Abstract
Alopecia (hair loss) occurs in some nonhuman primates housed in captivity and is of concern to colony managers and veterinarians. Here we review the characteristics, potential causes, and treatments for this condition. Although we focus on nonhuman primates, relevant research on other mammalian species is discussed also, due to the relative paucity of studies on alopecia in the primate literature. We first discuss the cycle of hair growth and explain how this cycle can be disrupted to produce alopecia. Numerous factors may be related to hair loss and range from naturally occurring processes (for example, seasonality, aging) to various biologic dysfunctions, including vitamin and mineral imbalances, endocrine disorders, immunologic diseases, and genetic mutations. We also address bacterial and fungal infections, infestation by parasites, and atopic dermatitis as possible causes of alopecia. Finally, we examine the role of psychogenic factors, such as stress. Depending on the presumed cause of the hair loss, various treatment strategies can be pursued. Alopecia in nonhuman primates is a multifaceted disorder with many potential sources. For this reason, appropriate testing for various disease conditions should be completed before alopecia is considered to be related to stress.
Abbreviations: VDR, vitamin D receptor
Hair loss (alopecia) is a complex phenomenon that is not fully understood either in human or nonhuman primates. Hair loss can occur as the result of a congenital or genetic disorder, or it can develop during the lifetime of the animal. Hair loss occurring throughout life can be further divided into inflammatory and noninflammatory types. In this article, we focus on alopecia in nonhuman primates. We also will refer to humans and other mammals, given that mechanisms of hair loss and potential treatments for this disorder have been studied infrequently in nonhuman primates.
Patterns of hair loss can be categorized in various ways, and several different types of scoring systems have been developed for both animals and humans.44,45,77 Most of these systems focus either on hair volume (referring to the number of hairs per unit area and often estimated along a 3- to 5-point scale) or distribution (referring to the size and location of bald patches) or some combination of both. Using rhesus monkeys as an example, typical patterns of hair loss include: 1) substantial whole-body hair loss (balding on the head, back, legs, and arms); 2) substantial hair thinning in various body regions (that is, hair is present but sparse); 3) extensive patches of bare skin, often bilateral in presentation, interspersed with normal hair; and 4) 1 or 2 small patches of bare skin on normally haired monkeys. A third dimension of hair loss is duration: hair loss can be short-term (less than 3 mo), long-term (lasting anywhere from 3 to 24 mo), or permanent. In the following paragraphs, we consider the typical hair cycle and various factors that can disrupt this cycle to cause loss of hair.
The Hair Cycle
The hair follicle possesses a unique ability to regenerate itself throughout the life of the organism. Hair growth occurs in a series of stages, starting with the anagen (or growth) phase. During this period, cell division in the matrix of the hair bulb produces keratinocytes, which form the various layers of the individual hair shaft. Melanocytes in the hair bulb provide the keratinocytes with pigment. After the hair reaches a certain length, which varies depending on body region, it enters the catagen (or degradation) phase, when cell division and pigmentation cease. During the telogen (or resting) phase, a new follicle appears below the original follicle, the hair shaft grows back into the original follicle, and the previous hair shaft is shed. It is unclear whether the hair is lost simply as a result of the new hair shaft's upward trajectory or whether there is a separate active shedding (exogen) stage.92,106 Studies on human head hair growth have shown that at any point in time, most (80% to 90%) of the hairs are in the anagen phase, with 1% to 2% in the catagen phase and the remaining 10% to 20% in the telogen phase.117
Dysfunctions during the telogen or anagen stages appear to result in hair loss. Some forms of hair loss are associated with excessive shedding of hair, a prolongation of the telogen phase commonly referred to as telogen effluvium.43 Other forms of hair loss are related to a failure to produce viable hair during the anagen phase. Alopecia areata universalis is an autoimmune disease caused by a peri- and intrafollicular infiltratation of T lymphocytes and macrophages, thereby leading to defective anagen-stage hair shafts and miniaturization of the follicles.15,112 Similar anagen dysfunction appears to underlie the best-known form of hair loss in humans, androgenetic alopecia (male pattern baldness). In this case, a dysfunctional anagen stage and miniaturization of the follicles presumably is caused by the effects of dihydrotestosterone on genetically susceptible hair follicles.78
Causes of Alopecia
Given the complex pattern of hair growth, it is not surprising to discover that there are many different factors that can contribute to the onset and maintenance of excessive hair loss in nonhuman primates. Although we will discuss these factors separately, hair loss in nonhuman primates very likely is a multietiologic phenomenon involving as-yet unknown combinations of factors that vary across subjects and settings. Hair loss itself has not been the focus of much research in monkeys. However, regulations to promote the psychologic well-being of captive primates along with the recent focus by regulators on alopecia as an area of concern make understanding hair loss in these animals essential.
Naturally occurring hair loss.
Various naturally occurring changes, including seasonality and aging, are known to alter the hair cycle process.
Seasonal variation.
Many mammalian species undergo changes in hair production across seasons. A common pattern in some mammals (for example, ferrets)74 involves 2 molting periods, wherein hair or fur is shed in early autumn replacing a summer coat with a winter coat and again in the spring replacing a winter coat with a summer coat. Some species also undergo a change in hair color (for example, the arctic hare whose coat is brown in the summer and white in the winter). These seasonally induced changes in hair are associated both with variations in day length and alterations in the levels of certain hormones.47,74 In temperate regions, long days are associated with lower levels of melatonin and prolactin and shorter hair (that is, summer coat), whereas short days are associated with higher levels of both hormones and longer hair (that is, winter coat). Although little is known about the control of seasonal variation on pelage in nonhuman primates, the role of prolactin in affecting seasonal molting patterns has been well established for Djungarian hamsters,34 goats,32 sheep,75 and deer.25 Further support for the role of prolactin comes from an examination of hair regrowth cycles, which are delayed in prolactin-treated mice.23
Some species of monkeys appear to undergo seasonal changes in pelage. Seasonal hair loss has been reported for free-ranging vervet monkeys,47 peaking in the months of November through January and being more obvious in subordinate monkeys. Such seasonal changes have also been observed in rhesus monkeys,105 even when maintained under artificial light cycles. Hair loss was greatest during the winter and spring months and appeared to affect female rhesus monkeys more than males. Considerable individual differences in seasonal hair loss may occur, ranging from subjects that show no discernible pattern of hair loss (as determined by simple visual inspection) to others in which hair loss is noticeable and even extreme in nature. The causes underlying this individual variation are largely unknown. However, as noted previously, sex and dominance rank may explain some of the variance
Aging.
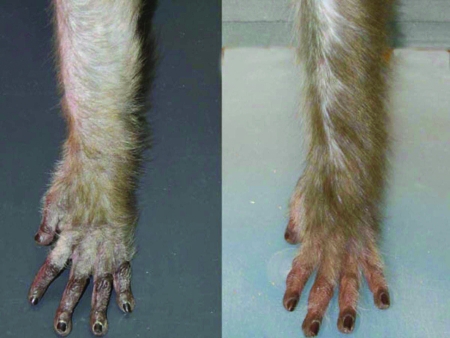
Hair loss has also been associated with the aging process. Even humans without visible hair loss show both a reduction in mean hair density and decreased growth rate of anagen hairs with increasing age.110,113 The hair thinning that is characteristic of aged rhesus monkeys is shown in Figure 1. Two studies45,46 have focused on age as a variable in hair loss in nonhuman primates. A comparison of geriatric and younger rhesus monkeys (mean ages, 25 and 10 y, respectively)46 revealed that older monkeys showed skin abnormalities (that is, increased areas of reddened skin, scaling, wrinkling, and subacute dermatitis) and thinning hair. These changes were not related to circulating concentrations of estradiol, thyroid stimulating hormone, triiodothyronine, thyroxine, and cortisol, nor were they a result of bacterial infections. An exploration of hair loss in a squirrel monkey colony45 revealed that hair loss in female monkeys was associated with both older age (9.6 y for monkeys with hair loss versus 4.7 y for monkeys with a normal coat) and increased parity (4.2 versus 2.0, respectively). Hair loss was unrelated to body weight, serum chemistry (for example, hemoglobin, serum glucose, BUN), or free thyroxine. Monkeys with hair loss had more telogen hairs than did normally haired monkeys, suggesting an alteration of the hair cycle process consistent with chronic telogen effluvium.
