Abstract
Giardia intestinalis is a common protozoan parasite that can infect many laboratory animal primates, although its role as a contributor to the induction of gastrointestinal disease remains unclear. This study sought to investigate the prevalence of Giardia in a colony of common marmosets by using a Giardia antigen-capture assay and to address the possible eradication of this infection by using tinidazole, an antiprotozoal similar to metronidazole but requiring fewer doses. Among 31 colony marmosets, 13 (42%) were positive for Giardia. Two doses of oral tinidazole eliminated the infection in all animals. Repeat testing of the 13 Giardia-positive monkeys 1 y later showed that 11 remained negative and that treated animals had a significant increase in weight at 1 y. Giardia antigen is common in common marmoset feces, and treatment using oral tinidazole is possible and highly effective.
Giardia intestinalis is a common zoonotic protozoan parasite causing diarrhea in humans and animals worldwide. Infection usually results from contact with the feces of an infected host or drinking water contaminated with Giardia cysts. As few as 10 cysts are necessary for infection in human subjects.36 Giardia causes both an acute disease and a chronic asymptomatic state. The most common clinical signs of acute disease are diarrhea, flatulence, foul stool, and abdominal cramps. In addition, Giardia has been implicated as a cause of cognitive impairment and stunted growth in infected children in developing countries.1,2,29
Giardiasis has previously been reported to occur in a variety of laboratory primates, including several species of neotropical monkeys such as marmosets and squirrel monkeys.13,20,28 Such infections may pose a zoonotic risk to animal handlers and potentially affect colony health. Common marmosets (Callithrix jacchus) frequently manifest chronic wasting and inflammatory bowel disease known as ‘wasting marmoset syndrome.’ The etiology of the intestinal disease is unknown, but marmosets often present clinically with skeletal muscle atrophy, marked weight loss, alopecia, and a history of intermittent diarrhea.5,11,16,21,35 Likely no single infectious agent or nutritional deficiency causes the clinical spectrum, but rather a combination of factors result in antigenic stimulation of the intestinal tract resulting in the chronic disease. The presence of Giardia cannot be ruled out as a cofactor.
Several studies have compared various diagnostic tools for detection of Giardia in fecal specimens. Multiple fecal tests for Giardial colonization are available, including antigen-detection enzyme immunoassays, immunochromatographic strips, and microscopy of wet-mounted stool after fecal flotation.9,24,37,38 Enzyme immunoassays are a rapid and precise tool for detecting Giardia in fecal specimens; test sensitivities and specificities have approached 100% in several studies.9,22,24,37 In addition, these studies have indicated that repeat stool sampling on different days may increase the yield of testing, because organisms are variably shed.9,14,36 The specific recommendation is to test 3 samples on alternate days or 3 samples within a 10-d span.38
Treatment options for Giardia infection are varied and include metronidazole, albendazole, quinacrine, furazolidone, and several other nitroimidazoles including tinidazole, secnidazole, ornidazole, and nimorazole.15 The most common treatment choice in veterinary medicine is metronidazole; however, this drug requires 5 to 8 d of treatment and ensuring animal compliance is difficult.27 Several of metronidazole's structural analogues, including tinidazole, are used as a single dose in the treatment of Giardiasis in humans with high cure rates (approximately 90%) and low complications.3,8,10,26,39
In this study we examined the use of a commercially available antigen-capture assay to diagnose Giardiasis in a large breeding colony of common marmosets. To address possible eradication of the infection, we describe the safe use and efficacy of tinidazole as a new treatment option in this species.
Materials and Methods
Animals.
All animals were housed at the New England Primate Research Center and maintained in accordance with the Guide for the Care and Use of Laboratory Animals.19 The facility is AAALAC-accredited, and all work was approved by Harvard Medical School's Standing Committee on Animals. Animals received commercial marmoset chow (New World Primate Chow 8791, Harlan Teklad, Indianapolis, IN) supplemented with a combination of fresh fruits, vegetables, seeds, eggs, and/or mealworms daily. Water was provided ad libitum in polycarbonate water bottles, with fresh water provided daily. Cage pans were cleaned 3 times each week, and cages were sanitized every other week. Each animal received environmental enrichment consisting of food enrichment, toys, nest boxes, and music. Room temperature was maintained at 25.6 ± 2.2 °C, with a relative humidity of 30% to 70%.
Collection of fecal samples.
