Abstract
Data implicating mucosal cytokines in the pathogenesis of canine inflammatory bowel disease (IBD) are limited. The aims of the present study were to report new findings of intestinal cytokine expression in dogs with IBD and to compare these data with previous studies through meta-analysis. Cytokine mRNA abundance in intestinal biopsies collected prospectively was evaluated by using a semiquantitative RT-PCR technique. For meta-analysis, an electronic database search revealed 3 clinical trials, all of which were nonrandomized (type III) case series. Prospective analysis showed that the intestines of healthy dogs and those with IBD express numerous cytokines and that a proinflammatory expression profile is not a feature of small or large-intestinal IBD. The meta-analysis data included 158 dogs characterized as healthy (n = 45), diarrheic nonIBD dogs (n = 6), nonresponders (n = 2), small-intestinal IBD (n = 41), colonic IBD (n = 25), and chronic enteropathy (n = 39). German shepherd dogs were overrepresented in 3 of the 4 studies. Healthy dogs showed mRNA expression for most cytokines including IL2, IL4, IL5, IL10, IL12, IFNγ, TNFα, and TGFβ. Only IL12 mRNA expression was increased consistently in small-intestinal IBD, whereas IBD colitis lacked consistent patterns of expression. In summary, dogs with IBD fail to express a predominant Th1- or Th2 cytokine bias in inflamed mucosa. Heterogeneity of results among these studies might be explained by numerous factors including the method of mRNA quantification, stage of disease, and demographic differences in study populations.
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IBD, inflammatory bowel disease; LP, lymphocytic–plasmacytic
Idiopathic inflammatory bowel disease (IBD) in dogs is a chronic immune-mediated disorder empirically defined by clinical, histologic, and therapeutic features.18,26,27,29,45 Evidence suggests that intestinal inflammation in IBD results from altered interaction between the resident microflora and mucosa in a susceptible host.48,53 Aggressive host immune responses directed against commensal bacteria play a central role in the pathogenesis of chronic mucosal inflammation. The concept of impaired immunoregulation in canine IBD is supported by observations of increased numbers of immunoglobulin-containing cells and T cells in inflamed tissues,16,28,30,49 upregulated mucosal and luminal expression of nitric oxide metabolites,20,29 and altered serum concentrations of select acute phase proteins, such as C-reactive protein, in diseased dogs.31 C-reactive protein is a marker of inflammation and tissue injury and is produced by the liver in response to stimulation by IL6, IL1β, and TNFα.14,51
Cytokines play a key role in the modulation of the mucosal immune system of humans. To maintain gut homeostasis, the normal mucosal immune system balances a network of inflammatory mediators, including proinflammatory, antiinflammatory, and regulatory cytokines.47 Cytokines are synthesized rapidly and secreted on stimulation and induce the production of adhesion molecules and other inflammatory mediators including reactive oxygen species, nitric oxide metabolites, and lipid products such as prostaglandins, leukotrienes, and platelet-activating factor. Cytokine-producing cells induce, amplify, prolong, and mediate intestinal mucosal injury.13 Disturbances in the balance of proinflammatory (Th1/Th17-derived) and immunoregulatory (Th2/Tr1-derived) cytokines occur in humans with IBD as well as numerous animal models of intestinal inflammation.16,39,44
Data evaluating the role of CD4+ T cells and mucosal cytokines in the pathogenesis of canine IBD are limited. Lymphocytes expressing CD4+ are either increased16 or decreased28 in dogs having small-intestinal IBD, whereas mucosal CD4+ T cells are increased in dogs with IBD colitis.30,49 A recent study15 described a balance between proinflammatory and antiinflammatory cytokine mRNA expression in dogs with small-intestinal enteropathies but included only 4 dogs diagnosed with IBD. A separate investigation44 evaluating mucosal cytokine mRNA expression in dogs with lymphocytic–plasmacytic colitis reported upregulated expression of proinflammatory cytokines IL2 and TNFα. Yet another study41 reported no difference in cytokine expression in the duodenal mucosa of dogs with or without chronic diarrhea; however, the dogs of this report were not subdivided in terms of response to therapy as having idiopathic IBD, antibiotic-responsive diarrhea, or food-responsive enteropathy. Because of these varied observations, it is unclear which cytokines, if any, control or enhance the local immune response of canine IBD because 1) few dogs with IBD have been evaluated, 2) German shepherd dogs with enteropathies were over-represented in most studies, 3) various measures of cytokine mRNA expression were used, and (4) distinctly variable patterns of cytokine expression were present among these earlier studies.
The objectives of this study were to 1) assess cytokine mRNA expression in the intestinal mucosa of dogs diagnosed with small- and large-intestinal IBD by using an RT-PCR technique and 2) compare these data with previously published data to determine the putative role of cytokine expression in the pathogenesis of canine IBD and other forms of chronic enteropathy through meta-analysis of combined findings.
Materials and Methods
Animals.
Study population.
Dogs referred to the Veterinary Teaching Hospital of Iowa State University for diagnostic investigation of gastroenteritis from July 1999 to March 2004 were eligible for enrollment in the study. All dogs were cared for in accordance with protocols approved by the Iowa State University Institutional Animal Care and Use Committee.
The case animals.
Forty-eight dogs were diagnosed with small-intestinal (n = 37) or large-intestinal (n = 11) IBD according to published criteria,27,29 including persistent (duration longer than 3 wk) gastrointestinal signs; failed responses to dietary (commercial intact protein elimination diet, hydrolysate elimination diet) or symptomatic therapies (paraciticides, antibiotics, anticholinergics, gastrointestinal protectants) alone; thorough diagnostic evaluation with failure to document other causes for gastroenteritis; and histopathologic diagnosis of benign intestinal inflammation. Each dog was fed the elimination diet for a minimum of 3 wk to rule out adverse food reactions (for example, food-responsive enteropathy) as a cause for gastrointestinal signs. In addition, metronidazole or amoxicillin with clavulinic acid was administered to every dog for 14 d if the dogs failed to respond to dietary intervention alone. The minimal diagnostic evaluation performed in all dogs included a complete blood count, serum biochemical profile, urinalysis, direct (wet mount) or indirect (flotation) examination of feces for nematode and protozoan parasites, and survey abdominal radiographs. In some instances, additional tests such as contrast radiography, abdominal ultrasonography, serum trypsin-like immunoreactivity, and serum vitamin assays for folate and cobalamin were performed as deemed appropriate. Additional inclusion criteria were absence of extraalimentary tract inflammation (based on results obtained from initial diagnostic testing) and failure to have received immunomodulating drug therapy (for example, corticosteroids, metronidazole, and sulfasalazine) within 10 d before referral.