Figure 1.
The left arms of 2 female rhesus monkeys, a 24-y-old (left) and a 9-y-old (right). Neither monkey has conspicuous bald spots on its torso. However, the aged monkey has thinner and shorter hair, which is particularly noticeable on her arms. The areas of skin seen on the 9-y-old monkey's arm are not due to alopecia but instead represent a normal parting of the hair produced by hair growing over the arm in one direction and under the arm in the other direction. This part, which varies in width, has been present in all rhesus monkey arms that we have photographed to date.
Nutritional and hormonal imbalances.
Nutritional factors.
Although vitamin and mineral imbalances have been posited as causes of hair loss,98 the nutritional parameters that might regulate hair production have not been evaluated comprehensively. Much of the focus has been on zinc, vitamin D, and protein. Studies of the role of zinc in hair loss demonstrate the principle that both too little and too much of a substance can have deleterious effects. Moderate to severe zinc deficiency has been associated with alopecia in rhesus and bonnet macaques,109 marmosets,20 talapoin monkeys,48 and children.4 In all of these cases, addition of zinc to the food or drinking water led to a substantial improvement of skin and hair. A milder deficiency that was sufficient to retard skeletal growth and maturation in adolescent rhesus monkeys did not reliably produce alopecia.40 Conversely, exposure to toxic levels of environmental zinc has also been associated with alopecia, usually in combination with anemia and achromatricia (also called ‘white monkey syndrome’). In 1 report,38 elevated zinc levels and white monkey syndrome were found in young baboons housed in 1 of several galvanized metal and concrete cage units. This particular unit received significantly lower levels of sunlight than did other units, and the authors hypothesized that zinc leaching from the galvanized cage during cage rinsing led to contaminated standing water that did not dissipate quickly because of the low illumination. Removal of the animals from this particular cage led to a reversal of clinical signs in most cases.
Although vitamin D is thought to play a role in hair production, most of the evidence for this hypothesis comes from studies of mice lacking the vitamin D receptor (VDR) and humans with VDR mutations.16,51 Targeted removal of the VDR in mice produces a cluster of clinical signs (including hypercalcemia, hyperparathyroidism, and rickets) that includes alopecia.61 The alopecia appears to be the result of an inadequate response of hair follicles to anagen initiation.100 Selectively restoring VDR to the keratinocytes by crossing VDR knockout mice with transgenic mice expressing the human VDR successfully prevented the development of alopecia.21 Because the effect of the VDR on hair loss is ligand-independent,99 the actual role of vitamin D in regulating hair growth remains elusive. Furthermore, little is known about the role of the VDR in hair loss in nonhuman primates.
Alopecia has also been associated with other deficiencies and overdoses. A syndrome of alopecia, weight loss, and hypoalbuminemia in Western lowland gorillas eventually was traced to protein deficiency.70 This finding is consistent with the reported hair loss and hypoalbuminemia in young baboons experimentally exposed to a protein-deficient diet.22 Hair loss has also been linked to folacin deficiency in squirrel monkeys.93
Two other nutritional factors merit some discussion, even though the influence of these factors has not yet been studied in nonhuman primates. An extensive literature in nonmenopausal women suggests that iron deficiency can result in alopecia.28,98 However, reduced iron stores do not account for all forms of hair loss. One study50 found a significant association of iron levels and hair loss in cases of androgenetic alopecia and alopecia areata but not in cases of alopecia universalis or telogen effluvium. Vitamin A also may have an effect on hair loss. Rats fed a diet deficient in vitamin A developed a constellation of clinical signs that included anemia and alopecia.66 Conversely, a syndrome of severe emaciation and alopecia in calves was thought to be due to a vitamin A overdose.118 Whether low iron stores or vitamin A levels affect hair growth in nonhuman primates is largely unknown. However, the designated requirements for vitamin A in Old World monkeys have recently come under scrutiny as possibly being too high.68,85,86
Hormonal imbalances or changes.
Changes in hormone levels have long been known to play a role in hair loss, particularly in humans. Perhaps the best known example is male pattern baldness (androgenetic alopecia), which is caused by the conversion of androgens into dihydrotestosterone within genetically vulnerable hair follicles. Dihydrotestosterone shortens the anagen phase and causes follicles to become miniaturized.78 This condition appears to have no nonhuman primate homologue, although the stumptailed macaque with its characteristic bald forehead has been used as a model for studying the effects of minoxidil on hair growth.30,31 However, other hormones can be linked to hair loss in both humans and animals. Here we focus on the possible roles of hypothyroidism, tumors of the pituitary or adrenal causing hyperadrenocorticism, and pregnancy in hair loss.
Hypothyroidism has been most well studied in dogs in part because of the high prevalence of this endocrine disorder in a variety of breeds.24,33 Primary hypothyroidism, which is associated with a gradual decline in functional thyroid tissue,37 gives rise to alopecia in about 25% of cases.82 The hair loss appears to be a result of telogen effluvium, and treatment with thyroxine generally induces hair regrowth and improved skin.24 In comparison to humans with hypothyroidism, dogs with this endocrinopathy require a substantially higher dose of thyroxine because of decreased rates of absorption and increased rates of clearance.72 Less is known about hypothyroidism and its link to hair loss in other species. However, hypothyroid-related hair loss has been reported for a gorilla,60 an orangutan,108 and a chimpanzee.67 Treatment with thyroid hormone reversed the alopecia in all cases except for the chimpanzee, for which hormone treatment was planned, but the effects were not described.
Hyperadrenocorticism (Cushing syndrome) has been studied extensively in both humans and canines. In dogs, this endocrinopathy is associated with excess glucocorticoids either from therapeutic administration of glucocorticoids (iatrogenic disease) or from excess adrenocorticotropic hormone secretion by the anterior pituitary, most often due to tumor of the pars distalis region. One of the classic signs of pituitary-dependent hyperadrenocorticism in dogs is a symmetrical pattern of hair loss that affects nearly the entire body except for the head or lower extremities.37 Typically pituitary-dependent hyperadrenocorticism is differentiated from stress-induced elevations in cortisol by means of several screening procedures, including the adrenocorticotropic hormone stimulation and dexamethasone suppression tests. Treatment of pituitary-dependent hyperadrenocorticism generally involves surgical removal of the tumor in humans, whereas both surgical treatment and drug administration have been used in dogs. Dogs have been treated successfully with lysodren (a relative of DDT),88 which suppresses the production of cortisol in the adrenal cortex and causes progressive destruction of adrenal tissue. Dogs must be monitored closely, and cortisone supplements must be available to reverse the suppression if necessary. Currently, trilostane is used increasingly to treat pituitary-dependent hyperadrenocorticism and appears to have fewer side effects than does lysodren.95 Although the incidence of pituitary-dependent hyperadrenocorticism in nonhuman primates is largely unknown, a recent case report of hair loss in a Japanese macaque was attributed to hyperadrenocorticism on the basis of changes in skin morphology and various endocrine and hematologic parameters.56
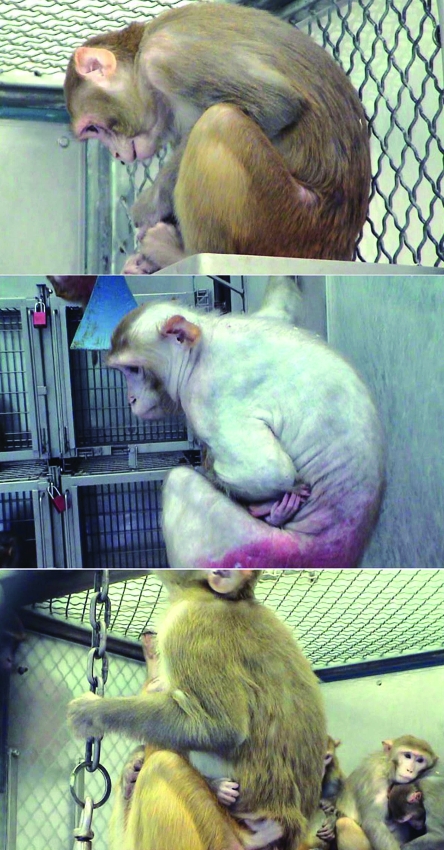
Hair loss has been associated with pregnancy and the postpartum period in both humans and animals. Pregnancy can induce telogen effluvium in some women;71 however, this form of hair loss is associated more commonly with the postpartum period, occurring in about 30% to 50% of women.27 In most women, the hair loss is temporary, and regrowth occurs in about 3 to 6 mo. Little is known about the causes or possible treatments for this condition.35 Both pregnancy and lactation have been associated with diffuse telogen effluvium in some breeds of dog.19 Furthermore, a recent report26 links hair loss to pregnancy in some rhesus monkeys. During gestation, 10 breeding female monkeys, housed in an indoor-outdoor run, showed substantial hair loss that was unrelated to seasonal influences. After parturition, hair grew quickly, and full coats were restored within 2 mo (Figure 2). As for humans, there is no established treatment for this condition in nonhuman primates, but vitamin, mineral, and hormonal imbalances should be ruled out.
