = 0.485) in their effects on secondary follicles. As in previous studies,16 VCD did not affect antral follicles at either dose compared with control values in either age group (for adults: VCD40, P = 0.07; VCD80, P = 0.30; for peripubertal rats: VCD40, P = 0.10; VCD80, P = 0.44). In terms of percentage reduction in follicle number, VCD40 decreased primordial follicles by approximately 48% in adult rats and approximately 37.9% in peripubertal rats while reducing primary follicles by approximately 41.9% in adult rats and approximately 19% in peripubertal rats. VCD80 decreased the numbers of primordial follicles by approximately 90.7% in adult rats and approximately 81.4% in peripubertal rats and of primary follicles by approximately 87.5% in adult rats and 68.3% in peripubertal rats. Although the magnitude of VCD's effect was similar in both age groups, the adult rats likely started the experiment with fewer follicles since a clear decrease (P < 0.05) in primordial follicle number with age can be seen in vehicle-treated animals of different ages across the three studies (Figure 4).
Figure 3.
Effect of VCD dose and age on primordial, primary, and secondary ovarian follicles in Sprague–Dawley rats. Adult rats (mean age, 269 d) or peripubertal rats (age, 28 d) were given either vehicle, VCD at 40 mg/kg, or VCD at 80 mg/kg IP for 30 d (n = 10 for each group, except n = 9 for adult rats treated with VCD at 80 mg/kg). Data are expressed as number of follicles per ovary (mean ± SE).
Figure 4.
Effect of age on primordial follicles in Sprague–Dawley rats. Vehicle-treated animals across the 3 studies (2 preliminary dosing studies and the main, age-related study) are shown by age: 28 d (n = 10); 107 to 148 d (n = 5); 134 to 190 d (n = 6); and 205 to 319 d (n = 10). Data are expressed as number of follicles per ovary (mean ± SE).
Body weight.
Intramuscular dosing study.
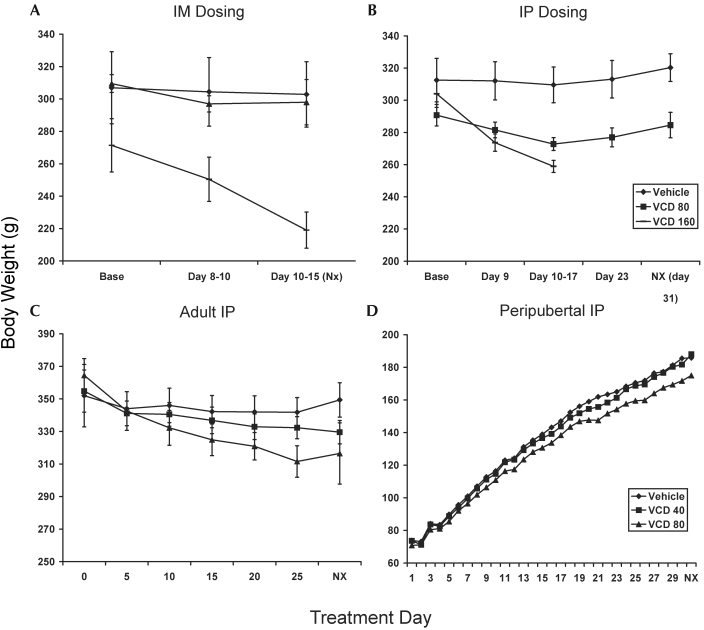
Rats treated with 160 mg/kg VCD IM (Figure 5 A) lost nearly 19% of their body weight after VCD treatment (after treatment, 219 ± 8.7 g; before treatment, 271.4 ± 16.4 g; P < 0.01 compared with vehicle or 80 mg/kg), whereas vehicle administration was not associated with significant weight loss (–1%), nor was VCD administration at 80 mg/kg (–4%).
Figure 5.
Effect of route of administration and dose of VCD on body weight in Sprague–Dawley rats. (A) Adult rats (mean age, 129 d) given either vehicle (n = 5) or VCD at 80 mg/kg (n = 2) for 15 d or VCD at 160 mg/kg (n = 3) for 12 or 13 d, delivered intramuscularly. (B) Adult rats (mean age, 154 d) given either vehicle (n = 6) or VCD at 80 mg/kg (n = 6) for 30 d or VCD at 160 mg/kg (n = 6) for 10 to 13 d, delivered intraperitoneally. For both A and B, animals in the VCD160 group were euthanized before the end of the study (day 12 or 13 for intramuscular study and days 10 to 13 for intraperitoneal study). (C) Adult rats (mean age, 269 d) in the age-comparison study were given either vehicle (n = 10), VCD at 40 mg/kg (n = 10), or VCD at 80 mg/kg (n = 9) IP for 30 d. (D) Peripubertal rats (age, 28 d) were given either vehicle (n = 10), VCD at 40 mg/kg (n = 10), or VCD at 80 mg/kg (n = 10) IP for 30 d. Data are expressed as mean body weight (g) ± SE. Error bars are not included for peripubertal rats because there were no significant effects due to treatment in this age group.
Intraperitoneal dosing study.
Adult Sprague–Dawley rats treated with 160 mg/kg VCD IP (Figure 5 B) experienced significant weight loss by day 9 of treatment (approximately 11% of baseline weight, −29.23 g, P = 0.006 compared with vehicle) and by days 10 to 13, approximately 16% of baseline body weight had been lost. Rats in the 80 mg/kg VCD group had a nonsignificant trend (P = 0.17) for lower baseline body weight than the other groups. Those rats experienced a 5% decrease in body weight at days 16 to 17 compared with their baseline body weight and weighed less than vehicle-treated rats at the end of the study (P = 0.02 compared with controls at necropsy). However, this dose did not produce a significant change in body weight at the end of the experiment (–8.73 ± 6.9 g, P = 0.22).
Main study: adult compared with peripubertal rats.
The effect of VCD on body weight in adult (Figure 5 C) and peripubertal rats (Figure 5 D) in the main study was similar to that seen in the preliminary intraperitoneal study. Body weight in the peripubertal rats was not significantly affected by VCD treatment (vehicle, +55%; VCD40, +54.6%; VCD80, +53.7%; P = 0.3629). For adult rats, time and treatment condition interacted significantly (P < 0.0001), with vehicle-treated rats showing a slight weight gain (approximately 0.69%) from baseline, VCD40-treated rats losing approximately 7.1% body weight, and VCD80 losing approximately 11% body weight. Because of the significant interaction with time, comparisons to determine significant effects of treatment could not be made.