The initial data set represented feces collected from the bottom of cages of pair-housed marmosets. Feces were placed into a cryovial, and a sterile culturette (BBL CultureSwab Liquid Amies, Becton Dickinson, Franklin Lakes, NJ) was inserted into the cryovial until coated in fecal material. The swab then was removed and sealed into its culture medium, and the cryovial was closed tightly. Both the vial and swab were labeled and frozen at −80 °C.
Subsequent fecal samples were collected from a separate group of marmosets that were individually housed temporarily. Before treatment, 3 samples were collected over 6 d; after treatment with tinidazole, 5 samples were collected over 20 d for a total of 8 fecal specimens from each animal. The pretreatment samples were collected over the course of 6 d with 2 to 3 d between collections. These samples were subsequently pooled to determine whether pooling of serial fecal specimens is feasible for diagnosing Giardial infection. The posttreatment samples were collected as follows: 1 sample 1 to 2 d after each of 2 treatments and 3 samples between 8 and 17 d posttreatment.
Follow-up samples from marmosets previously identified as Giardia-positive were collected by fecal swab during routine preventative healthcare 1 y after initial diagnosis. The animals had been placed back into groups or pairs during the interim, preventing multiple collections over the course of several days.
Giardia antigen-capture assay.
Samples were prediluted in small tubes before addition to ELISA microplate wells coated with rabbit antiGSA65 antibody (Remel ProSpecT Giardia Microplate Assay, Remel, Lenexa, KS). One milliliter of dilution buffer was added to a small tube, and 1 fecal coated swab was stirred vigorously in the dilution buffer to suspend the fecal material. According to instructions from the manufacturer, negative and positive control samples were added to the first wells of the plate, and 0.2 mL prediluted specimen was added to each test well. The plate was incubated at room temperature for 60 min, after which samples were removed by shaking and the wells washed with 400 µL wash buffer (0.1% thimerosal) 3 times. Two hundred microliters of peroxidase-labeled mouse monoclonal antiGSA antibody enzyme conjugate was added to each well, and the plate was incubated at room temperature for 30 min and then washed 5 times with wash buffer. Two hundred microliters of color substrate (3, 3′, 5, 5′-tetramethylbenzidine in buffer) was added to each well for 10 min before the color development was stopped with 50 µL 1.0 N H2SO4. An automatic plate reader was used to determine optical density at 450 µm, and a positive test was interpreted as an optical density greater than 0.050.
Treatment.
In all, 31 animals representing a variety of ages and sexes were treated in this study (Table 1). Regardless of Giardia status and after Giardia antigen-capture assay, all animals were dosed twice with tinidazole (Tindamax tablets, 250 mg, Presutti Laboratories, Arlington Heights, IL), a nitroimidazole antiprotozoal agent used to treat Giardia in both people and companion animals.25,33 Tablets were divided and crushed prior to being dissolved in 2 mL warm water. Tinidazole was administered by gastric gavage through a pediatric feeding tube. For the initial treatment, animals received 1/4 of a tablet (approximately 62.5 mg total or 150 mg/kg) per animal. This dose is higher than the recommended human pediatric dose of 50 mg/kg and adult dose of 2 g (27 mg/kg for a 75-kg adult) but within a range tolerated by human volunteers: doses as large as 3 g given 3 times daily for 14 d have been well tolerated by people.32,33 When performing dose calculation based on allometric scaling to account for variation in body size and metabolic rate, the dose of tinidazole administered to marmosets (900 mg/m2) was slightly lower than the reported pediatric dose (1250 mg/m2) and adult human dose (960 mg/m2).7 With this understanding, we also based the dose size on the ability to divide tablets accurately prior to administration and to ensure adequate drug dosage for the first treatment. The second treatment was 4 d after the first, and 1/8 of a tablet (approximately 31 mg or 77 mg/kg) was administered. This dose was reduced to prevent side effects from treatment. Treatment was well tolerated in all animals, with no evidence of discomfort or clinical effect.
Table 1.
Incidence of Giardia in marmosets
| No. positive/total no. tests |
|||
| before treatment | after treatmenta | ||
| Juvenile (age, <2 y) | 4/7 (57%) | 0/7 (0%) | |
| male | 2/5 (40%) | ||
| female | 2/2 (100%) | ||
| Adult (age, 2 to 7 y) | 7/21 (33%) | 0/21 (0%) | |
| male | 3/9 (33%) | ||
| female | 4/12 (33%) | ||
| Aged adult (age, >7 y) | 2/3 (66%) | 0/3 (0%) | |
| male | 2/3 (66%) | ||
Each value represents 5 samples collected from days 1 through 20 after treatment.
Weight measurement and fecal scoring.