Figure 2.
Depicted is the change in hair condition in a female rhesus monkey over the course of her pregnancy. The top and bottom photos, in which the hair condition is normal, were taken at the time of conception and 2 mo after parturition, respectively. The middle photo, showing total-body alopecia, was taken during the month when parturition occurred. These photographs are courtesy of E Davis (NIH Animal Center, National Institute of Child Health and Human Development, Poolesville, MD).
Immunologic and genetic factors.
Autoimmune hair disorders.
Alopecia areata is an immunologic disorder that generally affects men and women between the ages of 20 and 40 y.117 The disease starts with rapid patchy hair loss, which can spread to the entire scalp (alopecia areata totalis). In rarer cases, alopecia areata can involve hair loss over the entire body (alopecia universalis).84 The typical pattern involves the development of only a few patches that disappear very slowly as a result of regrowth from the periphery. Alopecia areata is characterized by a dysfunctional immune response, in which the hair follicle is invaded by lymphocytes (primarily CD4-positive T lymphocytes) and macrophages that target specific antigens of the anagen hair follicle. The occurrence of a similar kind of immune-mediated hair disease has been noted both in rhesus monkeys and chimpanzees. One reported case involved a female rhesus monkey presenting with total hair loss since infancy.15 Based on clinical examination, serologic testing, and histologic and immunocytochemical examinations of the skin showing perifollicular lymphocytic infiltration, the authors concluded that the monkey had alopecia universalis. A similar conclusion was reached in determining the cause of total body hair loss in a female chimpanzee.36
Several other autoimmune disorders are associated with alopecia. Lichen planus is an inflammatory disorder affecting the follicles and inducing hair loss.6 Lupus erythematosus is associated with nonscarring alopecia in 40% of cases, but in a small percentage of cases (14%), a scarring alopecia can develop secondary to discoid lesions.119 At present, lichen planus has not been reported in nonhuman primates; however, a systemic lupus erythematosus-like syndrome occurred in a rhesus macaque,5 and a similar syndrome was observed in cynomolgus monkeys maintained on a 40% alfalfa sprouts diet.64
Mutations of the hairless gene.
Certain forms of hair loss have been linked to mutations in the hairless (hr) gene in mice and humans. Atrichia with papular lesions is a rare genetic disorder in which organisms are born with hair which, once shed, is never replaced.2,18 An examination of the skin reveals the absence of normal hair follicles and the presence of papular cysts. Permanent and total hair loss occurs between days 14 to 21 of postnatal life in mice and during the first year of life in humans.83 Mutations of hr also can produce hair loss in rhesus macaques. An infant rhesus macaque was born fully haired at the Tulane Primate Center but lost all of its hair and eyelashes within the first week of life.94 Analysis of its skin showed an absence of hair follicles and the presence of dermal cysts. Subsequent genetic evaluation revealed mutations in the hr gene.1
Inflammatory hair loss.
A variety of skin conditions are associated with inflammation and pruritis and may also result in hair loss. These etiologies include bacterial infections, either primary or secondary to some other disease process, parasitic infections, and allergic processes (for example, dermatitis).
Bacterial infections.
Several different bacteria affect the skin, producing lesions and hair loss. Staphylococcus bacteria can produce a scarring alopecia (folliculitis decalvans) in middle-aged adults.79 Staphylococcus infections have also been shown to produce skin lesions and alopecia in sheep,59 horses,29 and dogs.90 Furthermore, induction of chronic salmonellosis in guinea pigs was associated with hair loss that varied across subjects as a function of the route of administration and the strain of Salmonella.102 Although bacterial infections should be ruled out as a possible source of hair loss, no available report suggests that bacterial infections are a leading cause of hair loss in captive nonhuman primates.
Bacterial skin infections also can develop as a secondary consequence of other diseases. Most relevant for nonhuman primates are the skin infections that can develop in monkeys with diabetes. Humans with either type 1 or type 2 diabetes are at increased risk for skin infections that can lead to hair loss.69 Skin infections were noted in mandrills with a syndrome similar to type 2 diabetes.91
Parasitic infections.
Fungal infections can cause hair loss that generally is associated with pruritis. Tinea capitus is a disorder that produces scalp eruptions and hair loss in both children and adults.65 The infection typically is caused either by Microsporum canis or Trichophyton tonsurans. Fungal infections (usually caused by M. canis) have also been detected in rhesus monkeys,13,81 chimpanzees,57 and gibbons.111 Treatment with oral antifungal preparations (for example, griseofulvin and itraconazole) was generally effective in reducing or eliminating the infection. Recent research suggests that topical treatment with a sustained-release preparation of clotrimazole can effectively eliminate M. canis infection in gibbons.12
Several insect parasites can produce oozing skin eruptions and hair loss. Prominent among these is the sarcoptic mange mite, which has a worldwide distribution. Sarcoptes scabiei mites parasitize many different mammals. However, evidence suggests that mites have species-specific hosts and that cross-transference to a nonspecific host produces a pseudosarcoptosis, which is transient and self-limiting.11 Diagnosis can be established from microscopic examination of skin scrapings. Sarcoptic mange is quite rare in nonhuman primates and appears to be limited to monkeys maintained in natural or seminatural settings. Sacaroptic mange was identified in 5 juvenile, human-habituated mountain gorillas in Bwindi Impenetrable National Park, Uganda,41 and in an adult female bonnet monkey maintained in an open enclosure in a seminatural environment.73 In both cases, sarcoptic mange was treated successfully with ivermectin.
Atopic dermatitis.
Atopic dermatitis, a common cause of hair loss in cats and dogs, is a chronic skin condition that appears to be precipitated by exposure to allergens. The condition is associated invariably with pruritus, which sometimes results in oozing sores that can lead to secondary bacterial infections. The dermatitis may be caused by airborne allergens (for example, dust mites), food sensitivity, or direct skin contact with allergens in cleaning agents, toys, and bedding products. Atopic dermatitis was identified as the cause of skin inflammation and alopecia in 2 case studies involving rhesus monkeys.63,80 An allergen-specific IgE antibody test was used to screen for potential allergens. In 1 case, a possible latex allergy was identified and confirmed through skin prick tests. Replacement of latex gloves with vinyl gloves led to a marked improvement in the skin condition of this monkey.63 In the other case, the source of the allergic reaction could not be identified with an allergen-specific IgE antibody test.80 This outcome is not uncommon, in that the source of the allergic reaction can be difficult to identify. Despite this difficulty, several treatments have proven somewhat effective. Essential fatty acids are now being used to treat atopic dermatitis. This treatment appears to have few side effects and is effective in reducing inflammation, skin lesions, and pruritis in dogs99 and humans.49,58 Furthermore, oral administration of a fatty acid (dihomo-gamma-linolenic acid) to NC/Nga mice that spontaneously developed atopic dermatitis resulted in a marked dose-dependent reduction in the severity of the lesions.54 Steroids have also been used to treat atopic dermatitis and may be the pharmacotherapy of choice in some cases, despite the possibility of considerable side effects.3 However, recent reports indicate that cyclosporine, a macrolide calcineurin inhibitor, may be as potent as glucocorticoids in eliminating atopic dermatitis.76 Cyclosporin successfully reduced skin lesions and pruritus and produced hair growth in dogs,76 cats,114 and in the described rhesus monkey for which no specific allergen was identified.80
Psychologic factors and hair loss.