The control group.
The control group consisted of 9 adult, mixed-breed dogs of random-source origin (that is, research-facility–derived) that were free of gastrointestinal signs for at least 42 d before gastrointestinal sampling. Control dogs were judged to be healthy on the basis of normal results on physical examination, complete blood count, serum biochemical analysis, urinalysis, multiple fecal examinations, and dirofilarial antigen assay.
Tissue sampling.
After enrollment, multiple (10 to 15 per organ evaluated) mucosal biopsy specimens were procured endoscopically from the stomach, small intestine, or large intestine of diseased dogs for microscopic review. Thirty-six dogs having upper gastrointestinal signs (vomiting, small bowel diarrhea, anorexia, weight loss) underwent esophagogastroduodenoscopy, whereas 5 dogs having lower gastrointestinal signs (tenesmus, hematochezia, mucoid feces, or frequent defecation) only underwent full colonoscopy to the level of the cecum. Upper and lower endoscopic examinations were performed in 7 dogs having mixed signs of enterocolitis. Biopsy specimens were obtained directly from mucosal lesions of increased granularity, friability, or erosions as well as normal-appearing mucosa in all IBD dogs. Biopsy specimens were obtained from the stomach, small intestine, and colon of each control dog also. Tissues for histopathologic examination were placed in 10% neutral buffered formalin, whereas biopsies for cytokine mRNA analysis (3 to 5 specimens per dog) were snap-frozen in liquid nitrogen and stored at –70 °C until analysis. In addition, positive-control tissues (for example, tissues known to show constitutive expression of proinflammatory and regulatory cytokine mRNA) consisting of mesenteric lymph node specimens were collected from healthy control dogs.
Histopathologic examination of all tissues was performed by a single pathologist (MA), who graded endoscopic specimens and assigned a lesion severity score for each dog. No information regarding history, clinical signs, or endoscopic observations was made available to the pathologist. A histologic grading system based on the extent of architectural disruption and mucosal epithelial changes was used to score severity of lesions as previously described.1,16,27,31 Similar histopathologic criteria have recently been proposed by the World Small Animal Veterinary Association gastrointestinal standardization group for diagnosis of gastrointestinal inflammation in the dog and cat.10
RNA extraction and RT-PCR.
Two to 4 intestinal biopsies each from control and IBD dogs were thawed and homogenized with a mechanical tissue homogenizer. RNA was extracted (RNeasy extraction kit, Qiagen, Valencia, CA) according to the manufacturer's instructions. After elution of sample RNA from the spin columns with 40 μL RNase-free water treated with diethyl polycarbonate (Sigma, St Louis, MO), each sample was treated with 1 unit DNAase I to remove genomic DNA, according to the manufacturer's protocol. The RNA concentration was quantified by UV absorbance at 260 µm, with an OD260:OD280 ratio of greater than 1.8 required for adequate purity. Eluted RNA was stored at –70 °C.
Primers were designed with a computer software package (MacVector version 6, MacVector, Cary, NC) by using the available canine specific GenBank sequences for IL1α (403782), IL1β (403974), IL2 (30710), IL4 (187322), IL5 (AF331919), IL10 (U33843), IL12p40 (AF091134), TNFα (Z70046), IFNγ (AF126247), and TGFβ (L34956). Primers for the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were based on previously published sequences.41 Crossreactive oligonucleotides were eliminated by screening against known sequences (BLAST). cDNA generated from 1 μg RNA (Reverse Transcriptase System, Promega, Madison, WI) was diluted to 200 μL in RNAase-free water. One microgram of heparinized RNA was diluted to 200 μL without reverse transcription as a negative control. The starting quantity of cDNA for each sample was normalized based on the expression of the GAPDH gene in each sample. Further, each PCR was optimized by using cDNA derived from samples of canine mesenteric lymph node.
Gene-specific cDNA amplification was performed with AmliTaq Gold PCR Buffer (Perkin-Elmer, Cambridge, UK) by using 5 μL of correctly diluted positive control or sample cDNA and 200 nM of each primer in a final reaction volume of 40 μL. The PCR was performed in a thermal cycler under the following conditions for IFNγ, IL5, and IL10: initial incubation (94 °C for 11 min), then 40 cycles of denaturing at 94 °C for 45 sec, annealing at 55 °C and 45 sec, and finally extension at 72 °C for 45 sec. The PCR conditions for GAPDH, IL1α, and IL1β, IL2, IL4, IL12, TGFβ, and TNFα were: initial incubation at 94 °C for 11 min, then 30 cycles for GAPDH but 40 cycles for the remaining cytokines of denaturing at 94 °C for 45 s, annealing at 60 °C for 45 s, and extension at 72 °C for 45 s.
The optimal conditions for each primer pair were established in pilot trials. Amplification products were electrophoresed in 1.2% agarose gels, stained with ethidium bromide, and viewed under UV light, and images captured digitally by using a charge-coupled device video camera. The optical density of each PCR product was calculated by using Scion Software (Scion Corporation, Frederick, MD). Data were expressed in a semiquantitative fashion by calculating the ratio of cytokine expression to constitutive GAPDH expression. Only samples positive for GAPDH were used in this study.
Confirmation of experimental protocol and PCR product specificity.
To minimize variability between samples, individual dogs, and dog groups, normalization of cytokine cDNA to GAPDH expression was performed first such that consistent and equivalent concentrations of starting cDNA were used for all PCR amplifications. Single bands of the expected size for each cytokine were seen on PCR gels as previously described.15,44 Purification and sequencing of each PCR product confirmed the reaction specificity for the intended target cDNA sequence.
Data analysis.
To describe cytokine mRNA levels in each group (control and IBD), the mean was determined. Permutation tests for nonparametric data were used to compare the mean values of cytokine gene transcripts in healthy intestinal mucosa to the amount present in intestinal mucosa obtained from dogs diagnosed with IBD.43 This same approach to analysis was used for any subgroup analysis, such as compartmentalized comparisons for small- and large-intestinal IBD. Nonparametric (Kruskal–Wallis) ANOVA and Spearman rank correlation were used to assess associations between cytokine concentration and histology severity score. A P value of less than 0.05 was considered statistically significant.
Search strategy for evidence-based data.
On the basis of the findings from the preceding experiments, a second analysis of data was performed by using a literature review with meta-analysis to compare our findings of cytokine mRNA expression in intestinal biopsies from IBD dogs to cytokine expression in dogs diagnosed with IBD and other forms of chronic enteropathy.