Food intake.
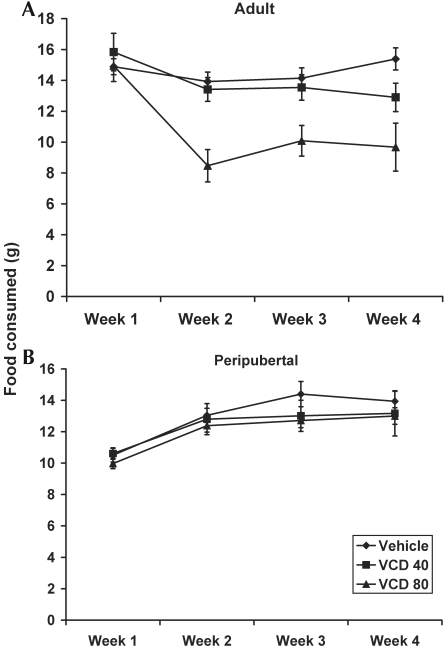
The effect of VCD on food intake for adult rats in the main study is shown in Figure 6. Peripubertal and adult rats were analyzed separately for food intake because peripubertal rats were expected to increase food intake over time as a result of the increased caloric requirement related to growth, whereas food intake was expected to remain fairly constant over time for adult rats. Peripubertal rats showed a significant effect of treatment on food intake (P = 0.001), with the greatest effect at week 3. Peripubertal rats treated with VCD at 80 mg/kg ate significantly less than those treated with either vehicle (P = 0.0005) or VCD at 40 mg/kg (P = 0.0023). In terms of percentage change from baseline, peripubertal rats in the vehicle group increased their mean food intake by 32% at the end of the study compared with baseline; peripubertal rats in the VCD40 group increased their food intake by approximately 29.2%, and peripubertal rats in the VCD80 group increased their food intake by approximately 27.4%. Adult rats showed significant (P = 0.0003) time × treatment interaction, indicating that the effect of VCD on body weight varied between groups and time points during the 30-d study. Overall during the 30-d treatment period, adult rats in the vehicle group daily consumed 14.80 ± 0.21 g; those in the VCD40 group consumed 13.81 ± 0.39 g; and those in the VCD80 group consumed 10.42 ± 0.45 g.
Figure 6.
Effect of VCD on food intake (g) for adult (mean age, 269 d) and peripubertal (age, 28 d) Sprague–Dawley rats. Animals were injected intraperitoneally daily for 30 d with either vehicle, VCD at 40 mg/kg, or VCD at 80 mg/kg. Data are expressed as weekly food intake (g; mean ± SE).
Complete blood counts.
For peripubertal rats, there were no main effects of treatment on any of the hematology measures evaluated (white blood cells, platelets, hemoglobin, hematocrit, neutrophils, and lymphocytes). The effect of treatment on platelets in peripubertal rats approached significance (P = 0.0543), in which rats treated with VCD at 80 mg/kg had fewer platelets at each time point than did the other peripubertal rats. Importantly, all of the hematology measures for peripubertal rats remained within normal clinical limits throughout the duration of the study.28,42,45
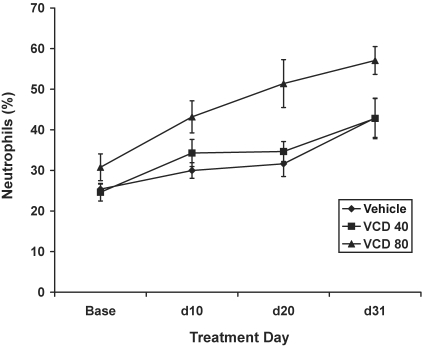
In contrast to its effect in peripubertal rats, VCD80 treatment led to a marked inflammatory response in adult rats. Compared with control values, neutrophil counts were increased in the VCD80 group (P = 0.002) but not in the VCD40 group (P = 0.8; Figure 7). In addition, both hemoglobin and hematocrit were lower in adult rats treated with VCD at 80 mg/kg compared with controls (hemoglobin, P = 0.01; hematocrit, P = 0.007).
Figure 7.
Effect of VCD on a marker of inflammation (percentage neutrophils) in adult (mean age, 269 d) Sprague–Dawley rats. Adult rats were injected intraperitoneally daily for 30 d with either vehicle, VCD at 40 mg/kg, or VCD at 80 mg/kg. Data are expressed as percentage neutrophils (mean ± SE).
Clinical chemistry measures.
Measures of renal function (creatinine, blood urea nitrogen [BUN]) and liver function (alanine aminotransferase) were assessed for all rats in the main study. Peripubertal rats treated with VCD80 had significantly lower serum creatinine concentrations compared with control rats (P = 0.0368, data not shown), but BUN, a related measure of renal function, showed no effect of treatment (P = 0.35). Creatinine levels for all rats remained within normal range (0.4 to 1.4 mg/dL) for Sprague–Dawley rats of this age.28,42,45 VCD40 treatment had no effect on creatinine in peripubertal rats (P = 0.15). In contrast, adult rats showed significant interaction between time and treatment condition for both creatinine (P < 0.0001) and BUN (P = 0.003). Visual inspection of the data (not shown) for adult rats also suggested a trend toward increases in both BUN and creatinine near the end of the study, but these results are likely attributable to the removal and euthanasia of 4 adult rats from the VCD80 group. These 4 rats had marked increases in BUN and creatinine levels (59 to 125 mg/dL and 1.54 to 2.19 mg/dL, respectively) that exceeded the normal ranges for both (BUN, 21 ± 3.4 mg/dL; creatinine, 0.70 ± 0.13 mg/dL).28 Neither dose of VCD affected liver enzymes in either age group (peripubertal rats, P = 0.6189; adult rats, P = 0.6669).
Results of necropsy and histology.
Intramuscular dosing study.