The role of psychologic factors in hair loss is not well understood. Here we focus on various behavioral characteristics that may lead to hair loss and examine the concept of stress-related hair loss.
Self-induced hair loss.
Monkeys and apes can develop an abnormal pattern of behavior in which they pluck their hair.14 Some animals pull their hair, out creating bald patches, whereas other hair pullers show little evidence of hair loss. This disorder shares some similarities with the human pathologic disorder trichotillomania.116 In both cases, the act tends to be highly ritualized and repetitive: after the hair is pulled, it usually is manipulated and then ingested. Some investigators speculate that hair pulling is brought about by environmental stressors or by exposure to impoverished captive environments.96 Indeed, 14% of rhesus monkeys housed in individual cages developed this pathology.62 However, other medical conditions may underlie the development of hair pulling, and therefore a health exam is necessary to rule out physical causes. This point was underscored in a recent study of psychogenic alopecia in cats. Cats that had previously been diagnosed with psychogenic alopecia (an apparent compulsive licking or grooming associated with hair loss) were reevaluated. Of the 21 cats in the study, 16 actually had a medical disorder, the most common being atopic dermatitis, leading the authors to conclude that psychogenic alopecia is overdiagnosed.115
Currently, no established treatment for hair pulling reliably reduces or eliminates this behavior. Little substantiated evidence indicates that environmental enrichment routinely alleviates this behavior, although exercise cages107 or social housing.104 may provide some benefit. The failure to reduce or eliminate this perplexing behavior in captive primates underscores the need for future research to determine the underlying causes and identify effective treatments.
Hair pulling by others.
Nonhuman primates living in social groups may be vulnerable to having their hair pulled out by other animals. In a study of 2 breeding groups of rhesus monkeys, monkeys pulled hair at the rate of 2.3 episodes per hour. Most of the episodes were directed to other monkeys rather than to self (97% versus 3% respectively).97 Reduction in socially directed hair pulling in group-housed monkeys was noted after provision of a foraging task.17 However, in our experience, the problem may be more difficult to extinguish in pair-housed monkeys and may not necessarily be eliminated with new partners. In this situation, the only known successful treatment is separation of animals; therefore, the risk of alopecia induced by hair pulling has to be weighed against the cost of individual cage housing.
Stress.
Increasing evidence suggests that stress may be associated with hair loss. This notion began with early uncontrolled studies and, in some cases, anecdotal reports that alopecia areata in humans might be due to stressful life events. A review of this body of information89 pointed out the noteworthy limitations of such approaches. Subsequent studies that were better controlled did find some evidence for a role of stress in precipitating episodes of hair loss. Nevertheless, the authors of the review89 urged caution regarding assuming a definite association between stress and either the onset or exacerbation of alopecia areata.
Thee possible relationships between stress and hair loss in humans have been proposed:42 1) stress (either acute or chronic) could be a primary causal factor in triggering hair loss by telogen effluvium; 2) stress could be a secondary factor in exacerbating hair loss due to a preexisting disorder, such as endocrine imbalance, toxin exposure, nutrient deficiency, or autoimmune disease; and 3) stress could be induced by the psychologic effects of losing one's hair, which then might exacerbate the problem in a classic ‘vicious circle.’ Only the first 2 hypotheses would seem to pertain to nonhuman primates, because the relevance of alopecia as a stressor for nonhuman primates is questionable at best.
The primary effects of stress on hair growth or loss have been explored most fully in mice. Mice exposed to footshock or noise exhibit hair loss as a result of prolonging the telogen phase, thereby delaying anagen phase induction,7,52 or by prematurely terminating the anagen phase, leading to more rapid development of the catagen phase.9 The mechanisms responsible for these effects appear to be complex, involving multiple biologic pathways.10 At a cellular level, various stressors may induce a local inflammatory response that leads to an inhibition of keratinocyte proliferation as well as an increase in apoptotic death of keratinocytes in telogen-stage hair follicles.8 The signaling pathways mediating these effects are thought to involve the peptide substance P acting on neurokinin 1 receptors,9,52 nerve growth factor,87 and possibly glucocorticoids due to activation of the hypothalamic–pituitary–adrenocortical axis. Moreover, elements of the skin (including hair follicles) appear to have their own endocrine-like system that includes local synthesis and expression of corticotropin-releasing hormone and its receptors, adrenocorticotropic hormone, and cortisol.10,103 Some evidence indicates that this system is upregulated in affected areas of skin in patients with alopecia areata.53,55
Alopecia in captive macaque monkeys, at least as related to hair-pulling behavior, has been attributed to stress.44 Nevertheless, currently little, if any, evidence supports a direct association between stress and alopecia in nonhuman primates and certainly no mechanistic information is available. In fact, 1 recent study found a reduction rather than an increase in fecal levels of the cortisol metabolite 11-oxoetiocholanolone in rhesus monkeys with alopecia.105 Clearly, more research in this area is needed before any reliable conclusions can be drawn.
If alopecia is due to stress, pharmacologic treatment might be beneficial. One report39 cites anecdotal data suggesting that alopecia areata may respond positively to treatment with antidepressant or anxiolytic drugs, but the authors also note the need for appropriately controlled clinical trials to substantiate such observations. A retrospective study of 11 cats investigated psychogenic alopecia related to overgrooming (potential biologic causes of hair loss were ruled out by examining veterinarians). Consistent with the anecdotal reports regarding alopecia areata, most of these animals responded positively to antidepressant or anxiolytic drug administration.101 We know of no reports of successful treatment of alopecia in nonhuman primates with psychoactive medications.
Managing Alopecia in Laboratory Primates
Because monkeys in captivity can develop alopecia, it is important to determine the likely causes and identify effective treatments. As we have discussed, many different factors contribute to hair loss in nonhuman primates. But clearly some of these factors are quite rare (for example, mutations of the hairless gene), whereas others may be more common. Here we present a possible strategy for determining the causes of and managing alopecia in nonhuman primates.
The first step in the process is assessment. As part of regular health exams, animals' hair and skin conditions should be routinely assessed. Quantifying the hair loss and evaluating the skin surface may be useful in determining whether the condition is improving or deteriorating from one health exam to the next. Photographs to document progression can be a useful tool. The second step is to determine whether immediate medical evaluation is necessary. In this regard, dividing cases of alopecia into 2 categories may be helpful. The first is the ‘wait and watch’ category which would include cases of minor hair loss (one or more small patches) as well as hair loss associated with seasonal or reproductive factors that would be expected to resolve on its own without significant consequence. The second is a medical evaluation category which would include alopecia associated with reddened and inflamed skin, extensive whole-body hair loss that cannot be attributed to seasonal and reproductive factors, and excessive hair plucking. For cases requiring medical evaluation, the third step is to obtain skin biopsies for evaluation of inflammation, bacterial, fungal, or parasitic infections. Biopsies also can provide information on hair follicle condition. This information should be supplemented with behavioral observations to determine the incidences of scratching and presence or absence of hair pulling by self or others. This determination can often be made by animal care technicians, who are most familiar with the animals, but these determinations also can be made by environmental enrichment technicians by using direct observation or video recordings of subjects with alopecia along with room-matched controls. The fourth step, if necessary, is to obtain blood samples for routine screening and assessment of relevant hormone levels. The final step is to narrow the range of possibilities and consider treatment options. Here we consider 3 scenarios representing what might be the more common causes of hair loss in nonhuman primates and possible treatments.
Scenario 1.