A broad-based bibliographic search of online databases including Medline (PubMed beginning in 1998), Commonwealth Agricultural Bureau (http://www.cabdirect.org/), and the Veterinary Information Network (http://www.vin.com/) was performed in December 2007. Search terms used included 1 or more of the following: inflammatory bowel disease, cytokine mRNA, mucosal cytokines, RT-PCR, canine, dog, population, and chronic enteropathy. Additional searches were performed for relevant articles or research abstracts found within the scientific proceedings of the American College of Veterinary Internal Medicine and the European College of Veterinary Internal Medicine since 1998. Citations were considered relevant if they were published in a peer-reviewed journal, described observational studies on dogs with chronic enteropathy, and reported some measure of intestinal cytokine expression. Once relevant citations were identified, the full manuscript was obtained and evaluated. The full manuscript was reviewed for essential materials, such as the study inclusion criteria, patient definition, method of IBD diagnosis, and type of control animals; these factors were considered necessary for evaluation of either the validity or interpretation of study outcome. Further, each study was classified according to the type of experimental design using the US Food and Drug Administration's 4-component rating system as follows: randomized, controlled intervention trials (study design type I); prospective observational cohort studies (study design type II); nonrandomized intervention trials with concurrent or historical controls or case-control studies (study design type III); and cross-sectional studies or analyses of secondary disease endpoints in intervention trials or case series (study design type IV).50 The data extracted from each study also included study population and analysis methods for cytokine expression and histopathology.
Results
Population demographics.
Breeds represented in the IBD group included mixed breeds (n = 20), cocker spaniel (n = 4), Labrador retriever (n = 3), golden retriever (n = 3), Siberian husky (n = 2), American Eskimo (n = 2), rottweiler (n = 2), German shorthair pointer (n = 2), Brittany spaniel (n = 2), and one each of vizsla, dachshund, Doberman pinscher, Pekingese, maltese, shi tzu, miniature pinscher, and Chihuahua. The mean age of the IBD dogs at diagnosis was 6.8 y (range, 0.8 to 14.6 y). The control group comprised healthy, adult mixed-breed dogs younger than 2 y.
Histopathologic evaluation of mucosal biopsies.
All 48 dogs diagnosed with IBD had variable degrees of lymphocytic–plasmacytic (LP) inflammation in small or large-intestinal biopsy specimens. Of the 7 dogs presenting with signs of enterocolitis and undergoing both upper and lower gastrointestinal endoscopy, 6 dogs had a final histopathologic of LP colitis, whereas the remaining dog was diagnosed as having LP enteritis. Based on the lesion severity score, 37 of 48 dogs had moderate-to-severe mucosal inflammation. The final histologic diagnoses for the IBD group were mild LP enteritis (n = 9), moderate-to-severe LP enteritis (n = 28), mild LP colitis (n = 2), and moderate-to-severe LP colitis (n = 9). Three dogs had intestinal inflammation of varying severity in both the small- and large-intestinal mucosa. Histopathologic lesions of inflammation were not observed in gastric, duodenal, or colonic mucosal biopsies from control dogs.
Cytokine mRNA expression in control dogs.
Levels of cytokine mRNA expression were altered in biopsies of duodenal and colonic mucosa from control dogs. In small-intestinal samples, IL1α, IL1β, IL5, IL10, IL12p40, TNFα, IFNγ, and TGFβ mRNA transcripts were detected in 6 of 9 (68%) control dogs. Expression of IL4 and IL2 mRNA was detected in samples from 1 and 5, respectively, of the 9 control dogs. Cytokine mRNA expression was detected less frequently in colonic biopsies, with only IL1β, IL5, and TNFα detectable in samples from at least 4 of 9 control dogs. Neither IL1α nor IL4 mRNA transcripts were detected in any control samples, whereas mRNAs for IL12p40 and IFNγ were detected in samples from 1 control dog only. The presence of histologically normal intestinal tissues was associated with mRNA expression of IL10 (P = 0.040) and TGFβ (P = 0.001)
Cytokine mRNA expression in dogs with IBD.
Cytokine mRNA was expressed frequently in duodenal samples from dogs with IBD. Transcripts for IL1α, IL1β, IL2, IL5, IL12p40, TNFα, and TGFβ was present in at least 27 of 37 (73%) dogs. Only IL4 was detected infrequently (that is, 12 of 37 dogs) and in very low levels. Colitic dogs having detectable levels of cytokine transcripts were less frequent, with only IL1β, IL4, IL5, TNFα, and TGFβ noted in biopsies from at least 5 of 11 dogs. Transcripts for IL10 and IL12p40 were detected in only 1 dog, whereas mucosal expression of IFNγ was not detected in colonic specimens from any IBD dog. Severity (mild versus severe) of histopathologic inflammation in IBD dogs was not associated with the expression of mRNA encoding IL1α (P = 0.719), IL1β (P = 0.058), IL2 (P = 0.306), IL4 (P = 0.068), IL5 (P = 0.587), IL12 (P = 0.144), TNFα (P = 0.554), or IFNγ (P = 0.205).
Comparisons of cytokine expression between dog groups.