Weights were obtained from each animal at the time of the first treatment and at 3 wk and 1 y after treatment during routine colony preventative healthcare. Fecal scores according to a 5-point scale (Figure 1) were obtained in a blinded fashion on days −3 and −1 before treatment and days 12, 17, and 21 after treatment.
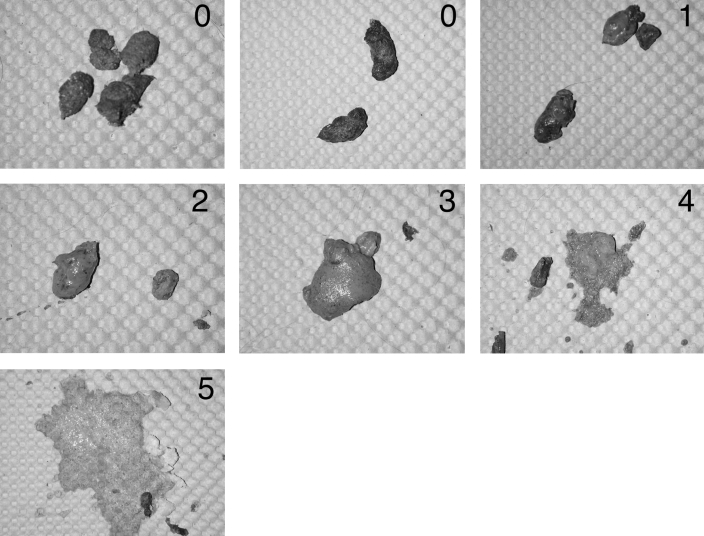
Figure 1.
Fecal scoring system. Grade 0 represents normal marmoset feces. Grade 1 feces are more moist than normal but still formed. Grade 2 feces are beginning to lose form and are moister than grade 1 material. Grade 3 fecal material is diarrhea that has lost much of its form and is very watery with a soft consistency. Grade 4 indicates a further continuation of this process, with loss of almost all of the fecal form. Grade 5 represents severe, watery diarrhea with complete loss of formed feces.
Statistical analysis.
Clinical and experimental data were compared between groups. Fecal scores were compared between affected and unaffected animals at the time of diagnosis by using the Mann–Whitney rank-sum test. Body weights were compared between affected and unaffected animals and before and after treatment by using the paired Student t test. To rule out the possibility of increases in weight due to normal age-related growth, a group of 25 (14 female, 11 male) untreated age-matched control animals from the same colony as those treated were chosen at random. The weights of these control animals, which were obtained during routine colony preventative healthcare, were compared with those of treated animals by using the Student t test. The weights were obtained at the equivalents of day 21 and 1 y, during quarterly preventative healthcare examinations. Statistical analysis was performed using the SigmaStat statistical analysis package (Systat Software, San Jose, CA).
Results
Incidence of Giardia in the colony.
The initial study tested 33 cages of pair housed animals for Giardia at 2 time points. The test identified 18 of 33 (55%) cages in which Giardia antigen was present in at least 1 of the 2 samples obtained from each, but we were unable to determine which specific animal was positive because the marmosets were pair housed. No additional data on the initial group of animals were available, including weights and follow-up Giardia test results, and treatment was not attempted.
Subsequent data were collected from marmosets temporarily housed individually. The second data set was collected almost 3 y after the primary set and involved a different group of marmosets than that initially. The incidence of Giardia in the individually housed marmosets is summarized in Table 2. Overall, 13 of 31 (42%) of samples testing by using antigen capture were positive for Giardia intestinalis in at least 1 of a series of 3 fecal samples. Most (9 of 13) of these samples were positive at multiple time points. The Giardia-positive animals represented a variety of ages (Table 1), and prevalence did not differ between juvenile animals and adults (Fischer exact test, P = 0.381). Both sexes were represented equally (males: 7 of 17 positive, 41%; females: 6 of 14 positive, 43%). Marmosets that had been paired together previously were not necessarily both positive for Giardia. Taken with the initial pilot data, these results suggest that marmoset colonies may be persistently infected with Giardia over prolonged periods.
Table 2.