If increased scratching is observed along with reddened skin, and biopsies have ruled out bacterial, fungal, or parasitic infections, then the most likely diagnosis is atopic dermatitis. If the source of the allergen cannot be identified and removed, then several treatment options are available depending on the research protocol. For nonhuman primates, treatment with oral steroids or cyclosporine should be considered. We successfully treated 1 aged female monkey with pronounced total-body hair loss, inflamed skin, and pruritis by administering dexamethasone orally for a period of several months, during the latter part of which the dosage was gradually reduced. Treatment was effective in restoring full body hair, and her coat has been maintained for well more than 1 year after treatment.
Scenario 2.
If the hair loss is self-induced (by hair pulling) and skin biopsies have ruled out inflammation and bacterial, fungal, and parasitic infections, then a tentative diagnosis is psychogenic or stress-induced hair loss. If the stressor cannot be identified and removed (possible sources include experimental procedures, husbandry practices, and other animals, among others), then 2 strategies might be useful. The first is an enrichment strategy in which the environment is modified to provide the animal with greater stimulation. Such changes might include pair housing. The second is a pharmacotherapeutic strategy in which the animal is treated for anxiety by using anxiolytic drugs such as diazepam. However, no systematic studies show that either of these strategies is completely effective in eliminating hair-pulling behavior. Moreover, any proposed treatments for alopecia must be compatible with the research protocol under which the animal is being studied.
Scenario 3.
If behavioral correlates of the hair loss are not apparent and skin biopsies reveal no evidence of skin inflammation or the presence of bacterial, fungal, or parasitic infections or alterations in hair follicle biology, then other factors have to be considered in the diagnosis. Three such factors include seasonal variation, reproduction, and age. Seasonal or pregnancy related-hair loss should resolve within 4 to 8 mo without treatment. If none of these factors is related to the hair loss or if the hair loss does not resolve, then hormonal imbalances and nutritional deficits should be ruled out. If the hair loss appears to be a natural consequence of aging, no treatment is currently available to ameliorate this condition in nonhuman primates.
Conclusions
A variety of factors are known to produce hair loss in mammals and, more specifically nonhuman primates. Some of these factors are rare, accounting for only a small percentage of monkeys that show alopecia; others are probably much more common. In general, little is known about the prevalence of different types of hair loss in captive nonhuman primates, in part because of a tendency to view hair loss in this mammalian order as a unitary phenomenon related to stress. Identifying the causes of hair loss in individual primates depends on an assessment approach involving health exams, skin biopsies, blood screening, and behavioral observations. Knowing the cause is crucial to establishing effective treatment regimens. However, hair loss also can result from several causative factors working in concert. Furthermore, much remains unknown about treatment. Whereas some forms of alopecia can be treated (for example, hair loss associated with hypothyroidism), well established treatments are not available for other forms (for example, hair loss associated with hair pulling). In fact, simply monitoring the animal may be the best approach when underlying medical causes are ruled out and other clinical signs such as dermatitis or pruritus are absent. Finally, some forms of hair loss cannot be remediated (for example, mutations of the hairless gene, hair loss in some animals as a result of old age), and therefore alopecia most likely will never completely be eliminated from captive nonhuman primate populations. These problems underscore the need for future research on the causes of hair loss in nonhuman primates, not only to benefit their condition but also to develop new biomedical models to advance our understanding of alopecia in humans.
Acknowledgments
The preparation of this manuscript was supported by grants RR11122 (to MAN) and RR00168 (to the New England Primate Research Center) from the National Institutes of Health.
References
- 1.Ahmad W, Ratterree MS, Panteleyev AA, Aita VM, Sundberg JP, Christiano AM. 2002. Atrichia with popular lesions resulting from mutations in the rhesus macaque (Macaca mulatta) hairless gene. Lab Anim 36:61–67 [DOI] [PubMed] [Google Scholar]
- 2.Ahmad W, Zlotogorski A, Panteleyev AA, Lam H, Ahmad M, ul Haque MF, Abdallah HM, Dragan L, Christiano AM. 1999. Genomic organization of the human hairless gene (HR) and identification of a mutation underlying congenital atrichia in an Arab Palestinian family. Genomics 56:141–148 [DOI] [PubMed] [Google Scholar]
- 3.Akhavan A, Rudikoff D. 2008. Atopic dermatitis: systemic immunosuppressive therapy. Semin Cutan Med Surg 27:151–155 [DOI] [PubMed] [Google Scholar]
- 4.Alhaj E, Alhaj N, Alhaj NE. 2007. Diffuse alopecia in a child due to dietary zinc deficiency. Skinmed 6:199–200 [DOI] [PubMed] [Google Scholar]
- 5.Anderson ST, Klein EC. 1993. Systemic lupus erythematosus in a rhesus macaque. Arthritis Rheum 36:1739–1742 [DOI] [PubMed] [Google Scholar]
- 6.Annessi G, Lombardo G, Gobello T, Puddu P. 1999. A clinicopathologic study of scarring alopecia due to lichen planus: comparison with scarring alopecia in discoid lupus erythematosus and pseudopelade. Am J Dermatopathol 21:324–331 [DOI] [PubMed] [Google Scholar]
- 7.Aoki E, Shibasaki T, Kawana S. 2003. Intermittent foot shock stress prolongs the telogen stage in the hair cycle of mice. Exp Dermatol 12:371–377 [DOI] [PubMed] [Google Scholar]
- 8.Arck PC, Handjiski B, Hagen E, Joachim R, Klapp BF, Paus R. 2001. Indications for a ‘brain–hair follicle axis (BHA)’: inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. FASEB J 15:2536–2538 [DOI] [PubMed] [Google Scholar]
- 9.Arck PC, Handjiski B, Peters EMJ, Peter AS, Hagen E, Fischer A, Klapp BF, Paus R. 2003. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol 162:803–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arck PC, Slominski A, Theoharides TC, Peters EMJ, Paus R. 2006. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol 126:1697–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arlian LG, Morgan MS, Arends JJ. 1996. Immunologic cross-reactivity among various strains of Sarcoptes scabiei. J Parasitol 82:66–72 [PubMed] [Google Scholar]
- 12.Avni-Magen N, Elad D, Friedman M, Gati I, Kaufman E, Lavy E. 2008. Use of a sustained release preparation of clotrimazole to treat dermatophytosis in a siamang (Hylobates syndactylus). J Zoo Wildl Med 39:115–117 [DOI] [PubMed] [Google Scholar]