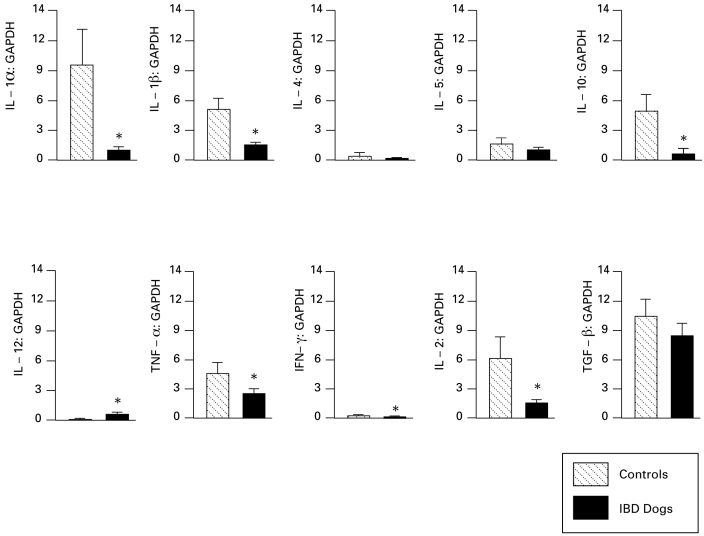
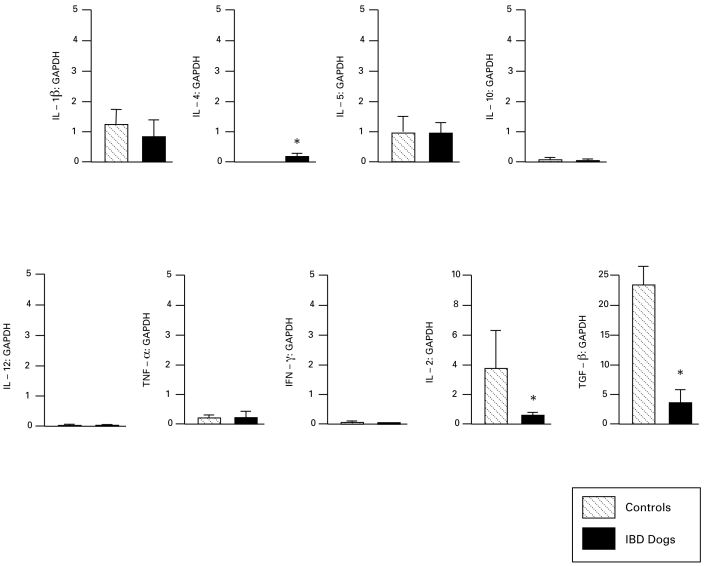
In dogs with small-intestinal IBD, mRNA levels of IL1α, IL1β, IL2, IL10, TNFα, and IFNγ were decreased (P < 0.05 for all), whereas expression of IL12p40 was increased (P < 0.05) as compared with that in control dogs (Figure 1). Dogs with colonic IBD showed decreased expression of IL2 and TGFβ (P < 0.05 for both) but increased IL4 expression (P < 0.05) compared with that in control dogs (Figure 2). A comparison of the compartmentalized (for example, small intestine versus colon) differences in cytokine mRNA transcripts showed that the small-intestinal mucosa of healthy dogs expressed greater levels of IL2, IL4, and IL10 than did their colonic mucosa, whereas the colonic mucosa of healthy dogs expressed greater amounts of TGF than did their small-intestinal mucosa.
Figure 1.
Expression of cytokine mRNA relative to GAPDH in small-intestinal mucosa of control dogs (n = 9) and dogs with IBD (n = 37). Data shown as mean ± SEM for each cytokine. *, P < 0.05 compared with value for controls.
Figure 2.
Expression of cytokine mRNA relative to GAPDH in colonic mucosa of control dogs (n = 9) and dogs with IBD colitis (n = 11). Data shown as mean ± SEM for each cytokine. *, P < 0.05 compared with value for controls.
Results of meta-analysis.
The initial electronic database search revealed a total of 5 citations. One citation12 (a research abstract) was excluded because details regarding study inclusion criteria, method of IBD diagnosis, and type of control animals were omitted. Once the full manuscript was evaluated only 3 of the remaining 4 resources provided relevant data describing mucosal cytokine mRNA expression in dogs having IBD15,44 or other forms of chronic enteropathy.41
Inclusion criteria and strength of studies.
Like the present study, each of the 3 previous studies provided commentary on study design, contained a population of healthy control animals for comparison to diseased dogs, and included histopathologic evaluation of intestinal samples obtained from diseased dogs (Table 1). The 4 studies evaluated were all type III investigations.
Table 1.
Overview of studies evaluating intestinal cytokine mRNA expression in dogs
| Reference | IL1 | IL2 | IL4 | IL5 | IL6 | IL10 | IL12 | IL18 | IFNγ | TNFα | TGFβ |
| 15 | NA | ↑ | – | ↑ | NA | – | ↑ | NA | – | ↑ | ↑ |
| 44 | NA | ↑ | – | NA | – | – | – | NA | – | ↑ | – |
| 41 | NA | – | – | – | – | – | – | – | – | – | – |
| Current study, small intestinal data | ↓ | ↓ | – | – | NA | ↓ | ↑ | NA | ↓ | ↓ | – |
| Current study, colonic data | – | ↓ | ↓ | – | NA | – | – | NA | – | – | ↓ |
The level of cytokine mRNA expression in IBD dogs relative to that in healthy dogs is shown.
↑, increased cytokine expression in diseased tissues; ↓, decreased cytokine expression in diseased tissues; –, no difference in transcript expression between diseased and control tissues; NA, not evaluated.
Summary of the evidence with results.
The 4 studies evaluated were all nonrandomized, prospective case control studies. These studies included data on a total of 158 dogs which were clinically categorized as healthy controls (n = 45; the present study and references 15, 41, and 44), diarrheic nonIBD dogs (n = 6),44 nonresponders to medical therapy (n = 2),15 small-intestinal IBD (n = 41; the present study and reference 15), large-intestinal IBD (n = 25; the present study and reference 44), and chronic enteropathy (n = 39).41 Two studies (the present study and reference 15) specifically evaluated dogs diagnosed with small-intestinal IBD, and 2 studies (the present study and reference 44) evaluated dogs diagnosed with IBD colitis. One study15 compared cytokine mRNA expression profiles in IBD dogs to a cohort group (n = 10 dogs) diagnosed with small-intestinal bacterial overgrowth. Another investigation41 characterized duodenal cytokine mRNA expression in dogs with chronic enteropathy and compared it with mucosal cytokine expression in healthy dogs. In that study,41 diseased dogs were defined simply on the criterion as having chronic diarrhea and were not subdivided according to therapeutic response into IBD or other causes for enteritis. German shepherd dogs were strongly represented in most study populations and accounted for 4 of 4 small-intestinal IBD dogs,15 5 of 14 IBD colitis dogs,44 and 12 of 39 dogs diagnosed with chronic enteropathy,41 in separate investigations, respectively.