Beginning on injection days 12 and 13, rats treated with 160 mg/kg VCD IM were lethargic and exhibited labored breathing, and 1 animal had difficulty using its hindlegs. Immediately after this observation, all rats in the 160-mg/kg treatment group (n = 3) and 3 vehicle-treated controls were euthanized for diagnostic necropsy. The main gross finding was hemorrhage and necrosis at the VCD injection site. Histologic evaluation revealed extensive, severe, necrotizing myositis and sciatic nerve degeneration (Figure 1 A through C). Comparative sections of muscle were collected from animals treated with vehicle or 80 mg/kg for 15 d. The vehicle-treated rats had mild, focal fasciitis, and those treated with 80 mg/kg VCD had moderate, focal necrosis and hemorrhage with fibrin in the fascia and adipose tissue at the injection site. Small numbers of myofibers were moderately atrophied, and the sciatic nerve near the injection site showed degeneration. Myofiber degeneration was noted also in these lesions. None of the other organs sampled from the other treatment groups showed any other VCD-related lesions. Age-related and incidental lesions were mild and included renal tubular mineralization and lymphocytic hepatitis.
Intraperitoneal dosing study.
Similar to the intramuscular study, adult rats treated with 160 mg/kg VCD IP became ill and were euthanized between days 10 and 13 of the study. The final morphologic diagnosis for all of the rats was severe diffuse fibrinous peritonitis with foreign material (assumed to be unabsorbed injected substance, that is VCD and oil). In several of the rats, fibrous adhesions were present between the liver, spleen, and kidney. Rats treated with vehicle and 80 mg/kg VCD had histologic evidence of mild peritonitis (inflammation of the peritoneal serous membrane), but no gross evidence of fibrous adhesions was seen.
Main study: adult compared with peripubertal rats.
In the main study comparing peripubertal and adult rats, 4 of the adult rats (age, 211 to 223 d) treated with VCD at 80 mg/kg had decreased food intake, abdominal wall thickening, and signs of dehydration as early as day 11. Rats were treated with subcutaneous fluids and analgesics (ketoprofen, 5 mg/kg once daily for 2 to 3 d). By day 25 to 27, all 4 rats had labored breathing, were lethargic, and had lost 10% to 24% of baseline body weight. Because they did not respond to supportive therapy, these 4 adult rats were euthanized. At necropsy, 3 of the 4 animals presented with elevated markers of renal function (BUN, 59 to 125 mg/dL; creatinine, 1.54 to 2.19 mg/dL) and inflammatory leukograms [that is, elevated white blood cell (7.94 to 21.62) and neutrophil (5.16 to 15.07) counts]. Levels of the liver enzyme alanine aminotransaminase were not increased in any of these rats.
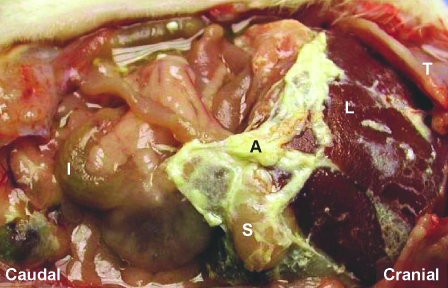
The main gross findings at necropsy for rats euthanized early were fibrinous adhesions involving multiple organs including diaphragm, stomach, spleen, liver, and kidney (Figure 8). Histologic examination of abdominal organs and tissues revealed peritonitis involving the liver capsule and abdominal wall muscle at the site of injection, as well as incidental age-associated changes in the kidney (glomerulonephropathy, tubular mineralization proteinosis). Uterine necrosis was present in 1 rat.
Figure 8.
Abdominal inflammation (peritonitis) in a representative adult Sprague–Dawley rat treated with 80 mg/kg VCD once daily for 25 d. Fibrinous adhesions (A) involving the liver (L) and stomach (S) are depicted. I, intestine; T, thorax.
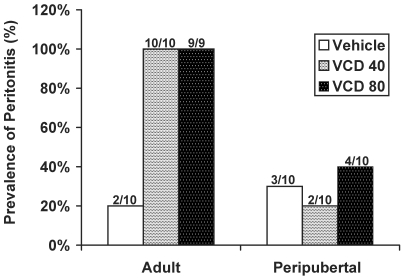
Among rats that completed the study, only 1 rat had gross evidence of peritonitis (fibrinous adhesions involving multiple organs). Histologic evidence of mild peritonitis (inflammation of the serosa surrounding 1 or more organs but no fibrinous adhesions) was present in 100% of adult rats treated with VCD and 20% to 40% of the VCD-treated peripubertal rats (Figure 9). Inflammation of the abdominal muscle and underlying peritoneum was seen in 21 of the 29 adult rats and 18 of the 30 peripubertal rats. A representative image of the inflammatory process present in the abdomen of a rat that completed the 30-d experiment and of 1 of the rats that was euthanized early is depicted in Figure 10. The only other noteworthy lesions present in the older rats were variable age-related glomerular changes in the kidney and metastatic calcification in the lungs of some; we considered this change to be secondary to renal dysfunction. No other marked gross or histologic lesions were present in any of the other organs examined for either young or adult rats.
Figure 9.
Prevalence of peritonitis in rats treated with VCD. Data are expressed as percentage occurrence per treatment group in adult (mean age, 269 d) and peripubertal (28 d) Sprague–Dawley rats. Fractions above the bars indicate the number of animals with peritonitis out of the total number of animals in the treatment group.
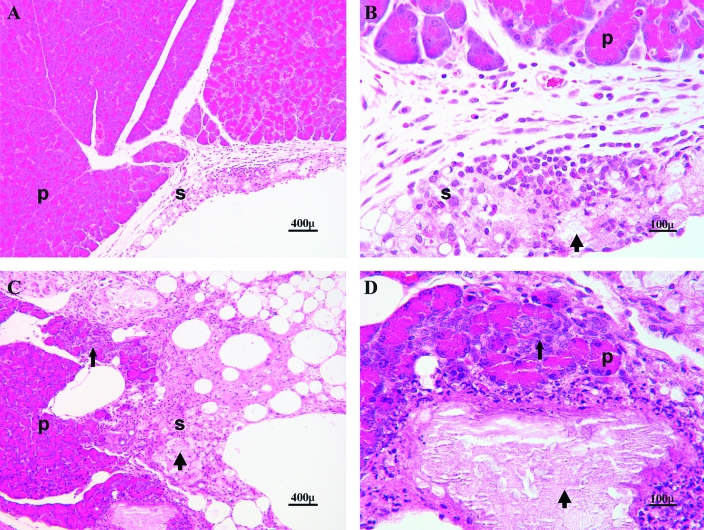
Figure 10.