- 13.Baker HJ, Bradford LG, Montes LF. 1971. Dermatophytosis due to Microsporum canis in a rhesus monkey. J Am Vet Med Assoc 159:1607–1611 [PubMed] [Google Scholar]
- 14.Bayne KAL, Novak MA. 1998. Psychological disorders, p 485–500 : Bennett BT, Abee CR, Henrickson R, editors Nonhuman primates in biomedical research, vol. II: Diseases New York: Academic Press [Google Scholar]
- 15.Beardi B, Wanert F, Zoller M, Freyschmidt-Paul P, Kaup F-J. 2007. Alopecia areata in a rhesus monkey (Macaca mulatta). J Med Primatol 36:124–130 [DOI] [PubMed] [Google Scholar]
- 16.Bikle DD, Elalieh H, Chang S, Xie Z, Sundberg JP. 2006. Development and progression of alopecia in the vitamin D receptor-null mouse. J Cell Physiol 207:340–353 [DOI] [PubMed] [Google Scholar]
- 17.Boccia ML, Hijazi AS. 1998. A foraging task reduces agonistic and stereotypic behaviors in socially housed pigtail macaques. Lab Primate Newsl 28:18–19 [Google Scholar]
- 18.Cachon-Gonzalez MB, Fenner S, Coffin JM, Moran C, Best S, Stoye JP. 1994. Structure and expression of the hairless gene of mice. Proc Natl Acad Sci USA 91:7717–7721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerundolo R. 1999. Symmetrical alopecia in the dog. In Pract 21:350–359 [Google Scholar]
- 20.Chadwick DP, May JC, Lorenz D. 1979. Spontaneous zinc deficiency in marmosets, Saguinus mystax. Lab Anim Sci 29:482–485 [PubMed] [Google Scholar]
- 21.Chen CH, Sakai Y, Demay MB. 2001. Targeting expression of the human vitamin D receptor to the keratinocytes of vitamin D receptor null mice prevents alopecia. Endocrinology 142:5386–5389 [DOI] [PubMed] [Google Scholar]
- 22.Coward DG, Whitehead RG. 1972. Experimental protein-energy malnutrition in baby baboons. Br J Nutr 28:223–237 [DOI] [PubMed] [Google Scholar]
- 23.Craven AJ, Nixon AJ, Ashby MG, Ormandy CJ, Blazek K, Wilkins RJ, Pearson AJ. 2006. Prolactin delays hair regrowth in mice. J Endocrinol 191:415–425 [DOI] [PubMed] [Google Scholar]
- 24.Credille KM, Slater MR, Moriello KA, Nachreiner RF, Tucker KA, Dunstan RW. 2001. The effects of thyroid hormones on the skin of Beagle dogs. J Vet Intern Med 15:539–546 [DOI] [PubMed] [Google Scholar]
- 25.Curlewis JD, Loudon ASI, Milne JA, McNeilly AS. 1988. Effects of chronic long-lasting bromocryptine treatment on liveweight voluntary food intake, coat growth, and breeding season in nonpregnant red deer hinds. J Endocrinol 119:413–420 [DOI] [PubMed] [Google Scholar]
- 26.Davis EB, Suomi SJ. 2006. Hair loss and replacement cycles in socially housed, pregnant, rhesus macaques. Am J Primatol 68:58. [DOI] [PubMed] [Google Scholar]
- 27.Dawber RPR, Connor BL. 1971. Pregnancy, hair loss, and the pill. Br Med J 4:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deloche C, Bastien P, Chadoutaud S, Galan P, Bertrais S, Hercberg S, de Lacharriere O. 2007. Low iron stores: a risk factor for excessive hair loss in nonmenopausal women. Eur J Dermatol 17:507–512 [DOI] [PubMed] [Google Scholar]
- 29.Devriese LA, Vlaminck K, Nuytten J, Keersmaecker P. 1983. Staphylococcus hyicus in skin lesions of horses. Equine Vet J 15:263–265 [DOI] [PubMed] [Google Scholar]
- 30.Diani AR, Mills CJ. 1994. Immunocytochemical localization of androgen receptors in the scalp of the stumptail macaque monkey, a model of androgenetic alopecia. J Invest Dermatol 102:511–514 [DOI] [PubMed] [Google Scholar]
- 31.Diani AR, Shull KL, Zaya MJ, Brunden MN. 1995. The penetration enhancer SEPA augments stimulation of scalp hair growth by topical minoxidil in the balding stumptail macaque. Skin Pharmacol 8:221–228 [DOI] [PubMed] [Google Scholar]
- 32.Dicks P, Russel AFJ, Lincoln GA. 1994. The role of prolactin in the reactivation of hair follicles in relation to moulting in cashmere goats. J Endocrinol 143:441–448 [DOI] [PubMed] [Google Scholar]
- 33.Dixon RM, Reid SW, Mooney CT. 1999. Epidemiological, clinical, haematological and biochemical characteristics of canine hypothyroidism. Vet Rec 145:481–487 [DOI] [PubMed] [Google Scholar]
- 34.Duncan MJ, Goldman BD. 1984. Hormonal regulation of the annual pelage color cycle in the Djungarian hamster, Phodopus sungorus. II. Role of prolactin. J Exp Zool 230:97–103 [DOI] [PubMed] [Google Scholar]
- 35.Eastham JH. 2001. Postpartum alopecia. Ann Pharmacother 35:255–258 [DOI] [PubMed] [Google Scholar]
- 36.Eichberg JW, De Villez RL. 1984. Alopecia totalis in a chimpanzee. J Med Primatol 13:81–88 [PubMed] [Google Scholar]
- 37.Frank LA. 2006. Comparative dermatology canine endocrine dermatoses. Clin Dermatol 24:317–325 [DOI] [PubMed] [Google Scholar]
- 38.Frost PA, Hubbard GB, Dammann MJ, Snider CL, Moore CM, Hodara VL, Giavedoni LD, Rohwer R, Mahaney MC, Butler TM, Cummins LB, McDonaold TJ, Nathanielsz PW, Schlabritz-Loutsevitch NE. 2004. White monkey syndrome in infant baboons (Papio species). J Med Primatol 33:197–213 [DOI] [PubMed] [Google Scholar]
- 39.García-Hernández MJ, Ruiz-Doblado S, Rodriguez-Pichardo A, Camacho F. 1999. Alopecia areata, stress and psychiatric disorders: a review. J Dermatol 26:625–632 [DOI] [PubMed] [Google Scholar]
- 40.Golub MS, Keen CL, Gershwin E, Styne DM, Takeuchi PT, Ontell F, Walter RM, Hendrickx AG. 1996. Adolescent growth and maturation in zinc-deprived rhesus monkeys. Am J Clin Nutr 64:274–282 [DOI] [PubMed] [Google Scholar]
- 41.Graczyk TK, Mudakikwa AB, Cranfield MR, Ellenberger U. 2001. Hyperkeratotic mange caused by Sarcoptes scabiei (Acariformes: Sarcoptidae) in juvenile human-habituated mountain gorillas (Gorilla gorilla beringei). Parasitol Res 87:1024–1028 [DOI] [PubMed] [Google Scholar]
- 42.Hadshiew IM, Foitzik K, Arck PC, Paus R. 2004. Burden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J Invest Dermatol 123:455–457 [DOI] [PubMed] [Google Scholar]
- 43.Headington JT. 1993. Telogen effluvium. New concepts and review. Arch Dermatol 129:356–363 [DOI] [PubMed] [Google Scholar]
- 44.Honess PE, Gimpel JL, Wolfensohn SE, Mason GJ. 2005. Alopecia scoring: the quantitative assessment of hair loss in captive macaques. Altern Lab Anim 33:193–206 [DOI] [PubMed] [Google Scholar]
- 45.Horenstein VD-P, Williams LE, Brady AR, Abee CR, Horenstein MG. 2005. Age-related diffuse chronic telogen effluvium-type alopecia in female squirrel monkeys (Saimiri boliviensis boliviensis). Comp Med 55:169–174 [PubMed] [Google Scholar]
- 46.Huneke RB, Foltz CJ, VandeWoude S, Mandress TD, Garman RH. 1996. Characterization of dermatologic changes in geriatric rhesus macaques. J Med Primatol 25:404–413 [DOI] [PubMed] [Google Scholar]
- 47.Isbell LA. 1995. Seasonal and social correlates of changes in hair, skin, and scrotal condition in vervet monkeys (Cercopithecus aethiops) of Amboseli National Park, Kenya. Am J Primatol 36:61–70 [DOI] [PubMed] [Google Scholar]
- 48.Juan-Salles C, Prats N, Verges J, Ruiz M, Valls X, Gine J, Marco A. 2001. Dermatosis in talapoin monkeys (Miopithesus talapoin) with response to zinc and animal protein. Vet Rec 149:24–25 [DOI] [PubMed] [Google Scholar]