In all 4 of the studies evaluated, diagnosis of IBD or chronic enteropathy was based on the presence of chronic gastrointestinal signs or diagnostic evaluation to rule out conditions similar to IBD by using objective histopathologic evaluation of mucosal biopsies. Dietary trials were performed before endoscopic examination in 2 of the reports (the present study and reference 15), and 2 reports (the present study and reference 44) noted that dogs were excluded if they had received antiinflammatory medications 1 to 3 wk prior to referral. All dogs with IBD had various degrees of lymphocytic–plasmacytic inflammation in both small- and large-intestinal mucosal biopsy specimens. Furthermore, severity of histopathologic lesions was not correlated with mRNA expression for any cytokine in 2 studies (the present study and reference 41). These lesions in inflamed canine mucosa were dissimilar from the histologic hallmarks of transmural noncaseating granulomas and crypt abscessation seen in active Crohn disease and ulcerative colitis, respectively.53
Cytokine mRNA expression was evaluated by using both semiquantitative (the present study and references 15 and 44) and quantitative41 RT-PCR techniques. Overall, mRNA expression patterns were defined broadly as being mixed Th1:Th2 in small-intestinal IBD,15 proinflammatory Th1-like in IBD colitis,44 and neither Th1- or Th2-like in dogs having small or large-intestinal IBD (the present study) or chronic enteropathy,41 respectively. mRNAs for cytokines IL2, IL4, IL5, IL10, IL12 subunits, IFNγ, TNFα, and TGFβ were evaluated across all studies most frequently (Table 2). Control animals consistently showed mRNA expression for the majority of cytokines studied, with the exception of IL4. Compared with that in control dogs, only mRNA expression for IL12 subunits in dogs with small-intestinal IBD (the present study and reference 15) was consistently increased, whereas no consistent patterns of mRNA expression were noted for any cytokine in dogs having IBD colitis (the present study and reference 44; Table 2).
Table 2.
RT-PCR analysis of mucosal cytokines in various studies
| Tissues evaluated |
|||||
| Reference | Dogs in study | Type of RT-PCR | Small intestine | Colon | Histology done? |
| 15 | 12 disease-free 4 IBD 2 nonresponders 10 small intestine bacterial overgrowth | Semiquantitative | X | Yes | |
| 44 | 6 disease-free 14 IBD 6 nonIBD diarrheic | Semiquantitative | X | Yes | |
| 41 | 18 disease free 39 chronic enteropathy | Real-time | X | Yes | |
| Current study | 9 disease-free 48 IBD | Semiquantitative | X | X | Yes |
Objective grading criteria were used during histology to characterize mucosal inflammation.
Discussion
The present study sought to further define mucosal cytokine gene expression in intestinal biopsies from dogs having small- or large-intestinal IBD. The results document that the intestinal mucosa expresses mRNA for a diverse spectrum of proinflammatory, antiinflammatory, and regulatory cytokines and that expression differs between dogs with IBD and clinically normal dogs. In dogs with small-intestinal IBD, the expression of mRNA for IL1α, IL1β, IL10, TNFα, IFNγ, and IL2 was significantly than in healthy dogs less but that for IL12p40 was significantly more. Furthermore, compared with control dogs, dogs with colonic IBD showed significantly less expression of mRNA for IL2 and TGFβ but significantly more mRNA expression for IL4. These findings indicate that the normal canine intestine is an immunologically active site and that alterations in proinflammatory cytokine mRNA expression are not a salient feature of either small- or large-intestinal IBD in dogs. Some strengths of this study include the size of the study population with inclusion of IBD dogs diagnosed according to stringent criteria27,29 and in which tissue specimens were objectively graded by using uniform histopathologic scoring.1,16,27,31 Another strength is use of a well-validated and standardized technique of semiquantitative PCR for assessment of mucosal cytokine mRNA expression in endoscopic biopsy specimens.15,44
The underlying etiology of canine IBD is unknown, and comparisons have been made to the human inflammatory bowel diseases, ulcerative colitis and Crohn disease.7,29 Canine IBD closely resembles human IBD in many respects including the clinical, diagnostic, pathologic, and therapeutic features of the disease (Table 3).27,29 Similar to that in humans, genetic factors likely contribute to the pathogenesis of canine IBD, because an increased risk for disease is associated with various breeds including German shepherd dogs, soft-coated wheaton terriers, basenjis, and French bulldogs.16,19,29 Dogs spontaneously develop chronic progressive enterocolitis with diarrhea and weight loss, which is most prevalent in middle-aged animals. This chronic inflammation persists throughout life, with episodic acute flares. Histologic features include infiltration of the lamina propria with mononuclear cells and goblet cell depletion, crypt hyperplasia, and epithelial erosion or ulceration.19,29 T lymphocytes likely mediate intestinal inflammation, because mucosal populations of CD3+, CD4+, and CD8+ T cells are increased relative to those in control dogs.16,28,30,49 Upregulated expression of nitric oxide metabolites appears to be a mechanism of disease in canine IBD, like in Crohn disease.20,29,33 A role for luminal bacteria is strongly suggested by observations that therapeutic levels of metronidazole attenuate clinical disease and by reports of increased numbers of mucosally associated bacteria in dogs with IBD.23,29,54 The well-documented spontaneous acute flares of canine disease closely resemble human IBD, as does the chronic enteritis or colitis sequence. Furthermore, this model is well suited to pharmaceutical and dietary studies, given that clinical criteria of disease activity have recently been defined.31
Table 3.
A comparison of the etiopathologic features between human and canine IBD
| Feature | Human IBD | Canine IBD |
| Genetic basis | Yes | Suspected |
| Etiology | Unknown | Unknown |
| Commensal bacterial role | Yes | Suspected |
| Rectal bleeding | Yes | Yes |
| Diarrhea | Yes | Yes |
| Definitive diagnosis | Gastrointestinal biopsy | Gastrointestinal biopsy |
| Disease activity assessment | Clinical indices Serologic markers (C-reactive protein) | Clinical indices Serologic markers (C-reactive protein) |
| Response to antiinflammatory drugs | Yes | Yes |
| Spontaneous ‘flares’ in gastrointestinal signs | Yes | Yes |
A breakdown of immunologic tolerance with development of a dysregulated immune response against components of the normal resident microflora is thought to be crucial to IBD pathogenesis.19,21,29,48,53 Indeed, studies in humans indicate a predominant role for Th1-derived cytokines, including IFNγ and IL2, which are produced in response to stimulation with TNF, IL12, and IL18 and promote chronic mucosal inflammation associated with Crohn disease.13,35,38,39,42 Considerable evidence supports that Th2-derived cytokines, such as IL4 and IL5, are important in the pathogenesis of ulcerative colitis.13,39 A thorough understanding of the cytokine network of canine IBD might yield valuable information regarding the specific biologic pathways involved in disease progression in both humans and dogs. In addition, these data may identify potential targets for therapeutic intervention and provide useful comparative information to the human condition.
