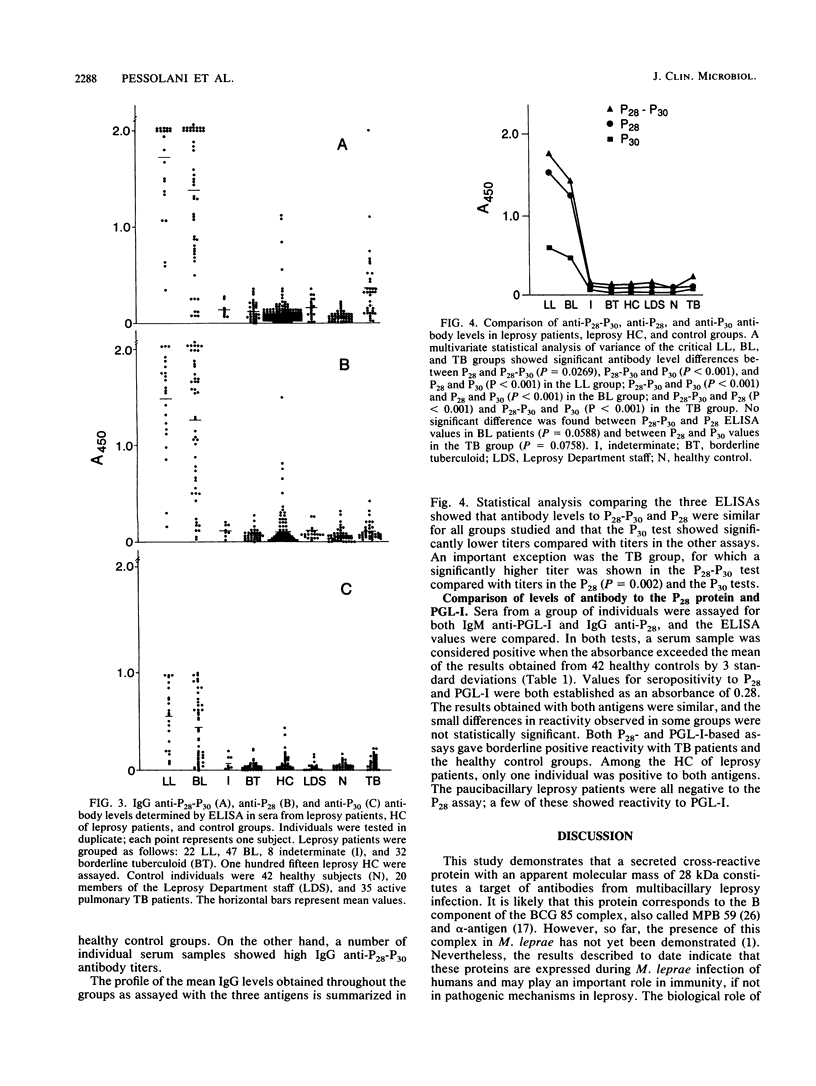
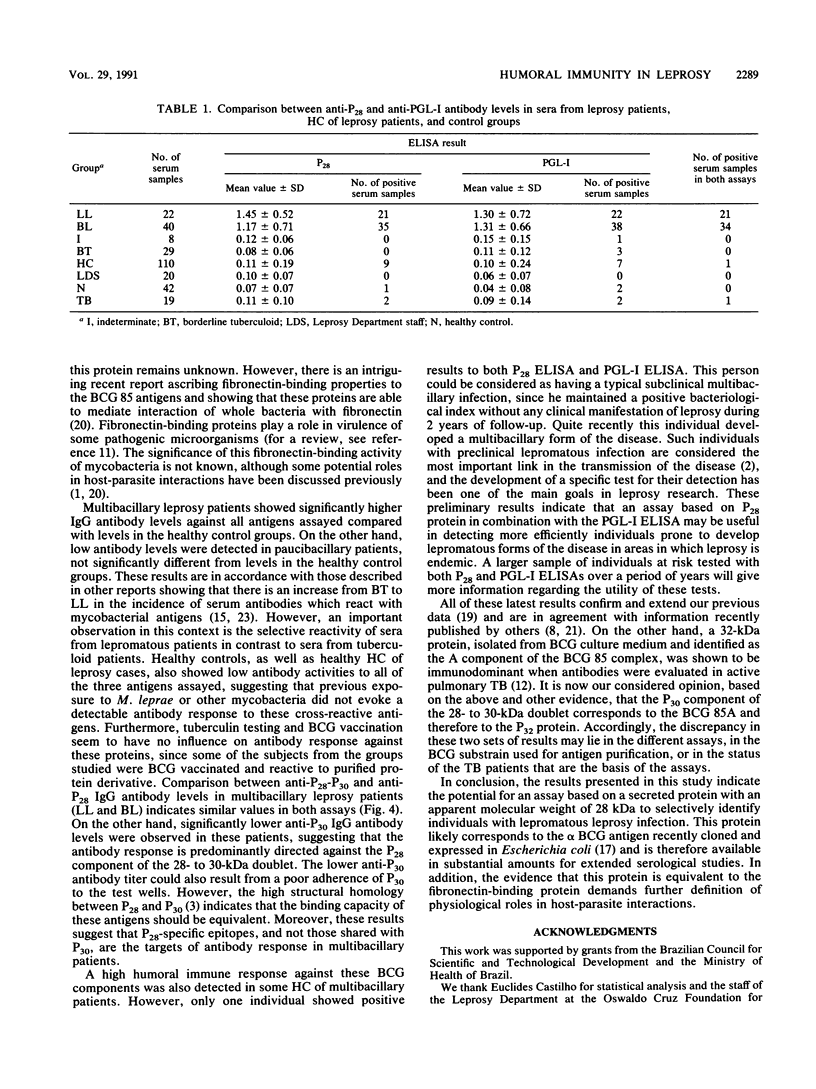
Abstract
Two major proteins from Mycobacterium bovis BCG culture filtrates with molecular masses of 28 kDa (P28) and 30 kDa (P30), identified as components of the BCG 85 complex, were purified and used in enzyme-linked immunosorbent assays (ELISAs) for the determination of specific immunoglobulin G (IgG) levels in patients with leprosy or tuberculosis or with exposure to these diseases. High reactivity to both antigens was observed with sera from lepromatous leprosy patients, whereas antibody levels in sera from paucibacillary leprosy patients were not significantly different from those in sera from healthy individuals from an area in which leprosy is endemic. High IgG responses were also found in some contacts of lepromatous leprosy patients. A comparison of the levels of anti-P28 and anti-P30 within the multibacillary leprosy patient group showed much higher IgG reactivity to P28 than to P30, suggesting that the antibody response of lepromatous patients is directed predominantly against the 28-kDa protein. A high degree of correlation in values of ELISAs based on P28 and on the phenolic glycolipid of Mycobacterium leprae was observed in all groups analyzed. The potential use of an assay based on the 28-kDa protein to selectively distinguish individuals destined to develop multibacillary leprosy is discussed, as also is the likelihood that the 28-kDa-30-kDa complex, part of the fibronectin-binding family, is an important component of M. leprae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Ratliff T. L., Wiker H. G., Harboe M., Bennedsen J., Rook G. A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988 Dec;56(12):3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Godal T. Selective primary health care: strategies for control of disease in the developing world. V. Leprosy. Rev Infect Dis. 1983 Jul-Aug;5(4):765–780. doi: 10.1093/clinids/5.4.765. [DOI] [PubMed] [Google Scholar]
- Borremans M., de Wit L., Volckaert G., Ooms J., de Bruyn J., Huygen K., van Vooren J. P., Stelandre M., Verhofstadt R., Content J. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect Immun. 1989 Oct;57(10):3123–3130. doi: 10.1128/iai.57.10.3123-3130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J. The carbohydrate-containing antigens of Mycobacterium leprae. Lepr Rev. 1986 Dec;57 (Suppl 2):39–51. [PubMed] [Google Scholar]
- Cherayil B. J., Young R. A. A 28-kDa protein from Mycobacterium leprae is a target of the human antibody response in lepromatous leprosy. J Immunol. 1988 Dec 15;141(12):4370–4375. [PubMed] [Google Scholar]