Giardiaantigen capture results.
| No. | Day –22 | Day –19 | Day –17 | Pooled | Day 2 | Day 5 | Day 11 | Day 16 | Day 20 | 1 Year |
| 1 | 0.002 | 0.001 | 0.000 | 0.002 | 0.015 | 0.002 | 0.002 | 0.000 | 0.000 | na |
| 2 | 0.098a | 0.069a | 0.002 | 0.005 | 0.000 | 0.000 | 0.002 | 0.002 | 0.000 | 0.021 |
| 3 | 1.625a | 1.423a | 0.611a | 1.760a | 0.017 | 0.006 | 0.004 | 0.000 | 0.002 | 0.007 |
| 4 | 0.000 | 0.002 | 0.000 | 0.003 | 0.000 | 0.000 | 0.003 | 0.000 | 0.001 | 0.002 |
| 5 | 0.001 | 0.000 | 0.000 | 0.003 | 0.000 | 0.007 | 0.006 | 0.000 | 0.002 | 0.006 |
| 6 | 0.000 | 0.000 | 0.001 | 0.003 | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 | na |
| 7 | 0.000 | 0.004 | 0.001 | 0.002 | 0.019 | 0.000 | 0.007 | 0.002 | 0.002 | na |
| 8 | 0.000 | 0.000 | 0.000 | 0.000 | 0.004 | 0.002 | 0.006 | 0.000 | 0.000 | 0.000 |
| 9 | 0.002 | 0.002 | 0.000 | 0.007 | 0.006 | 0.004 | 0.004 | 0.002 | 0.001 | na |
| 10 | 0.000 | 0.001 | 0.000 | 0.003 | 0.000 | 0.005 | 0.000 | 0.002 | 0.000 | na |
| 11 | 0.006 | 0.000 | 0.046 | 0.028 | 0.004 | 0.006 | 0.003 | 0.000 | 0.000 | 0.002 |
| 12 | 0.645a | 0.066a | 0.420a | 0.753a | 0.001 | 0.010 | 0.005 | 0.000 | 0.000 | 0.013 |
| 13 | 0.000 | 0.000 | 0.000 | 0.007 | 0.005 | 0.000 | 0.001 | 0.000 | 0.000 | na |
| 14 | 0.007 | 0.004 | 0.001 | 0.004 | 0.002 | 0.006 | 0.006 | 0.000 | 0.001 | 0.006 |
| 15 | 0.313a | 1.444a | 0.996a | 1.060a | 0.004 | 0.005 | 0.002 | 0.000 | 0.000 | 0.013 |
| 16 | 0.332a | 1.783a | 0.480a | 2.477a | na | na | na | na | na | 0.014 |
| 17 | 0.038 | 0.017 | 0.004 | 0.033 | 0.004 | 0.000 | 0.002 | 0.000 | 0.012 | na |
| 18 | 0.001 | 0.076a | 0.002 | 0.013 | 0.000 | 0.016 | 0.000 | 0.000 | 0.000 | 0.009 |
| 19 | 0.258a | 0.065a | 0.535a | 0.414a | 0.012 | 0.006 | 0.004 | 0.004 | 0.021 | 0.015 |
| 20 | 0.010 | 0.001 | 0.000 | 0.004 | na | na | na | na | na | 0.012 |
| 21 | 0.125a | 0.025 | 0.072a | 0.066a | 0.005 | 0.004 | 0.001 | 0.018 | 0.004 | 0.008 |
| 22 | 0.005 | 0.000 | 0.003 | 0.005 | 0.007 | 0.007 | 0.003 | 0.000 | 0.000 | na |
| 23 | 0.034 | 0.005 | 0.310a | 0.200a | 0.003 | 0.007 | 0.005 | 0.000 | 0.002 | 0.017 |
| 24 | 0.004 | 0.000 | 0.007 | 0.001 | 0.001 | 0.004 | 0.003 | 0.000 | 0.000 | 0.014 |
| 25 | 0.013 | 0.268a | 0.005 | 0.234a | 0.006 | 0.001 | 0.004 | 0.000 | 0.004 | 0.138a |
| 26 | 0.007 | 0.000 | 0.069a | 0.011 | 0.009 | 0.016 | 0.002 | 0.000 | 0.000 | 0.075a |
| 27 | 0.001 | 0.000 | 0.022 | 0.007 | 0.004 | 0.004 | 0.000 | 0.000 | 0.001 | 0.010 |
| 28 | 0.147a | 0.012 | 0.720a | 0.032 | 0.005 | 0.003 | 0.004 | 0.000 | 0.002 | 0.006 |
| 29 | 0.972a | 0.318a | 2.238a | 1.533a | 0.009 | 0.005 | 0.001 | 0.000 | 0.002 | 0.001 |
| 30 | 0.007 | 0.005 | 0.003 | 0.006 | 0.001 | 0.006 | 0.001 | 0.000 | 0.000 | na |
| 31 | 0.042 | 0.004 | 0.046 | 0.022 | 0.000 | 0.007 | 0.001 | 0.000 | 0.000 | 0.001 |
Pooled samples contain material from the 3 previous test points. Treatment occurred on days 0 and 4.
na, information not available (no test run at this time).