Photomicrograph of pancreas (p) and peripancreatic tissue (serosa, s) in an adult Sprague–Dawley rat that completed 30 d of treatment with 80 mg/kg VCD (A, B) and a rat that was necropsied due to inappetance and dehydration after 25 d of the same treatment (C, D). A and B depict mild peritonitis with localized areas of inflammatory cells (foamy macrophages, lymphocytes, neutrophils and fat necrosis; thick arrows); C and D depict a more extensive lesion infiltrating into the pancreatic parenchyma (thin arrows) and replacement of peripancreatic fat and acini with fibrous tissue. Hematoxylin and eosin stain; magnification, ×100 (A, C), ×400 (B, D).
Organ weights.
Organ weights were normalized by necropsy body weight to allow comparison among age groups. Means are represented as g per 100 g of body weight (Table 1).
Table 1.
The effect of VCD on organ weight in adult and peripubertal Sprague–Dawley rats
| Initial intraperitoneal study |
Adult rats |
Peripubertal rats |
||||||||
| Liver | Brain | Liver | Brain | Ovaries | Uterus | Liver | Brain | Ovaries | Uterus | |
| Vehicle | 3.634 ± 0.10 | 0.670 ± 0.02 | 3.540 ± 0.10 | 0.596 ± 0.02 | 0.045 ± 0.004 | 0.300 ± 0.07 | 4.683 ± 0.09 | 0.949 ± 0.02 | 0.054 ± 0.003 | 0.266 ± 0.04 |
| VCD40 | NA | NA | 3.573 ± 0.15 | 0.624 ± 0.02 | 0.038 ± 0.003 | 0.175 ± 0.01 | 4.642 ± 0.08 | 0.977 ± 0.03 | 0.055 ± 0.003 | 0.195 ± 0.02 |
| VCD80 | 3.953 ± 0.09a | 0.718 ± 0.02 | 4.021 ± 0.13a | 0.657 ± 0.02 | 0.039 ± 0.004 | 0.233 ± 0.05 | 4.782 ± 0.12a | 0.996 ± 0.02 | 0.049 ± 0.005 | 0.221 ± 0.02 |
| VCD160 | 4.371 ± 0.05a | 0.803 ± 0.02b | NA* | NA | NA | NA | NA | NA | NA | NA |
Not applicable because there was not a VCD160 group in the main study.
Organ weights are expressed as g/100 g of body weight, and values are mean ± SE. Group sizes were 6 per treatment for the preliminary intraperitoneal dosing study and 10 per treatment for the adult–peripubertal study.
P < 0.05 for VCD80 or VCD160 compared with vehicle.
P = 0.005 for VCD160 compared with vehicle.
Intraperitoneal dosing study.
In the preliminary intraperitoneal dosing study, liver weights were greater with both doses of VCD than in vehicle-treated animals (vehicle, n = 6, 3.63 g/100 g body weight; 80 mg/kg VCD: n = 6, 3.95 g, P = 0.03; 160 mg/kg VCD: n = 2, 4.37 g/100 g body weight, P = 0.006). The brain was heavier (P = 0.005) in rats treated with 160 mg/kg VCD (0.803 g /100 g body weight) than in vehicle-treated rats (0.670 g/ 100 g body weight).
Main study: adult compared with peripubertal rats.
In the main study, there was a significant overall effect of treatment on liver weight (P = 0.04). Regardless of age, animals treated with VCD at 80 mg/kg had significantly heavier livers than did animals treated with vehicle only (P = 0.03) or VCD at 40 mg/kg (P = 0.02). Treatment and age interacted significantly for brain weight (P = 0.02). In this interaction, peripubertal rats treated with VCD at 80 mg/kg had heavier brains than did peripubertal rats treated with vehicle only (P = 0.02), but adult rats treated with VCD at 80 mg/kg had lighter brains than did adult rats treated with vehicle only (P = 0.03). However, these differences became nonsignificant when Bonferroni correction was used (P = 0.12 for peripubertal rats treated with VCD at 80 mg/kg compared with peripubertal rats treated with vehicle only; P = 0.19 for adult rats treated with VCD at 80 mg/kg compared with adult rats treated with vehicle only). The effect of treatment on ovarian weight was nonsignificant (P = 0.77), whereas the effect of VCD on uterine weight approached significance (P = 0.06), with VCD40 rats having significantly lighter uteruses than rats treated with vehicle only (P = 0.02). Uterine weight did not differ (P = 0.10) between rats treated with vehicle only and VCD at 80 mg/kg.
Discussion
The use of VCD to induce ovarian failure in rats and mice provides an ovary-intact model for studying the perimenopausal transition and menopause. Furthermore, the model provides a platform for testing interventions that might delay or inhibit the development of diseases (for example, osteoporosis, cognitive decline, cardiovascular disease) that often accompany aging and menopause and comprise the majority of the health burden in older women. However, despite the fact that many of these disease processes are manifested later in life, the majority of VCD studies to date have used peripubertal animals, which may confound the interpretation of results.5,9,10,23,37,47,52 Accordingly, the current study was designed to determine how the effects of VCD compared in adult and peripubertal animals. In addition, because nearly all VCD studies have used Fisher 344 rats, preliminary dosing studies and clinical and pathologic outcomes were investigated in the Sprague-Dawley rat, a model widely used in aging-related research.