- 49.Kanehara S, Ohtani T, Uede K, Furukawa F. 2007. Clinical effects of undershirts coated with borage oil on children with atopic dermatitis: a double-blind, placebo-controlled clinical trial. J Dermatol 34:811–815 [DOI] [PubMed] [Google Scholar]
- 50.Kantor J, Kessler DG, Cotsarelis G. 2003. Decreased serum ferritin is associated with alopecia in women. J Invest Dermatol 121:985–988 [DOI] [PubMed] [Google Scholar]
- 51.Katavetin P, Katavetin P, Wacharasindhu S, Shotelersuk V. 2006. A girl with a novel splice site mutation in the VDR supports the role of a ligand-independent VDR function on hair cycling. Horm Res 66:273–276 [DOI] [PubMed] [Google Scholar]
- 52.Katayama M, Aoki E, Suzuki H, Kawana S. 2007. Foot shock stress prolongs the telogen stage of the spontaneous hair cycle in a nondepilated mouse model. Exp Dermatol 16:553–560 [DOI] [PubMed] [Google Scholar]
- 53.Katsarou-Katsari A, Singh LK, Theoharides TC. 2001. Alopecia areata and affected skin CRH receptor upregulation induced by acute emotional stress. Dermatology 203:157–161 [DOI] [PubMed] [Google Scholar]
- 54.Kawashima H, Tateishi N, Shiraishi A, Teraoka N, Tanaka T, Tanaka A, Matsuda H, Kiso Y. 2008. Oral administration of dihomo-gamma-linolenic acid prevents development of atopic dermatitis in NC/Nga mice. Lipids 43:37–43 [DOI] [PubMed] [Google Scholar]
- 55.Kim HS, Cho DH, Kim HJ, Lee JY, Cho BK, Park HJ. 2006. Immunoreactivity of corticotrophin-releasing hormone, adrenocorticotropic hormone and α-melanocyte-stimulating hormone in alopecia areata. Exp Dermatol 15:515–522 [DOI] [PubMed] [Google Scholar]
- 56.Kimura T. 2008. Systemic alopecia resulting from hyperadrenocorticism in a Japanese monkey. Lab Primate Newsl 47:5–9 [Google Scholar]
- 57.Klokke AH, de Vries GA. 1963. Tinea capitis in chimpanzees caused by Microsporum canis Bodin 1902 resembling M. obesum Conant 1937. Sabouraudia 2:268–270 [Google Scholar]
- 58.Koch C, Dölle S, Metzger M, Rasche C, Jungclas H, Rühl R, Renz H, Worm M. 2008. Docosahexaenoic acid (DHA) supplementation in atopic eczema: a randomized, double-blind, controlled trial. Br J Dermatol 158:786–792 [DOI] [PubMed] [Google Scholar]
- 59.Koutinas AF, Saridomichelakis MN, Argyroudis S, Koutinas CK, Karatzanos P, Giadinis N. 2007. Clinical, histopathological and therapeutic considerations in a flock of sheep with facial staphylococcal-associated dermatitis. Vet Dermatol 18:211–216 [DOI] [PubMed] [Google Scholar]
- 60.Lair S, Crawshaw GJ, Mehren KG, Perrone MA. 1999. Diagnosis of hypothyroidism in a Western lowland gorilla (Gorilla gorilla gorilla) using human thyroid-stimulating hormone assay. J Zoo Wildl Med 30:537–540 [PubMed] [Google Scholar]
- 61.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. 1997. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA 94:9831–9835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lutz C, Well A, Novak MA. 2003. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. Am J Primatol 60:1–15 [DOI] [PubMed] [Google Scholar]
- 63.Macy JD, Huether MJ, Beattie TA, Findlay HA, Zeiss C. 2001. Latex sensitivity in a macaque (Macaca mulatta). Comp Med 51:467–472 [PubMed] [Google Scholar]
- 64.Malinow MR, Bardana EJ, Pirofsky B, Craig S, McLaughlin P. 1982. Systemic lupus erythematosus-like syndrome in monkeys fed alfalfa sprouts: role of a nonprotein amino acid. Science 216:415–417 [DOI] [PubMed] [Google Scholar]
- 65.Martin ES, Elewski BE. 2003. Tinea capitis in adult women masquerading as bacterial pyoderma. J Am Acad Dermatol 49:177–179 [DOI] [PubMed] [Google Scholar]
- 66.Mejia LA, Hodges RE, Rucker RB. 1979. Clinical signs of anemia in vitamin A-deficient rats. Am J Clin Nutr 32:1439–1444 [DOI] [PubMed] [Google Scholar]
- 67.Miller RE, Albert SG, Boever WJ. 1983. Hypothyroidism in a chimpanzee. J Am Vet Med Assoc 183:1326–1328 [PubMed] [Google Scholar]
- 68.Mills JP, Tanumihardjo SA. 2006. Vitamin A toxicity in wild-caught African green vervet monkeys (Chlorocebus aethiops) after 2 years in captivity. Comp Med 56:421–425 [PubMed] [Google Scholar]
- 69.Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, Rutten GE. 2005. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis 41:281–288 [DOI] [PubMed] [Google Scholar]
- 70.Mundy NI, Ancrenaz M, Wickings J, Lunn PG. 1998. Protein deficiency in a colony of Western lowland gorillas (Gorilla g. gorilla). J Zoo Wildl Med 29:261–268 [PubMed] [Google Scholar]
- 71.Murray JC. 1990. Pregnancy and the skin. Dermatol Clin 8:327–334 [PubMed] [Google Scholar]
- 72.Nachreiner RF, Refsal KR, Ravis WR, Hauptman J, Rosser EJ, Pedersoli WM. 1993. Pharmacokinetics of L-thyroxine after its oral administration in dogs. Am J Vet Res 54:2091–2098 [PubMed] [Google Scholar]
- 73.Nagarajan P, Venkatesan R, Kumar JM, Majumda SS. 2004. Sarcoptes scabiei infection in a bonnet monkey (Macaca radiata). Inter J Appl Res Vet Med 2:123–125 [Google Scholar]
- 74.Nixon AJ, Ashby MG, Saywell DP, Pearson AJ. 1995. Seasonal fiber growth cycles of ferrets (Mustela putorious furo) and long-term effect of melatonin treatment. J Exp Zool 272:435–445 [DOI] [PubMed] [Google Scholar]
- 75.Nixon AJ, Ford CA, Wildermouth JE, Craven AJ, Ashby MG. 2002. Regulation of prolactin receptor expression in ovine skin in relation to circulating prolactin and wool follicle growth status. J Endocrinol 172:605–614 [DOI] [PubMed] [Google Scholar]
- 76.Olivry T, Steffan J, Fisch RD, Prélaud P, Guaguère E, Fontaine J, Carlotti DN, European Veterinary Dermatology Cyclosporine Group 2002. Randomized controlled trial of the efficacy of cyclosporine in the treatment of atopic dermatitis in dogs. J Am Vet Med Assoc 221:370–377 [DOI] [PubMed] [Google Scholar]
- 77.Olsen EA, Hordinsky MK, Price VH, Roberts JL, Shapiro J, Canfield D, Duvic M, King LE, Jr, McMichael AJ, Randall VA, Turner ML, Sperling L, Whiting DA, Norris D, National Alopecia Areata Foundation 2004. Alopecia areata investigational assessment guidelines–Part II. National Alopecia Areata Foundation. J Am Acad Dermatol 51:440–447 [DOI] [PubMed] [Google Scholar]
- 78.Otberg N, Finner AM, Shapiro J. 2007. Androgenetic alopecia. Endocrinol Metab Clin North Am 36:379–398 [DOI] [PubMed] [Google Scholar]
- 79.Otberg N, Kang H, Alzolibani AA, Shapiro J. 2008. Folliculitis decalvans. Dermatol Ther 21:238–244 [DOI] [PubMed] [Google Scholar]
- 80.Ovadia S, Wilson SR, Zeiss CJ. 2005. Successful cyclosporine treatment for atopic dermatitis in a rhesus macaque (Macaca mulatta). Comp Med 55:192–196 [PubMed] [Google Scholar]
- 81.Pal M, Matsusaka N. 1991. Dermatophytosis in a rhesus monkey (Macaca mulatta) and animal attendant caused by Microsporum canis. Erkrakungen der Zootiere 33:261–264 [Google Scholar]
- 82.Panciera DL. 1994. Hypothyroidism in dogs: 66 cases (1987–1992). J Am Vet Med Assoc 204:761–767 [PubMed] [Google Scholar]