Positive ELISA test.
No clinical signs were associated with infection.
Physical exams were performed on all animals, and no noteworthy clinical problems were present in either group. Among the most common clinical indicators of infection in people,38 loose stool is the easiest to quantify. Fecal scores (scale, 0 to 5) were recorded for each monkey included in the study. The overall incidence of diarrhea in tested animals was low (average score before treatment, 0.43). Fecal scores were not significantly affected by Giardia status (mean score among positive animals, 0.54; mean among negative animals, 0.35; Mann–Whitney Rank Sum test, P = 0.590).
In humans, weight loss is an inconsistent finding with giardiasis and is seen in approximately 20% of patients.38 Body weights recorded for all animals in the study indicated an insignificant difference between groups. Giardia-positive marmosets had a mean body weight of 338 g, whereas negative animals had a mean body weight of 359 g (t test, P = 0.214).
Treatment with tinidazole eliminated evidence of Giardia infection.
Treatment with tinidazole was well tolerated by all animals. ELISA results indicated that all of the infected and treated marmosets were negative for Giardia in 5 sequential fecal samples collected from 1 to 17 d after treatment, including the first test, which was performed after only 1 dose (Table 2). One animal was unavailable for the initial posttreatment testing but was negative at the 1-y time point. One year after treatment, 11 of 13 previously positive animals remained negative. In addition, 9 of the 18 previously negative animals remained Giardia-free at the 1-y point; the remaining 9 previously negative animals were unavailable for retesting at this time point.
Mean posttreatment fecal scores did not differ significantly from mean pretreatment values for either group (Mann–Whitney rank sum test; positive animals: fecal score before treatment, 0.54; fecal score after treatment, 0.2; P = 0.562; negative animals: pretreatment score, 0.35; posttreatment score, 0.7; P = 0.152). Each marmoset was weighed 21 d after treatment (5 d after the final negative test) and 1 y later during routine preventative healthcare (2 animals were unavailable for weight measurement at the 1-y point). Monkeys previously positive for Giardia initially showed an insignificant increase in mean body weight (before treatment, 338 g; 21 d after treatment, 340 g; paired t test, P = 0.781). However, after 1 y, the mean weight had increased from 338 g before treatment to 360 g (paired t test, P = 0.0471). The negative group showed an initial statistically significant decline in mean body weight from 359 g to 348 g (paired t test, P < 0.001) immediately after treatment but a significant increase from 359 g to 381 g (paired t test, P = 0.042) 1 y after treatment.
To determine whether this increase in weight was a normal age-associated change in the colony aging or due to tinidazole treatment, weights of the treated marmosets were compared with an age-matched sample of normal colony animals collected during routine preventative healthcare over the same timeframe. A significant difference was present between treated animals and untreated colony animals, with treated animals gaining more weight over the 1 y time frame than untreated animals (control animals: n = 25; mean weight gain, 0 g; treated animals: n = 29; mean weight gain, 20.66 g; t test, P = 0.011).
Pooling fecal specimens.
The 3 fecal samples collected before treatment were pooled and tested for Giardia antigen. In addition, 3 samples collected between days 8 and 17 after treatment were pooled and tested. None of the pooled fecal samples yielded false-positive results, but 4 of the 13 marmosets (31%) that had at least 1 positive sample had a negative result for the pooled fecal sample (animals 2, 18, 26, and 28 in Table 2), indicating that pooling of samples decreased test sensitivity.
Discussion
Our data indicate that Giardia can cause a common and persistent infection in marmoset colonies and that treatment for this infection is feasible on a colony-wide basis. Specifically 40% of marmosets tested individually and 55% of the cages in the colony were positive for G. intestinalis, and the data indicate that the colony was persistently infected for a period of approximately 3 y. Multiple tests over the course of 1 wk were necessary to identify carrier animals, and repeated sampling should be common practice when trying to identify Giardia in marmoset colonies. Our data further indicate that pooling fecal samples to identify individual carriers would miss many Giardia-positive animals, because the sensitivity of testing pooled samples was only 69%. However, pooling serial fecal samples for multiple colony animals likely would identify the presence of Giardia in a colony.