A critical appraisal of the literature that used meta-analysis in establishing causation was undertaken to evaluate the potential role of mucosal cytokine mRNA expression in dogs with IBD. A comparison of the mucosal cytokine expression patterns of previously published canine IBD studies to the present study yields both similarities and differences. Like the previous studies evaluated,15,41,44 we have demonstrated constitutive expression of a number of cytokine mRNA transcripts in the healthy intestinal mucosa of dogs, suggesting that both proinflammatory and antiinflammatory cytokines are required for maintenance of normal mucosal homeostasis. Whereas earlier reports showed that dogs with IBD had a mixed Th1:Th215 or proinflammatory Th1-like44 cytokine response in their intestinal mucosa, we failed to confirm these results in the present investigation. Our finding that proinflammatory cytokines are not upregulated in IBD dogs is in accordance with earlier studies,12,41 both of which used real-time RT-PCR methods for mRNA quantification.
The main rationale for performance of the present investigation is the numerous incongruities noted between the previous studies to date, including the relatively small number of IBD dogs actually studied, overrepresentation of German shepherd dogs enrolled into clinical trials, molecular technique used for detection of cytokine mRNA transcripts, and contrasting conclusions regarding cytokine expression drawn from these separate clinical investigations. Although it circumvented some of these limitations by using a larger and more diverse study population, the present study too suffered from a similar study design limitation. If our aim is to understand the role of mucosal cytokine production in the pathogenesis of canine IBD, with the goal of potentially modifying production to prevent or treat disease, then the ideal study design is difficult to achieve. In the hierarchy of evidence-based medicine, randomized clinical trials are considered to provide the best evidence for efficacy of treatment.22,36 However, when establishing risk factors for the development of disease, randomized clinical trials may be impossible to conduct if the disease is multifactorial or no appropriate animal model exists. In this situation, as is likely for canine IBD, well-conducted cohort studies that document exposure to the risk factors before disease occurrence currently are considered to have the best evidentiary value.11,22
Case control studies, which were used by all 4 investigations identified in this review, suffer from several potential biases that should be considered when assessing the inference from these studies. First and foremost, case control studies are unable to show a temporal relationship between the exposure (in this case, mucosal cytokine expression) and outcome of disease. For example, it is not possible to know with certainty that the mucosal cytokine expression observed at a single time point is a cause or consequence of IBD. Therefore, readers are cautioned against concluding that an increased or decreased expression of a particular cytokine contributes to the pathogenesis of canine IBD. It would be necessary to enroll dogs before disease onset, establish baseline levels of mucosal cytokine expression, and follow dogs over time to determine whether cytokine expression is causally related to IBD. Unfortunately, such a study is unlikely to occur. Our review found no class I or class II studies, which would have provided the greatest amount of information about cytokine profiles under the evidence-based medicine paradigm.2,3 Rather, all investigations were nonrandomized, prospective clinical trials that fell into class III design status.3
Although the same study design was used, the results of the studies were not consistent, suggesting lack of consensus about the role of mucosal cytokines in canine IBD is available. Possible reasons for these disparate results include the difference in case definition in the studies and the difference in the time of outcome measurement. As discussed, all studies used a case control design, meaning that cases were in progress at the time of outcome determination and obscuring whether dogs were examined at the same temporal point (for example, early versus late phases of inflammation) of disease progression.34 If stage of disease expression (that is, age of lesion) is associated with the outcome, then this lack of consistency may be one source of the heterogeneity observed.
An important issue that differs between the studies is the approach to outcome measurement. Our study used a semiquantitative molecular technique to assess for relative differences in intestinal cytokine mRNA expression between healthy dogs and dogs with IBD. Different molecular analyses, including semiquantitative RT-PCR15,44 and real-time RT-PCR41 techniques, were used to measure intestinal expression of mRNA encoding a panel of mucosal cytokines in the previous and present studies. The present investigation extends our pilot observations32 and includes the use of an identical semiquantitative RT-PCR involving gel-based measurement of cytokine mRNA in mucosal biopsies. In this regard, PCR amplification conditions were optimized in pilot studies, with each primer pair yielding a prominent PCR product of the expected size; positive control cDNA and negative controls (water) were included in each PCR run; and purification and sequencing of each PCR product was used to confirm the reaction specificity for the intended target cDNA sequence. More recently, real-time RT-PCR has been recognized to be a more accurate and sensitive method for quantifying mRNA transcripts than are gel-based semiquantification techniques.8,9 Benefits of this benchmark technology include 1) the avoidance for post-PCR processing; 2) wide (>107-fold) dynamic range, which allows direct comparison between RNAs of varying abundance; and (3) the quantitative potential of the PCR reaction, which makes it a quantitative as well as qualitative assay.17 Although real-time RT-PCR is often considered as the ‘gold standard’, it too suffers from serious limitations, including variability of RNA templates, assay designs, and protocols, as well as inappropriate data normalization and inconsistent data analysis, which may confound results.40 A recent report describes the use of real-time RT-PCR applied to the duodenal mucosa of dogs with chronic enteropathy, which showed no difference in cytokine mRNA expression between dogs with and without chronic diarrhea.41
Study outcome may also be a source of heterogeneity between investigations. Study populations in previous investigations included a high percentage of German shepherd dogs diagnosed with IBD and other causes for canine chronic enteropathy. This breed appears to be predisposed to both IBD and antibiotic-responsive diarrhea, including small-intestinal bacterial overgrowth.5,6,19,46 One study15 showed that intestinal cytokine mRNA expression was greater in German shepherd dogs with enteropathies than in controls; however, dogs with IBD and small-intestinal bacterial overgrowth did not differ. Conversely, another study41 found no difference in cytokine mRNA expression between German shepherd dogs and other breeds with chronic diarrhea or between histologically normal and inflamed duodenal mucosa.
The variable patterns of cytokine expression previously observed might also be explained by factors other than the method of mRNA quantification, stage of disease, and demographic differences. For example, differences in age, gender, growth phase, and nutritional status have all been shown to influence cytokine mRNA expression in humans.18,24,37 Emerging data clearly indicate that in human IBD, the long-term outcome of an inflammatory response is strongly influenced by the local cytokine milieu.34 This milieu changes over time, and different mediators are involved in the inductive versus the effector phases of inflammation, which eventually acquires distinctive Th1, Th2, or Th17 profiles.25,52 Importantly, cytokine–chemokine mRNA expression profiles show considerable overlap in Crohn disease and ulcerative colitis and may not be associated with histologically established inflammatory lesions.4 It remains possible that differences in study populations, treatment protocols, disease duration, histopathologic grading criteria, and tissues collected for analysis may have contributed to the different results regarding cytokine patterns in canine IBD.15,29,41,44
In summary, a review of the evidence currently available indicates that healthy dogs constitutively express numerous cytokines that may play a critical role in mucosal homeostasis. Dogs with IBD fail to express a predominant Th1- or Th2-derived cytokine response within inflamed mucosa. The lack of increased expression of inflammatory cytokines in diseased dogs suggests that Th1-derived cytokine responses are less important in the pathogenesis of canine IBD, similar to human ulcerative colitis. Compared with that in healthy controls or dogs with nonIBD gastrointestinal disorders, the generally lower cytokine mRNA expression in biopsied tissue from IBD-affected dogs may reflect infiltration of the mucosa with inflammatory cells other than CD4+ T cells. The leading limitation of all studies, based on the choice of design, is the inability to determine whether differences in cytokine expression occur before disease occurrence and therefore likely are causally associated with IBD or whether expression occurs as a consequence of other mechanisms, making cytokines appropriate targets for interventions to assess therapy but perhaps not prevention.