- 83.Panteleyev AA, Paus R, Ahmad W, Sundberg JP, Christiano AM. 1998. Molecular and functional aspects of the hairless (hr) gene in laboratory rodents and humans. Exp Dermatol 7:249–267 [DOI] [PubMed] [Google Scholar]
- 84.Papadopoulos AJ, Schwartz RA, Janniger CK. 2000. Alopecia areata. Pathogenesis, diagnosis, and therapy. Am J Clin Dermatol 1:101–105 [DOI] [PubMed] [Google Scholar]
- 85.Penniston KL, Tanumihardjo SA. 2001. Subtoxic hepatic vitamin A concentrations in captive rhesus monkeys (Macaca mulatta). J Nutr 131:2904–2909 [DOI] [PubMed] [Google Scholar]
- 86.Penniston KL, Tanumihardjo SA. 2006. Vitamin A intake of captive rhesus monkeys exceeds National Research Council recommendations. Am J Primatol 68:1114–1119 [DOI] [PubMed] [Google Scholar]
- 87.Peters EMJ, Handjiski B, Kuhlmei A, Hagen E, Bielas H, Braun A, Klapp BF, Paus R, Arck PC. 2004. Neurogenic inflammation in stress-induced termination of murine hair growth is promoted by nerve growth factor. Am J Pathol 165:259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peterson ME, Kintzer PP. 1997. Medical treatment of pituitary-dependent hyperadrenocorticism. Vet Clin North Am Small Anim Pract 27:255–272 [DOI] [PubMed] [Google Scholar]
- 89.Picardi A, Abeni D. 2001. Stressful life events and skin diseases: disentangling evidence from myth. Psychother Psychosom 70:118–136 [DOI] [PubMed] [Google Scholar]
- 90.Pin D, Carlotti D-N, Jasmin P, DeBoer DJ, Prelaud P. 2006. Prospective study of bacterial overgrowth syndrome in eight dogs. Vet Rec 158:437–441 [DOI] [PubMed] [Google Scholar]
- 91.Pirarat N, Kesdangsakolwut S, Chotiapisitkul S, Assarasakorn S. 2008. Spontaneous diabetes mellitus in captive Mandrillus sphinx monkeys: a case report. J Med Primatol 37:162–165 [DOI] [PubMed] [Google Scholar]
- 92.Randall VA. 2007. Hormonal regulation of hair follicles exhibits a biological paradox. Semin Cell Dev Biol 18:274–285 [DOI] [PubMed] [Google Scholar]
- 93.Rasmussen KM, Thenen SW, Hayes KC. 1979. Folacin deficiency and requirement in the squirrel monkey (Saimiri sciureus). Am J Clin Nutr 32:2508–2518 [DOI] [PubMed] [Google Scholar]
- 94.Ratterree MS, Baskin GB. 1992. Congenital hypotrichosis in a rhesus monkey. Lab Anim Sci 42:410–412 [PubMed] [Google Scholar]
- 95.Reine NJ. 2007. Medical management of pituitary-dependent hyperadrenocorticism: mitotane versus trilostane. Clin Tech Small Anim Pract 22:18–25 [DOI] [PubMed] [Google Scholar]
- 96.Reinhardt V. 2005. Hair pulling: a review. Lab Anim 39:361–369 [DOI] [PubMed] [Google Scholar]
- 97.Reinhardt V, Reinhardt A, Houser WD. 1986. Hair pulling and eating in captive rhesus monkeys. Folia Primatol 47:158–164 [DOI] [PubMed] [Google Scholar]
- 98.Rushton DH. 2002. Nutritional factors and hair loss. Clin Exp Dermatol 27:396–404 [DOI] [PubMed] [Google Scholar]
- 99.Saevik BK, Bergvall K, Holm BR, Saijonmaa-Koulumies LE, Hedhammar A, Larsen S, Kristensen F. 2004. A randomized, controlled study to evaluate the steroid sparing effect of essential fatty acid supplementation in the treatment of canine atopic dermatitis. Vet Dermatol 15:137–145 [DOI] [PubMed] [Google Scholar]
- 100.Sakai Y, Kishimoto J, Demay MB. 2001. Metabolic and cellular analysis of alopecia on vitamin D receptor knockout mice. J Clin Invest 107:961–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sawyer LS, Moon-Fanelli AA, Dodman NH. 1999. Psychogenic alopecia in cats: 11 cases (1993–1996). J Am Vet Med Assoc 214:71–74 [PubMed] [Google Scholar]
- 102.Singh BR, Alam J, Hansda D. 2005. Alopecia induced by salmonellosis in guinea pigs. Vet Rec 156:516–518 [DOI] [PubMed] [Google Scholar]
- 103.Slominski A, Wortsman J, Tuckey RC, Paus R. 2007. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol 265-266:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Statz LM, Borde M. 2001. Pairing success with male cynomolgus macaques after vasectomy. Contemp Top Lab Anim Sci 40:91 [Google Scholar]
- 105.Steinmetz HW, Kaumanns W, Dix I, Heistermann M, Fox M, Kaup F-J. 2006. Coat condition, housing condition and measurement of faecal cortisol metabolites -- a non-invasive study about alopecia in captive rhesus macaques (Macaca mulatta). J Med Primatol 35:3–11 [DOI] [PubMed] [Google Scholar]
- 106.Stenn K, Parimoo S, Prouty S. 1998. Growth of the hair follicle: a cycling and regenerating biological system, p 111–130 : Chuong CM, editor Molecular basis of epithelial appendage morphogenesis. Austin (TX): RG Landes [Google Scholar]
- 107.Storey PL, Turner PV, Tremblay JL. 2000. Environmental enrichment for rhesus macaques: a cost-effective exercise cage. Contemp Top Lab Anim Sci 39:14–16 [PubMed] [Google Scholar]
- 108.Suedmeyer WK. 1997. Hypothyroidism in an orangutan (Pongo pygmaeus pygmaeus): clinical management and long-term monitoring, p 71–72 Yulee (FL): American Association of Zoo Veterinarians [Google Scholar]
- 109.Swenerton H, Hurley LS. 1980. Zinc deficiency in rhesus and bonnet monkeys, including effects on reproduction. J Nutr 110:575–583 [DOI] [PubMed] [Google Scholar]
- 110.Tajima M, Hamada C, Takayuki A, Miyazawa M, Shibata R, Ishino A. 2007. Characteristic features of Japanese women's hair with aging and with progressing hair loss. J Dermatol Sci 45:93–103 [DOI] [PubMed] [Google Scholar]
- 111.Taylor RL, Cadigan FC, Jr, Chaicumpa V. 1973. Infections among Thai gibbons and humans caused by atypical Microsporum canis. Lab Anim Sci 23:226–231 [PubMed] [Google Scholar]
- 112.Tobin DJ. 2003. Characterization of hair follicle antigens targeted by the anti-hair follicle immune response. J Investig Dermatol Symp Proc 8:176–181 [DOI] [PubMed] [Google Scholar]
- 113.Van Neste D. 2004. Thickness, medullation, and growth rate of female scalp hair are subject to significant variation according to pigmentation and scalp location during aging. Eur J Dermatol 14:28–32 [PubMed] [Google Scholar]
- 114.Vercelli A, Raviri G, Cornegliani L. 2006. The use of oral cyclosporin to treat feline dermatoses: a retrospective analysis of 23 cases. Vet Dermatol 17:201–206 [DOI] [PubMed] [Google Scholar]
- 115.Waisglass SE, Landsberg GM, Yager JA, Hall JA. 2006. Underlying medical conditions in cats with presumptive psychogenic alopecia. J Am Vet Med Assoc 228:1705–1709 [DOI] [PubMed] [Google Scholar]
- 116.Walsh KH, McDougle CJ. 2001. Trichotillomania. Presentation, etiology, diagnosis and therapy. Am J Clin Dermatol 2:327–333 [DOI] [PubMed] [Google Scholar]
- 117.Wiedemeyer K, Schill WB, Loser C. 2004. Diseases on hair follicles leading to hair loss, part I: nonscarring alopecias. Skinmed 3:209–214 [DOI] [PubMed] [Google Scholar]
- 118.Yamamoto K, Sadahito K, Yoshikawa M, Nobuyuki O, Mikami O, Yamada M, Nakamura K, Yasuyuki N. 2003. Hyena disease (premature physeal closure) in calves due to overdose of vitamins A, D3, and E. Vet Hum Toxicol 45:85–87 [PubMed] [Google Scholar]
- 119.Yell JA, Mbuagbaw J, Burge SM. 1996. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol 135:355–362 [PubMed] [Google Scholar]