Infection in marmosets is not associated with persistent clinical signs of diarrhea. This finding is in contrast to studies in humans, in which 60% to 70% of infected persons show signs of diarrhea.38 Instead, the condition observed in the marmosets seems analogous to the chronic asymptomatic carrier state that occurs in humans and companion animals.4,17 These carrier monkeys may represent an important pathogen reservoir and serve as a source of infection for naïve animals. Episodes of self-limiting, intermittent diarrhea are common in marmoset colonies, and a cause is often not identified. Although Giardia infection was not associated with diarrhea in this study, treatment was associated with a statistically significant increase in body weight 1 y after therapy, suggesting that infection within the colony may be associated with mild clinical effects.
Well-documented reports of Giardia in other nonhuman primate species are scarce, although the disease has been described to occur in squirrel monkeys, rhesus macaques, and marmosets.13,20,28 A previous examination of this disease in marmosets showed that the disease was more common in animals younger than 1 y than in older animals, but association of infection with clinical signs was not discussed.20 We did not appreciate a similar age predilection in the present study. In squirrel monkeys, 33% of animals with clinical gastrointestinal disease and as many as 55% of clinically healthy animals tested positive for Giardia.13 In rhesus macaques, Giardia was not associated with the onset of diarrhea but was present at background levels within the colony examined.31 These reports concur in general with the results we present, suggesting that Giardia infection is a common subclinical infection of nonhuman primates.
Giardial genotypes in human infections have been characterized, and differences in molecular characteristics between symptomatic and asymptomatic human strains have been identified.12,23 The Giardia genotype present in this marmoset colony may represent a nonpathogenic protozoal strain resulting in few clinical signs. However, even asymptomatic giardiasis has been linked to clinical syndromes in humans, including stunted growth and cognitive impairment. Therefore eliminating infection in infected marmosets is important for overall colony health.1,2,29
As an antigenic stimulus, Giardia may contribute to the inflammatory bowel disease seen commonly in marmosets. Historically the incidence of inflammatory bowel disease in this colony of marmosets has been as high as 60%.21 The cause remains unclear but is likely multifactorial, and Giardia infection may be a contributing factor.
Giardial infection of marmosets is potentially important as a zoonotic infection in veterinary support staff. There are several reports of zoonotic transmission from animals to man and there are molecular similarities between isolates from companion animals and humans.6,34 However, the zoonotic potential of Giardia has recently come under question as molecular techniques have characterized multiple strains of the organism that are species specific and case analysis has revealed that contact with animals is not a risk factor for acquiring Giardia infection.18,30 To our knowledge, no animal or veterinary care staff have become infected with Giardia as a result of contact with marmosets; however, until further data clearly indicates that Giardia from marmosets is not a zoonotic agent, it should be treated as a possible human pathogen.
For these reasons, elimination of Giardia from the colony is desirable from both a colony health and research perspective. These results show tinidazole to be highly effective at treating Giardia infection. Among 5 posttreatment fecal samples obtained over 20 d, no sample tested positive for Giardia, and 11 of 13 treated animals remained negative 1 y later. In addition, 9 of 9 previously negative animals were still negative at the 1 y time point. The 2 animals that tested positive at 1 y likely reacquired infection from nearby animals or an environmental source. False negatives at the 1-y sample point are also possible. We did not pursue additional fecal samples to reduce any additional stress on this group of animals because they were no longer individually housed.
We noted initial nonsignificant weight loss in the animals which can be explained by separation from cagemates and stress from handling for treatment. However at 1 y, most animals had gained weight, and treated animals had gained more weight than age-matched untreated controls. That the only significant difference in weight noted was between treated and untreated animals at 1-y post treatment suggests that drug itself, not necessarily treatment of Giardia, had an effect on body weight. However, a single dose of tinidazole likely would not have long-lasting effects other than its antiprotozoal action.
Two treatments of tinidazole eliminated all evidence of Giardia infection from the cohort. The ease of dosing makes eradication of Giardia from a marmoset colony feasible and possible.
Compared with metronidazole, tinidazole offers a more convenient dosing schedule and reaches a higher cure rate.8,15 Here, tinidazole was 100% effective in eliminating evidence of infection. The results also suggest that 1 dose may be sufficient to clear infection. By using a 1-dose schedule, animals can be treated during preventative healthcare sessions, and the entire colony can be treated over the course of several days. This regimen offers a marked advantage over metronidazole, which requires 5 to 8 d of treatment. Not only is metronidazole time-consuming for technical staff to administer treatments, but the required regimen assumes that animals ingest metronidazole at each treatment, and compliance is often difficult to ensure given the drug's bitter taste. Tinidazole can be considered a favorable alternative to metronidazole for treatment of Giardia in marmosets.