- Cho S. N., Fujiwara T., Hunter S. W., Rea T. H., Gelber R. H., Brennan P. J. Use of an artificial antigen containing the 3,6-di-O-methyl-beta-D-glucopyranosyl epitope for the serodiagnosis of leprosy. J Infect Dis. 1984 Sep;150(3):311–322. doi: 10.1093/infdis/150.3.311. [DOI] [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Das P. K., Rambukkana A., Baas J. G., Groothuis D. G., Halperin M. Enzyme-linked immunosorbent assay for distinguishing serological responses of lepromatous and tuberculoid leprosies to the 29/33-kilodalton doublet and 64-kilodalton antigens of Mycobacterium tuberculosis. J Clin Microbiol. 1990 Feb;28(2):379–382. doi: 10.1128/jcm.28.2.379-382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Van Vooren J. P., Turneer M., Bosmans R., Dierckx P., De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988 Feb;27(2):187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Klatser P. R., De Wit M. Y., Kolk A. H. An ELISA-inhibition test using monoclonal antibody for the serology of leprosy. Clin Exp Immunol. 1985 Dec;62(3):468–473. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J Bacteriol. 1988 Sep;170(9):3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pessolani M. C., Rumjanek F. D., Marques M. A., de Melo F. S., Sarno E. N. Serological response of patients with leprosy to a 28- to 30-kilodalton protein doublet from early cultures of Mycobacterium bovis BCG. J Clin Microbiol. 1989 Oct;27(10):2184–2189. doi: 10.1128/jcm.27.10.2184-2189.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff T. L., McGarr J. A., Abou-Zeid C., Rook G. A., Stanford J. L., Aslanzadeh J., Brown E. J. Attachment of mycobacteria to fibronectin-coated surfaces. J Gen Microbiol. 1988 May;134(5):1307–1313. doi: 10.1099/00221287-134-5-1307. [DOI] [PubMed] [Google Scholar]
- Rumschlag H. S., Shinnick T. M., Cohen M. L. Serological responses of patients with lepromatous and tuberculoid leprosy to 30-, 31-, and 32-kilodalton antigens of Mycobacterium tuberculosis. J Clin Microbiol. 1988 Oct;26(10):2200–2202. doi: 10.1128/jcm.26.10.2200-2202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serological tests for leprosy. Lancet. 1986 Mar 8;1(8480):533–535. [PubMed] [Google Scholar]
- Sinha S., Sengupta U., Ramu G., Ivanyi J. Serological survey of leprosy and control subjects by a monoclonal antibody-based immunoassay. Int J Lepr Other Mycobact Dis. 1985 Mar;53(1):33–38. [PubMed] [Google Scholar]
- Touw J., Langendijk E. M., Stoner G. L., Belehu A. Humoral immunity in leprosy: immunoglobulin G and M antibody responses to Mycobacterium leprae in relation to various disease patterns. Infect Immun. 1982 Jun;36(3):885–892. doi: 10.1128/iai.36.3.885-892.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Lea T. E. Purification and characterization of two protein antigens from the heterogeneous BCG85 complex in Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81(4):298–306. doi: 10.1159/000234153. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S., Patarroyo M. E., Ramirez C., Cruz N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81(4):307–314. doi: 10.1159/000234154. [DOI] [PubMed] [Google Scholar]