Our results indicate that, when given similar doses, the magnitude of the VCD effect on primordial and primary follicles is not affected by age. After 30 daily injections of VCD at 80 mg/kg, the numbers of primordial follicles were reduced by approximately 85% to 90% in adult and approximately 81% in 28d Sprague–Dawley rats. However, untreated vehicle-control adult rats had nearly 2/3 fewer follicles present than did young rats, and adult rats were nearly devoid of primordial and primary follicles after VCD treatment. In addition, adult rats treated with a lower dose of VCD (40 mg/kg) had similar follicle numbers after treatment as did young rats treated with 80 mg/kg VCD. Therefore, it may be possible to use a lower dose of VCD in adult Sprague–Dawley rats to achieve ovarian failure. Finally, treatment of adult Sprague–Dawley rats (107 to 190 d of age) with 160 mg/kg VCD, a dose commonly used to cause ovarian failure in mice,19,32,33,36,56 resulted in nearly 100% loss of primordial follicles after only 10 to 13 daily injections given intramuscularly or intraperitoneally. However, this dose was not tolerated clinically by Sprague–Dawley rats.
In the preliminary dosing studies, rats treated with VCD at 160 mg/kg IP had significantly more secondary follicles than did animals treated with VCD at 80 mg/kg (Figure 2). However, although these numbers were significantly different statistically, this finding likely is not clinically relevant and is due to error variance related to the small number of animals used in these preliminary studies and the small number of secondary follicles in the ovary at any given time.
No persistent adverse clinical effects on young rats (28 to 58 d) treated with VCD (80 mg/kg for as long as 30 d) have been reported to date.5,11,17,24,37,47,48 However, in 1 report,19 C57Bl/6 mice dosed with 160 mg/kg VCD had reduced weight gain during treatment. This effect was attributed to an effect of VCD dosing on appetite, because weight returned to baseline levels soon after treatment ceased.19 In the present studies, adult rats treated with VCD lost weight during treatment in what appeared to be a dose-dependent manner (approximately 5% to 8% loss with 80 mg/kg, approximately 18% loss with 160 mg/kg), and food intake was decreased in both young and adult rats treated with VCD at 80 mg/kg. Although adult Sprague–Dawley rats clearly cannot tolerate VCD at 160 mg/kg, it is likely that, except for 4 rats that were clinically ill and removed from the study, rats treated with 80 mg/kg VCD would have returned to baseline weight after treatment ceased.
To further characterize potential clinical effects of VCD on Sprague–Dawley rats, markers of liver injury, renal function, and inflammatory profiles (complete blood counts) were measured. Consistent with previous reports of young B6C3F1 mice given 160 mg/kg,36 VCD did not affect a marker of hepatic damage (alanine aminotransferase) in peripubertal or adult Sprague–Dawley rats, and in peripubertal rats, all clinical measures remained within normal reference range. Conversely, adult rats treated with VCD at 80 mg/kg had a significant increase in total number of white blood cells characterized by neutrophilia, whereas those treated with 40 mg/kg VCD did not differ from controls. The neutrophilia most likely was the result of inflammation at the injection site and within the abdomen (peritonitis). Increases of serum creatinine and BUN above the normal reference range occurred in 4 adult rats treated with VCD 80 mg/kg. These rats were removed from the study early due to inappetance and lethargy, and dehydration was the most plausible explanation for the increase in BUN and creatinine.
Taken together, the data from the main study suggest that the majority of rats treated (30 of 30 peripubertal and 25 of 29 adult) did not have adverse clinical symptoms after administration of VCD at 40 or 80 mg/kg. However, VCD is a contact-irritant to tissues,7 and a subset of the adult rats (4 of 29) had severe inflammatory reactions, resulting in early removal from the study. Although the rats were anesthetized to avoid the complication, inadvertent injections into abdominal muscle, visceral fat, or organs may have caused these severe inflammatory reactions. Adult female Sprague–Dawley rats generally are much larger in body size than are adult female F344 rats,12,51,54 potentially increasing the technical difficulty of the intraperitoneal injection.
For the VCD rat model to be useful for perimenopausal studies, VCD must not have long-term effects in nonovarian tissues. In all 3 studies presented here, VCD did not result in abnormal pathologic changes in any tissues studied, other than an inflammatory reaction at the site of injection or superficially around abdominal organs. The severity of inflammatory reaction in the abdomen was greater in adults than in young rats and was most severe with the highest dose studied (160 mg/kg). There are several possible reasons for the increased prevalence of peritonitis in older Sprague–Dawley rats. First, adult rats tended to have more abdominal fat, and given that VCD is lipophilic, this attribute may have slowed the absorption of VCD into systemic circulation, resulting in a chemically induced inflammatory reaction. Because measuring circulating VCD concentrations was impossible (assay not currently available) and because only limited data are available on the pharmacokinetics of the parent compound, 4-vinylcyclohexene (VCH),46 we could not compare VCD absorption rates between young and adult rats. Second, strain differences in susceptibility to mount an inflammatory response to VCD treatment may have played a role. For example, a comparison of lipopolysaccharide-induced febrile responses in Sprague–Dawley and F344 females showed that Sprague–Dawley rats display a characteristic biphasic febrile response to lipopolysaccharide, but F344 rats exhibit marked attenuation of the second phase of the lipopolysaccharide-induced febrile response, indicating a blunted inflammatory response.50 This characteristic may account for the neutrophilia, often associated with a febrile response, seen in Sprague–Dawley rats treated with VCD - a response that has not been reported in F344 rats. Third, as noted earlier, pharmacokinetic studies to examine VCD clearance in animals are unavailable. Older animals may have cleared VCD more slowly than did peripubertal rats, explaining why VCD had more adverse effects on the older animals. It would be useful for future research to focus on VCD clearance in rodents of various ages, species, and strains. Finally, adult rats may have decreased renal function relative to growing rats, resulting in reduced clearance of VCD from systemic circulation. In this study, nearly all of the adult rats had an age-associated nephropathy, which may have impaired renal function. In rats, aging reportedly is associated with increased proteinuria and decreased urinary concentrating ability, as well as increased size, weight, and degree of cortical scarring of kidneys.40 Lesions due to chronic progressive nephropathy can be seen at 30 wk of age in Sprague–Dawley rats,40 and in a rat model of analgesic nephropathy (using Sprague–Dawley rats), decreases in glomerular filtration rate, sodium excretion, and urine osmolality were seen.1
In addition to gross and histopathologic examination of multiple organs, organ weights were recorded for both intraperitoneal studies. Liver weight was increased with VCD treatment in both young and adult rats. In the absence of histopathologic abnormalities, however, the most likely explanation for increased liver weight is the induction of metabolic enzymes and increased hepatic proteins in response to VCD exposure. Similar responses to other xenobiotic compounds in mice26 and Sprague–Dawley rats39 have been reported. Furthermore, increased liver weights have been observed in mice immediately after VCD treatment (160 mg/kg), whereas livers from mice examined 2 wk after treatment did not differ from controls.36 The effect of VCD on brain weight was inconsistent across our 3 studies, and no significant effect was seen in the main study. In addition, no pathologic abnormalities were found in the brains. However, further studies need to be done to determine whether VCD could have direct effects on neurobehavior, and a VCD-treated ovariectomized control group should be included in such experiments.