References
- 1.Allenspach K, Wieland B, Grone A, Gaschen F. 2007. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med 21:700–708 [DOI] [PubMed] [Google Scholar]
- 2.Aragon CL, Budsberg SC. 2005. Applications of evidence-based medicine: cranial cruciate ligament injury repair in the dog. Vet Surg 34:93–98 [DOI] [PubMed] [Google Scholar]
- 3.Aragon CL, Hofmeister EH, Budsberg SC. 2007. Systematic review of clinical trials of treatments for osteoarthritis in dogs. J Am Vet Med Assoc 230:514–521 [DOI] [PubMed] [Google Scholar]
- 4.Autschbach F, Giese T, Gassler N, Sido B, Heuschen G, Heuschen U, Zuna I, Schulz P, Weckauf H, Berger I, Otto HF, Meuer SC. 2002. Cytokine/chemokine mRNA expression profiles in ulcerative colitis and Crohn disease. Virchows Arch 441:500–513 [DOI] [PubMed] [Google Scholar]
- 5.Batt RM, Carter MW, Peters TJ. 1984. Biochemical changes in the jejunal mucosa of dogs with a naturally occurring enteropathy associated with bacterial overgrowth. Gut 25:816–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batt RM, Needham JR, Carter MW. 1983. Bacterial overgrowth associated with a naturally occurring enteropathy in the German shepherd dog. Res Vet Sci 35:42–46 [PubMed] [Google Scholar]
- 7.Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, Orsi RH, Wiedmann M, McDonough P, Kim SC, Berg D, Schukken Y, Scheri E, Simpson KW. 2007. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn disease involving the ileum. ISME J 1:403–418 [DOI] [PubMed] [Google Scholar]
- 8.Bustin SA. 2000. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 25:169–193 [DOI] [PubMed] [Google Scholar]
- 9.Bustin SA, Benes V, Nolan T, Pfaffl MW. 2005. Quantitative real-time RT-PCR—a perspective. J Mol Endocrinol 34:597–601 [DOI] [PubMed] [Google Scholar]
- 10.Day MJ, Bilzer T, Mansell J, Wilcock B, Hall EJ, Jergens A, Minami T, Willard M, Washabau R. 2008. International standards for the histopathological diagnosis of gastrointestinal inflammation in the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 138 Suppl 1:S1–S43 [DOI] [PubMed] [Google Scholar]
- 11.Egger M, Smith GD, Altman DG, editors 2001. Systematic reviews in health care: meta-analysis in context. London (UK): BMJ [Google Scholar]
- 12.Fugiwara S, Yasunaga H, Nakayama H, Doi K, Masuda K, Ohno K, Tsujimoto H. 2002. Quantitative analysis of cytokine mRNAs in the duodenal mucosa in dogs with small-intestinal inflammatory bowel disease. J Vet Intern Med 16:327 [Google Scholar]
- 13.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. 1996. Disparate CD4 + lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn disease LP cells manifest secretion of IFNγ, whereas ulcerative colitis LP cells manifest increased secretion of IL5. J Immunol 157:1261–1270 [PubMed] [Google Scholar]
- 14.Gabay C, Kushner I. 1999. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–454 [DOI] [PubMed] [Google Scholar]
- 15.German AJ, Hall EJ, Day MJ. 2001. Immune cell populations within the duodenal mucosa of dogs with enteropathies. J Vet Intern Med 15:14–25 [DOI] [PubMed] [Google Scholar]
- 16.German AJ, Helps CR, Hall EJ, Day MJ. 2000. Cytokine mRNA expression in mucosal biopsies from German shepherd dogs with small-intestinal enteropathies. Dig Dis Sci 45:7–17 [DOI] [PubMed] [Google Scholar]
- 17.Ginzinger DG. 2002. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol 30:503–512 [DOI] [PubMed] [Google Scholar]
- 18.Grimble RF. 2003. Inflammatory response in the elderly. Curr Opin Clin Nutr Metab Care 6:21–29 [DOI] [PubMed] [Google Scholar]
- 19.Guilford WG. 1996. Idiopathic inflammatory bowel diseases, p 451–486 : Guilford WG, Williams DA, Center SA, Meyer DJ, Strombeck DR, editors Strombeck's small animal gastroenterology. Philadelphia (PA): WB Saunders [Google Scholar]
- 20.Gunawardana SC, Jergens AE, Ahrens FA, Niyo Y. 1997. Colonic nitrite and immunoglobulin G concentrations in dogs with inflammatory bowel disease. J Am Vet Med Assoc 211:318–321 [PubMed] [Google Scholar]
- 21.Hanauer SB. 2006. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis 12 Suppl 1:S1–S9 [DOI] [PubMed] [Google Scholar]
- 22.Holmes MA. 2007. Evaluation of the evidence. Vet Clin North Am Small Anim Pract 37:447–462 [DOI] [PubMed] [Google Scholar]
- 23.Hostutler RA, Luria BJ, Johnson SE, Weisbrode SE, Sherding RG, Jaeger JQ, Guilford WG. 2004. Antibiotic-responsive histiocytic ulcerative colitis in 9 dogs. J Vet Intern Med 18:499–504 [DOI] [PubMed] [Google Scholar]
- 24.Huang H, Patel DD, Manton KG. 2005. The immune system in aging: roles of cytokines, T cells, and NK cells. Front Biosci 10:192–215 [DOI] [PubMed] [Google Scholar]
- 25.Iwakura Y, Ishagame H. 2006. The IL23–IL17 axis in inflammation. J Clin Invest 116:1218–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs G, Collins-Kelly L, Lappin M, Tyler D. 1990. Lymphocytic–plasmacytic enteritis in 24 dogs. J Vet Intern Med 4:45–53 [PubMed] [Google Scholar]
- 27.Jergens AE. 1999. Inflammatory bowel disease: current perspectives. Vet Clin North Am Small Anim Pract 29:501–521 [PubMed] [Google Scholar]
- 28.Jergens AE, Gamet Y, Moore FM, Niyo Y, Tsao C, Smith B. 1999. Colonic lymphocyte and plasma cell populations in canine lymphocytic-plasmacytic colitis: an immunohistochemical and morphometric study. Am J Vet Res 60:515–520 [PubMed] [Google Scholar]