Future work should include classification of the Giardia organism responsible for infecting the marmoset colony to determine its relation to other animal and human isolates and therefore its zoonotic potential, and to determine whether it shares characteristics with pathogenic or nonpathogenic strains. In addition, it is necessary to determine whether eradication of Giardia from this and other marmoset colonies is possible and, if so, whether the incidence of inflammatory bowel disease declines as a result.
Acknowledgments
The authors thank Shelly Sancricca, Larry Murphy, Cindy Pitard, and Cindy Dorval for their assistance with the marmoset colony and Elaine Roberts for technical assistance. This work was supported by NIH NCRR grant P51 RR000168-47.
References
- 1.Ali SA, Hill DR. 2003. Giardia intestinalis. Curr Opin Infect Dis 16:453–460 [DOI] [PubMed] [Google Scholar]
- 2.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. 2002. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet 359:564–571 [DOI] [PubMed] [Google Scholar]
- 3.Canete R, Escobedo AA, Gonzalez ME, Almirall P, Cantelar N. 2006. A randomized, controlled, open-label trial of a single day of mebendazole versus a single dose of tinidazole in the treatment of giardiasis in children. Curr Med Res Opin 22:2131–2136 [DOI] [PubMed] [Google Scholar]
- 4.Cedillo-Rivera R, Enciso-Moreno JA, Martinez-Palomo A, Ortega-Pierres G. 1989. Giardia lamblia: isoenzyme analysis of 19 axenic strains isolated from symptomatic and asymptomatic patients in Mexico. Trans R Soc Trop Med Hyg 83:644–646 [DOI] [PubMed] [Google Scholar]
- 5.Chalifoux LV, Bronson RT, Escajadillo A, McKenna S. 1982. An analysis of the association of gastroenteric lesions with chronic wasting syndrome of marmosets. Vet Pathol Suppl 19 Suppl 7:141–162 [PubMed] [Google Scholar]
- 6.Eligio-Garcia L, Cortes-Campos A, Jimenez-Cardoso E. 2005. Genotype of Giardia intestinalis isolates from children and dogs and its relationship to host origin. Parasitol Res 97:1–6 [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration, Center for Biologics Evaluation and Research Guidance for industry and reviewers: estimating the safe starting dose in clinical trials for therapeutics in adult healthy volunteers. available at http://www.fda.gov/cber/gdlns/dose.htm
- 8.Fung HB, Doan TL. 2005. Tinidazole: a nitroimidazole antiprotozoal agent. Clin Ther 27:1859–1884 [DOI] [PubMed] [Google Scholar]
- 9.Garcia LS, Shimizu RY. 1997. Evaluation of nine immunoassay kits (enzyme immunoassay and direct fluorescence) for detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimens. J Clin Microbiol 35:1526–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner TB, Hill DR. 2001. Treatment of giardiasis. Clin Microbiol Rev 14:114–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gore MA, Brandes F, Kaup FJ, Lenzner R, Mothes T, Osman AA. 2001. Callitrichid nutrition and food sensitivity. J Med Primatol 30:179–184 [DOI] [PubMed] [Google Scholar]
- 12.Guimaraes S, Sogayar MI, Franco MF. 2003. Protease activity in Giardia duodenalis trophozoites of axenic strains isolated from symptomatic and asymptomatic patients. Mem Inst Oswaldo Cruz 98:77–81 [DOI] [PubMed] [Google Scholar]
- 13.Hamlen HJ, Lawrence JM. 1994. Giardiasis in laboratory-housed squirrel monkeys: a retrospective study. Lab Anim Sci 44:235–239 [PubMed] [Google Scholar]
- 14.Hanson KL, Cartwright CP. 2001. Use of an enzyme immunoassay does not eliminate the need to analyze multiple stool specimens for sensitive detection of Giardia lamblia. J Clin Microbiol 39:474–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris JC, Plummer S, Lloyd D. 2001. Antigiardial drugs. Appl Microbiol Biotechnol 57:614–619 [DOI] [PubMed] [Google Scholar]