Because VCD is used to cause ovarian failure, it initially might seem counterintuitive that we did not find decreased ovarian and uterine weights in animals treated with VCD. However, although ovarian and uterine weights are typically decreased after VCD administration, these decreases are only observed if animals are allowed to reach complete ovarian failure (depletion of all follicle types).33,36 These losses in ovarian and uterine weights are due to loss of large antral follicles (for ovarian weight) and loss of tropic effects of ovarian 17β-estradiol on the uterus (uterine weight). Because the current experiment ended while secondary and antral follicles were still present and producing estradiol, it is not surprising that ovarian and uterine weights were not decreased. However, because the uterine weight data were not adjusted for the day of estrus cycle and because uterine weight can vary significantly across the estrus cycle,55 we cannot know for certain that there was no effect of VCD on uterine weight. Future studies should include daily vaginal swabbing of VCD-treated animals to track estrus.
A potential weakness of the current study is the assumption that VCD exposure did not affect the estrus cycles and estradiol concentrations. This assumption is supported by the finding that the numbers of secondary and antral follicles were not decreased. This finding is consistent with previous studies; for example, estradiol concentrations (measured at proestrus peak) of peripubertal female C57Bl/6 mice dosed with VCD (160 mg/kg, 15 d) did not differ from control.32 In addition, estrus cycle length (influenced by estrogen) was not different from control until 13 d after the final dose. However, without vaginal cytology and plasma estradiol concentrations, we cannot know for certain that VCD did not affect estrus cycles and steroid production, and because changes in estradiol can affect appetite, body weight gain, and inflammatory responses in rats, future investigations should include these measures.
In conclusion, as with young mice and F344 rats, VCD effectively destroys primordial and primary follicles in adult as well as peripubertal Sprague–Dawley rats, suggesting that the adult VCD-treated Sprague–Dawley rat is suitable for modeling perimenopause and menopause as they occur in aging women. Compared with younger rats, adult Sprague–Dawley rats were more sensitive to the irritant properties of VCD, and the use of a lower VCD dose (for example, 40 or 60 mg/kg) for longer periods, different vehicles, or more dilute mixtures may be warranted. Further studies to determine the time to onset of complete ovarian failure in adult Sprague–Dawley VCD-treated rats are needed.
Acknowledgments
This study was supported in part by grant R24 RR 022191 from the National Center for Research Resources and grant RO1 AG 027847 from the National Institute of Aging, National Institutes of Health. The study also was supported by grant R01 AG 021948 from the National Institute of Aging, National Institutes of Health (PBH), and Center Grant ES06694 (PBH). The authors would like to acknowledge the following people for their technical contributions to this work: Melissa Ayers, Dewayne Cairnes, Debbie Golden, Andrea Grantham, Margaret May, and Maryanne Post. The authors also would like to acknowledge Creative Communications of Wake Forest University for their assistance with the figures in this manuscript.
References
- 1.Ahmed MH, Ashton N, Balment RJ. 2003. Renal function in a rat model of analgesic nephropathy: effect of chloroquine. J Pharmacol Exp Ther 305:123–130 [DOI] [PubMed] [Google Scholar]
- 2.Alfinito PD, Huselton C, Chen X, Deecher DC. 2006. Pharmacokinetic and pharmacodynamic profiles of the novel serotonin and norepinephrine reuptake inhibitor desvenlafaxine succinate in ovariectomized Sprague–Dawley rats. Brain Res 1098:71–78 [DOI] [PubMed] [Google Scholar]
- 3.Arjmandi BH, Alekel L, Hollis BW, Amin D, Stacewicz-Sapuntzakis M, Guo P, Kukreja SC. 1996. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J Nutr 126:161–167 [DOI] [PubMed] [Google Scholar]
- 4.Bahr JM, Nakai M, Rivera A, Walsh J, Evans GL, Lotinum S, Turner RT, Black M, Jeffery EH. 2005. Dietary soy protein and isoflavones: minimal beneficial effects on bone and no effect on the reproductive tract of sexually mature ovariectomized Sprague–Dawley rats. Menopause 12:165–173 [DOI] [PubMed] [Google Scholar]
- 5.Borman SM, VanDePol BJ, Kao S, Thompson KE, Sipes IG, Hoyer PB. 1999. A single dose of the ovotoxicant 4-vinylcyclohexene diepoxide is protective in rat primary ovarian follicles. Toxicol Appl Pharmacol 158:244–252 [DOI] [PubMed] [Google Scholar]
- 6.Cayan S, Canpolat B, Cayan F, Yilmaz N, Kartal A, Oguz I, Akbay E. 2006. The effect of chronic inflammatory condition of the bladder and estrogen replacement therapy on bladder functions and histology in surgically menopause and chronic cystitis induced rats. Neurourol Urodyn 25:194–201 [DOI] [PubMed] [Google Scholar]
- 7.Chhabra R. 1989. Toxicology and carcinogenesis studies of 4-vinyl-1-cyclohexene diepoxide in F344 rats and B6C3F1 mice. National Toxicology Program, US Department of Health and Human Services, Public Health Service NC, Research Triangle Park. p 1–362 [Google Scholar]