- 29.Jergens AE, Moore FM, Haynes JS, Miles KG. 1992. Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987–1990). J Am Vet Med Assoc 201:1603–1608 [PubMed] [Google Scholar]
- 30.Jergens AE, Moore FM, Kaiser MS, Haynes JS, Kinyon JM. 1996. Morphometric evaluation of immunoglobulin A-containing and immunoglobulin G-containing cells and T cells in duodenal mucosa from healthy dogs and from dogs with inflammatory bowel disease or nonspecific gastroenteritis. Am J Vet Res 57:697–704 [PubMed] [Google Scholar]
- 31.Jergens AE, Schreiner CA, Frank DE, Niyo Y, Ahrens FE, Eckersall PD, Benson TJ, Evans R. 2003. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med 17:291–297 [DOI] [PubMed] [Google Scholar]
- 32.Jergens AE, Sonea IM, Kauffman L. 2003. Cytokine mRNA expression in intestinal biopsies of dogs with inflammatory bowel disease. J Vet Intern Med 17:413 [Google Scholar]
- 33.Kolios G, Valatas V, Ward SG. 2004. Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunology 113:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kugathasan S, Saubermann LJ, Smith L, Kou D, Itoh J, Binion DG, Levine AD, Blumberg RS, Fiocchi C. 2007. Mucosal T-cell immunoregulation varies in early and late inflammatory bowel disease. Gut 56:1696–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monteleone G, Biancone L, Marasco R, Morrone G, Marasco O, Luzza F, Pallone F. 1997. Interleukin 12 is expressed and actively released by Crohn disease intestinal lamina propria mononuclear cells. Gastroenterology 112:1169–1178 [DOI] [PubMed] [Google Scholar]
- 36.Moriello KA. 2003. Editor's commentary: introducing evidence-based clinical reviews in veterinary dermatology. Vet Dermatol 14:119–120 [DOI] [PubMed] [Google Scholar]
- 37.Moxley G, Posthuma D, Carlson P, Estrada E, Han J, Benson LL, Neale MC. 2002. Sexual dimorphism in innate immunity. Arthritis Rheum 46:250–258 [DOI] [PubMed] [Google Scholar]
- 38.Mullin GE, Lazenby AJ, Harris ML, Bayless TM, James SP. 1992. Increased interleukin 2 mRNA in the intestinal mucosal lesions of Crohn disease but not ulcerative colitis. Gastroenterology 102:1620–1627 [DOI] [PubMed] [Google Scholar]
- 39.Niessner M, Volk BA. 1995. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reverse transcribed polymerase chain reaction (RT-PCR). Clin Exp Immunol 101:428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nolan T, Hands RE, Bustin SA. 2006. Quantification of mRNA using real-time RT-PCR. Nat Protoc 1:1559–1582 [DOI] [PubMed] [Google Scholar]
- 41.Peters IR, Helps CR, Calvert EL, Hall EJ, Day MJ. 2005. Cytokine mRNA quantification in duodenal mucosa from dogs with chronic enteropathies by real-time RT-PCR. J Vet Intern Med 19:644–653 [DOI] [PubMed] [Google Scholar]
- 42.Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF, Jr, Foley E, Moskaluk CA, Bickston SJ, Cominelli F. 1999. IL18, a novel immunoregulatory cytokine, is upregulated in Crohn disease: expression and localization in intestinal mucosal cells. J Immunol 162:6829–6835 [PubMed] [Google Scholar]
- 43.Ramsey FL, Schafer DW. 2002. Alternatives to the t-Tools 4.3.1 permutation tests, p 95–97: Ramsey FL, Schafer DW, editors The statistical sleuth: a course in methods of data analysis. Pacific Grove (CA): Duxbury Press [Google Scholar]
- 44.Ridyard AE, Nuttall TJ, Else RW, Simpson JW, Miller HR. 2002. Evaluation of Th1, Th2, and immunosuppressive cytokine mRNA expression within the colonic mucosa of dogs with idiopathic lymphocytic–plasmacytic colitis. Vet Immunol Immunopathol 86:205–214 [DOI] [PubMed] [Google Scholar]
- 45.Roth L, Walton AM, Leib MS, Burrows CF. 1990. A grading system for lymphocytic–plasmacytic colitis in dogs. J Vet Diagn Invest 2:257–262 [DOI] [PubMed] [Google Scholar]
- 46.Rutgers HC, Batt RM, Elwood CM, Lamport A. 1995. Small-intestinal bacterial overgrowth in dogs with chronic intestinal disease. J Am Vet Med Assoc 206:187–193 [PubMed] [Google Scholar]
- 47.Sartor RB. 1995. Insights into the pathogenesis of inflammatory bowel diseases provided by new rodent models of spontaneous colitis. Inflamm Bowel Dis 1:64–75 [Google Scholar]
- 48.Sartor RB. 2006. Mechanisms of disease: pathogenesis of Crohn disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 3:390–407 [DOI] [PubMed] [Google Scholar]
- 49.Stonehewer J, Simpson JW, Else RW, Macintyre N. 1998. Evaluation of B and T lymphocytes and plasma cells in colonic mucosa from healthy dogs and dogs with inflammatory bowel disease. Res Vet Sci 65:59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.United States Food and Drug Administration Interim evidence-based ranking system for scientific data. [Cited 22 Jan 2007]Available at: http://www.cfsan.fda.gov/∼dms/hclmgui4.html
- 51.Vermeire S, van Assche G, Rutgeerts P. 2004. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis 10:661–665 [DOI] [PubMed] [Google Scholar]
- 52.Wynn TA. 2005. Th17: a giant step from Th1 and Th2. Nat Immunol 6:1069–1070 [DOI] [PubMed] [Google Scholar]
- 53.Xavier RJ, Podolsky DK. 2007. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448:427–434 [DOI] [PubMed] [Google Scholar]
- 54.Xenoulis PG, Palculict B, Allenspach K, Steiner JM, Van House AM, Suchodolski JS. 2008. Molecular–phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. FEMS Microbiol Ecol 66: 579–589 [DOI] [PubMed] [Google Scholar]