- 16.Hendricks EE, Ludlage E, Bussell S, George K, Wegner FH, Mansfield KG. 2004. Wasting syndrome and disruption of the somatotropic axis in simian immunodeficiency virus-infected macaques with Mycobacterium avium complex infection. J Infect Dis 190:2187–2194 [DOI] [PubMed] [Google Scholar]
- 17.Heresi G, Cleary TG. 1997. Giardia. Pediatr Rev 18:243–247 [DOI] [PubMed] [Google Scholar]
- 18.Hunter PR, Thompson RC. 2005. The zoonotic transmission of Giardia and Cryptosporidium. Int J Parasitol 35:1181–1190 [DOI] [PubMed] [Google Scholar]
- 19.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 20.Kalishman J, Paul-Murphy J, Scheffler J, Thomson JA. 1996. Survey of Cryptosporidium and Giardia spp. in a captive population of common marmosets. Lab Anim Sci 46:116–119 [PubMed] [Google Scholar]
- 21.Ludlage E, Mansfield K. 2003. Clinical care and diseases of the common marmoset (Callithrix jacchus). Comp Med 53:369–382 [PubMed] [Google Scholar]
- 22.Maraha B, Buiting AG. 2000. Evaluation of four enzyme immunoassays for the detection of Giardia lamblia antigen in stool specimens. Eur J Clin Microbiol Infect Dis 19:485–487 [DOI] [PubMed] [Google Scholar]
- 23.Mohamed NH, Salama MM, Moustafa MA, El-Wakil HS, Mohareb EW, Thabet HS. 2004. Molecular characterization of Egyptian Giardia lamblia isolates. J Egypt Soc Parasitol 34:213–226 [PubMed] [Google Scholar]
- 24.Oster N, Gehrig-Feistel H, Jung H, Kammer J, McLean JE, Lanzer M. 2006. Evaluation of the immunochromatographic CORIS Giardia-Strip test for rapid diagnosis of Giardia lamblia. Eur J Clin Microbiol Infect Dis 25:112–115 [DOI] [PubMed] [Google Scholar]
- 25.Papich MG. 2007. Saunders handbook of veterinary drugs. St Louis (MO): Saunders/Elsevier [Google Scholar]
- 26.Petri WA. 2005. Treatment of giardiasis. Curr Treat Options Gastroenterol 8:13–17 [DOI] [PubMed] [Google Scholar]
- 27.Plumb DC. 2002. Veterinary drug handbook. Ames (IA): Iowa State Press [Google Scholar]
- 28.Potkay S. 1992. Diseases of the Callitrichidae: a review. J Med Primatol 21:189–236 [PubMed] [Google Scholar]
- 29.Prado MS, Cairncross S, Strina A, Barreto ML, Oliveira-Assis AM, Rego S. 2005. Asymptomatic giardiasis and growth in young children: a longitudinal study in Salvador, Brazil. Parasitology 131:51–56 [DOI] [PubMed] [Google Scholar]
- 30.Savioli L, Smith H, Thompson A. 2006. Giardia and Cryptosporidium join the'Neglected Diseases Initiative. Trends Parasitol 22:203–208 [DOI] [PubMed] [Google Scholar]
- 31.Sestak K, Merritt CK, Borda J, Saylor E, Schwamberger SR, Cogswell F, Didier ES, Didier PJ, Plauche G, Bohm RP, Aye PP, Alexa P, Ward RL, Lackner AA. 2003. Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus macaques. Infect Immun 71:4079–4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobel JD, Nyirjesy P, Brown W. 2001. Tinidazole therapy for metronidazole-resistant vaginal trichomoniasis. Clin Infect Dis 33:1341–1346 [DOI] [PubMed] [Google Scholar]
- 33.Speelman P. 1985. Single-dose tinidazole for the treatment of giardiasis. Antimicrob Agents Chemother 27:227–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson RC. 2004. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet Parasitol 126:15–35 [DOI] [PubMed] [Google Scholar]
- 35.Tucker MJ. 1984. A survey of the pathology of marmosets (Callithrix jacchus) under experiment. Lab Anim 18:351–358 [DOI] [PubMed] [Google Scholar]
- 36.Vesy CJ, Peterson WL. 1999. Review article: the management of giardiasis. Aliment Pharmacol Ther 13:843–850 [DOI] [PubMed] [Google Scholar]
- 37.Weitzel T, Dittrich S, Mohl I, Adusu E, Jelinek T. 2006. Evaluation of seven commercial antigen detection tests for Giardia and Cryptosporidium in stool samples. Clin Microbiol Infect 12:656–659 [DOI] [PubMed] [Google Scholar]
- 38.Wolfe MS. 1992. Giardiasis. Clin Microbiol Rev 5:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaat JO, Mank T, Assendelft WJ. 2000. Drugs for treating giardiasis. Cochrane Database Syst Rev 2:CD000217. [DOI] [PubMed] [Google Scholar]