- 8.Cirpan T, Iscan O, Terek MC, Ozsener S, Kanit L, Pogun S, Zekioglu O, Yucebilgin S. 2006. Proliferative effects of different hormone regimens on mammary glands in ovariectomized rats. Eur J Gynaecol Oncol 27:256–261 [PubMed] [Google Scholar]
- 9.Devine PJ, Payne CM, McCuskey MK, Hoyer PB. 2000. Ultrastructural evaluation of oocytes during atresia in rat ovarian follicles. Biol Reprod 63:1245–1252 [DOI] [PubMed] [Google Scholar]
- 10.Devine PJ, Sipes IG, Hoyer PB. 2004. Initiation of delayed ovotoxicity by in vitro and in vivo exposures of rat ovaries to 4-vinylcyclohexene diepoxide. Reprod Toxicol 19:71–77 [DOI] [PubMed] [Google Scholar]
- 11.Devine PJ, Sipes IG, Skinner MK, Hoyer PB. 2002. Characterization of a rat in vitro ovarian culture system to study the ovarian toxicant 4-vinylcyclohexene diepoxide. Toxicol Appl Pharmacol 184:107–115 [PubMed] [Google Scholar]
- 12.Dorman DC, Struve MF, Gross EA, Brenneman KA. 2004. Respiratory tract toxicity of inhaled hydrogen sulfide in Fischer-344 rats, Sprague–Dawley rats, and B6C3F1mice following subchronic (90-day) exposure. Toxicol Appl Pharmacol 198:29–39 [DOI] [PubMed] [Google Scholar]
- 13.Draper HH, Sie T-L, Bergan JG. 1972. Osteoporosis in aging rats induced by high phosphorus diets. J Nutr 102:1133–1142 [DOI] [PubMed] [Google Scholar]
- 14.El-Bakri NK, Islam A, Zhu S, Elhassen A, Mohammed A, Winblad B, Adem A. 2004. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance. J Cell Mol Med 8:537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Z, Cheng Y, Zhang JT. 2004. Long-term effects of melatonin of 17β-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. J Pineal Res 37:198–206 [DOI] [PubMed] [Google Scholar]
- 16.Flaws JA, Doerr JK, Sipes G, Hoyer PB. 1994. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod Toxicol 8:509–514 [DOI] [PubMed] [Google Scholar]
- 17.Flaws JA, Salyers KL, Sipes IG, Hoyer PB. 1994. Reduced ability of rat preantral ovarian follicles to metabolize 4-vinyl-1-cyclohexene diepoxide in vitro. Toxicol Appl Pharmacol 126:286–294 [DOI] [PubMed] [Google Scholar]
- 18.Gallo D, Zannoni GF, Martinelli E, Ferlini C, Fabrizi M, Riva A, Morazzoni P, Bombardelli E, Sambia G. 2006. Estradiol and phytoestrogens differently influence the rodent postmenopausal mammary gland. Menopause 13:72–79 [DOI] [PubMed] [Google Scholar]
- 19.Haas JR, Christian PJ, Hoyer PB. 2007. Effects of impending ovarian failure induced by 4-vinylcyclohexene diepoxide on fertility in C57BL/6 female mice. Comp Med 57:443–449 [PubMed] [Google Scholar]
- 20.Heger S, Mastronardi C, Dissen GA, Lomniczi A, Cabrera R, Roth CL, Jung H, Galimi F, Sippell W, Ojeda SR. 2007. Enhanced at puberty 1 (EAP1) is a new transcriptional regulator of the female neuroendocrine reproductive axis. J Clin Invest 117:2145–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong SK, Yang JH, Kim TB, Kim SW, Paick JS. 2006. Effects of ovariectomy and oestrogen replacement on the function and expression of Rho kinase in rat bladder smooth muscle. BJU Int 98:1114–1117 [DOI] [PubMed] [Google Scholar]
- 22.Hoyer PB. 2005. Damage to ovarian development and function. Cell Tissue Res 322:99–106 [DOI] [PubMed] [Google Scholar]
- 23.Hoyer PB, Cannady EA, Kroeger NA, Sipes G. 2001. Mechanisms of ovotoxicity induced by environmental chemicals: 4-vinylcyclohexene diepoxide as a model chemical, p 73–81. Dansette PM, editor. Biological Reactive Intermediates VI. New York (NY): Kluwer Academic Plenum Publishers; [DOI] [PubMed] [Google Scholar]
- 24.Hoyer PB, Devine PJ, Hu X, Thompson KE, Sipes IG. 2001. Ovarian toxicity of 4-vinylcyclohexene diepoxide: a mechanical model. Toxicol Pathol 29:91–99 [DOI] [PubMed] [Google Scholar]
- 25.Hoyer PB, Sipes IG. 2007. Development of an animal model for ovotoxicity using 4-vinycyclohexene: a case study. Birth Defects Res B Dev Reprod Toxicol 80:113–125 [DOI] [PubMed] [Google Scholar]
- 26.Huang W, Zhang J, Washington M, Liu J, Parant JM, Lozano G, Moore DD. 2005. Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol 19:1646–1653 [DOI] [PubMed] [Google Scholar]
- 27.Kanter EM, Walker RM, Marion SL, Brewer M, Hoyer PB, Barton JK. 2006. Dual modality imaging of a novel rat method of ovarian carcinogenesis. J Biomed Opt 11:041123. [DOI] [PubMed] [Google Scholar]
- 28.Kohn DF, Clifford CB. 2002. Biology and diseases of rats, p 121–165. Fox JG, Anderson LC, Loew FM, Quimby FW, editors. Laboratory Animal Medicine. Orlando (FL): Academic Press [Google Scholar]
- 29.Le Saux M, Estrada-Camarena E, Di Paola T. 2006. Selective estrogen receptor-alpha but not -beta agonist treatment modulates brain alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. J Neurosci Res 84:1076–1084 [DOI] [PubMed] [Google Scholar]
- 30.Lee BH, Lee HH, Kim JH, Cho BR, Choi YS. 2007. Effects of a soluble fraction of soybean on lipid profiles in ovariectomized rats fed a cholesterolemic diet. J Med Food 10:521–525 [DOI] [PubMed] [Google Scholar]
- 31.Lee YH, Hyun SH, Choung SY. 2006. Effect of herbal extract mixture on menopausal urinary incontinence in ovariectomized rats. Biofactors 26:171–178 [DOI] [PubMed] [Google Scholar]
- 32.Lohff JC, Christian PJ, Marion SL, Arrandale A, Hoyer PB. 2005. Characterization of cyclicity and hormonal profile with impending ovarian failure in a novel chemical-induced mouse model of perimenopause. Comp Med 55:523–527 [PubMed] [Google Scholar]
- 33.Lohff JC, Christian PJ, Marion SL, Hoyer PB. 2006. Effect of duration of dosing on onset of ovarian failure in a chemical-induced mouse model of perimenopause. Menopause 13:482–488 [DOI] [PubMed] [Google Scholar]
- 34.Lovekamp-Swan T, Glendenning ML, Schreihofer DA. 2007. A high-soy diet enhances neurotropin receptor and Bcl-XL gene expression in the brains of ovariectomized female rats. Brain Res 1159:54–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucas EA, Chen TY, Chai SC, Devareddy L, Juma S, Wei CI, Tripathi YB, Daggy BP, Hwang DF, Arjmandi BH. 2006. Effect of vitamin E on lipid parameters in ovariectomized rats. J Med Food 9:77–83 [DOI] [PubMed] [Google Scholar]
- 36.Mayer LP, Devine PJ, Dyer CA, Hoyer PB. 2004. The follicle-depleted mouse ovary produces angrogen. Biol Reprod 71:130–138 [DOI] [PubMed] [Google Scholar]
- 37.Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, Marion SL, Sipes G, Hoyer PB. 2002. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod Toxicol 16:775–781 [DOI] [PubMed] [Google Scholar]
- 38.Nakai M, Cook L, Pyter LM, Black M, Sibona J, Turner RT, Jeffery EH, Bahr JM. 2005. Dietary soy protein and isoflavones have no significant effect on bone and a potentially negative effect on the uterus of sexually mature intact Sprague–Dawley female rats. Menopause 12:291–298 [DOI] [PubMed] [Google Scholar]
- 39.Newsholme SJ, Maleef BF, Steiner S, Anderson NL, Schwartz LW. 2000. Two-dimensional electrophoresis of liver proteins: characterization of a drug-induced hepatomegaly in rats. Electrophoresis 21:2122–2128 [DOI] [PubMed] [Google Scholar]
- 40.Owen RA, Heywood R. 1986. Age-related variations in renal structure and function in Sprague–Dawley rats. Toxicol Pathol 14:158–167 [DOI] [PubMed] [Google Scholar]
- 41.Pedersen T, Peters H. 1968. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil 17:555–557 [DOI] [PubMed] [Google Scholar]
- 42.Petterino C, Argentino-Storino A. 2006. Clinical chemistry and haematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp Toxicol Pathol 57:213–219 [DOI] [PubMed] [Google Scholar]
- 43.Rimoldi G, Christoffel J, Wuttke W. 2006. Morphologic changes induced by oral long-term treatmentw ith 8-prenylnaringenin in the uterus, vagina, and mammary gland of castrated rats. Menopause 13:669–677 [DOI] [PubMed] [Google Scholar]
- 44.Saito T, Kinoshita S, Fujii T, Bandoh K, Fuse S, Yamauchi Y, Koizumi N, Horiuchi T. 2004. Development of novel steroid sulfatase inhibitors; II. TZS-8478 potently inhibits the growth of breast tumors in postmenopausal breast cancer model rats. J Steroid Biochem Mol Biol 88:167–173 [DOI] [PubMed] [Google Scholar]
- 45.Sharp PE, La Regina MC. 1998. The laboratory rat. Boca Raton (FL): CRC Press [Google Scholar]
- 46.Smith BJ, Carter DE, Sipes IG. 1990. Comparison of the disposition and in vitro metabolism of 4-vinylcyclohexene in the female mouse and rat. Toxicol Appl Pharmacol 105:364–371 [DOI] [PubMed] [Google Scholar]
- 47.Springer LN, Flaws JA, Sipes IG, Hoyer PB. 1996. Follicular mechanisms associated with 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Reprod Toxicol 10:137–143 [DOI] [PubMed] [Google Scholar]
- 48.Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. 1996. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol 139:394–401 [DOI] [PubMed] [Google Scholar]
- 49.Stendig-Lindberg G, Koeller W, Bauer A. 2004. Prolonged magnesium deficiency causes osteoporosis in the rat. J Am Coll Nutr 23:704S–711S [DOI] [PubMed] [Google Scholar]
- 50.Taylor AN, Tio DL, Romeo HE. 2005. The febrile response to intraperitoneal lipopolysaccharide: strain and gender differences in rats. J Neuroimmunol 158:86–93 [DOI] [PubMed] [Google Scholar]
- 51.Thigpen JE, Setchell KDR, Padilla-Banks E, Haseman JK, Saunders HE, Caviness GF, Kissling GE, Grant MG, Forsythe DB. 2007. Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD1 mice and F344 rats but not in CD Sprague–Dawley rats. Environ Health Perspect 115:1717–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson KE, Bourguet SM, Christian PJ, Benedict JC, Sipes IG, Flaws JA, Hoyer PB. 2005. Differences between rats and mice in the involvement of the aryl hydrocarbon receptor in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss. Toxicol Appl Pharmacol 203:114–123 [DOI] [PubMed] [Google Scholar]
- 53.Ueyama T, Ishikura F, Matsuda A, Asanuma T, Ueda K, Ichinose M, Kasamatsu K, Hano T, Akasaka T, Tsuruo Y, and others 2007. Chronic estrogen supplementation following ovariectomy improves the emotional stress-induced cardiovascular responses by indirect action on the nervous system and by direct action on the heart. Circ J 71:565–573 [DOI] [PubMed] [Google Scholar]
- 54.Webb AA, Gowribai K, Muir GD. 2003. Fischer (F344) rats have different morphology, sensorimotor and locomotor abilities compared to Lewis, Long–Evans, Sprague–Dawley and Wistar rats. Behav Brain Res 144:143–156 [DOI] [PubMed] [Google Scholar]
- 55.White JO, Thrower S, Lim L. 1978. Intracellular relationships of the oestrogen receptor in the rat uterus and hypothalamus during the oestrous cycle. Biochem J 172:37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright LE, Christian PJ, Rivera Z, Van Alstine WG, Funk JL, Bouxsein ML, Hoyer PB. 2008. Comparison of skeletal effects of ovariectomy versus chemically induced ovarian failure in mice. J Bone Miner Res 23:1296–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]