Abstract
A bovine colostral antibody against verotoxin (VT) 2 of Escherichia coli O157:H7 was administered orally to beagle dogs. The antibody remained in the dogs’ small intestine for at least 2 h, whereas little serum antibody remained 1.5 h after administration. Furthermore, the antibody activity of secretory IgA did not change until 2 h after administration; however, the activity of IgG and IgM antibodies decreased by approximately 60% and 40% at 2 h after administration, respectively. Seven beagle dogs inoculated with Escherichia coli O157:H7 producing VT2 were administered bovine colostral antibody or bovine colostral whey without antibody. With administration of bovine colostral whey without antibody, the amount of VT2 in feces decreased gradually after administration and increased again at 5 d after inoculation, whereas bovine colostral antibody significantly reduced the amount of VT2 in feces on the day after administration. In addition, 9 beagle dogs were given bovine colostral antibody, bovine plasma antibody, or saline. The amount of VT2 in feces again decreased significantly more rapidly after administration of bovine colostral antibody than after administration of bovine plasma antibody or saline.
Abbreviations: EHEC, enterohemorrhagic Escherichia coli; VT, verotoxin
In July 1996, a widespread outbreak of enterohemorrhagic Escherichia coli (EHEC) O157:H7 infection occurred among schoolchildren in Sakai, Japan, followed by numerous other similar outbreaks of food poisoning throughout the country.4,19 Escherichia coli O157:H7 infection is monitored in Japan, in accordance with the Infection Diseases Control Law, and in 2005, 3589 cases were reported.10 Enterohemorrhagic Escherichia coli infection occurs in many industrialized nations21 and is an emergent infectious disease of significant clinical importance.12,13,23
Therapeutic approaches for EHEC infection are the subject of widespread discussion.9,25,31 Generally, the treatment for bacterial food poisoning is antibiotic administration. However, antibiotic therapy is not recommended for food poisoning caused by EHEC infection, because it increases the risk of serious complications, such as hemolytic uremic syndrome, due to the release of verotoxin (VT) from killed bacteria. Therefore, alternative therapeutic approaches, such as inhibiting VT activity or absorption from the intestine, are required. We previously obtained a colostral antibody against VT2 from cows immunized with the toxin and confirmed the neutralization efficacy of this reagent against VT2 in mice.15 However, before this bovine colostral antibody can be administered to patients infected with E. coli O157, its resistance to decomposition by intestinal proteases must be investigated. Each immunoglobulin class reportedly differs in its resistance to protease degradation in vitro,1,3,18, 22,26,28 but such resistance has not been confirmed in vivo. Furthermore, few animal models are available for evaluating for E. coli O157:H7 infection. The weaned immature mouse model has been used to study E. coli O157:H7 infection and VT,15 and beagle dogs pretreated with fradiomycin before inoculation with E. coli O157:H7 developed diarrhea. We chose to use this canine model in the current study.
In this study, we investigated the resistances of bovine colostral antibody and individual immunoglobulin classes to proteases in the small intestine of beagle dogs. We also evaluated the efficacy of this colostral antibody against VT2 in beagle dogs.
Materials and Methods
Microorganisms and VT2 detection.
The human isolate of E. coli O157:H7 producing VT2 used in this study was cultured by using brain heart infusion broth (Becton Dickinson, Franklin Lakes, NJ) for 48 h, and culture supernatant was obtained by centrifugation (1600 × g, 20 min). Microorganisms were suspended in sterile saline and diluted to 1 × 109 CFU/mL for administration to beagle dogs.
VT2 in culture medium and feces was measured by using a commercial kit (VTEC RLPA, Denka Seiken, Tokyo, Japan) based on reversed passive latex agglutination.
Animals.
Nine beagle dogs (8 male and 1 female; age, 1 y; Saitama Experimental Animals Supply, Saitama, Japan) and 23 male beagle dogs (age, 1 y; AQS, Chiba, Japan) were used. Four male Japanese white rabbits (Saitama Experimental Animals Supply) and 2 dairy cows (age, 6 to 8 y; 3 to 4 month prior to delivery) bred at a cattle farm in Shimane Prefecture, Japan, also were used.
Dogs and rabbits were reared individually in cages housed in an animal room maintained at a temperature of 22 ± 2 °C and humidity of 60% ± 10% with a 12:12-h light:dark cycle (lights on, 0700 to 1900) and 15 air changes per hour. Dogs were fed a commercial chow (Oriental Yeast, Tokyo, Japan) and were allowed free access to water.
All experiments conformed to Japanese regulations concerning animal care and use, as specified in the Guidelines for Animal Experimentation11 and were approved by the Animal Research Committee of Azabu University.
Immunization of cows with VT2.
Two pregnant dairy cows (ages, 6 and 8 y; 3 to 4 mo before delivery) were immunized15 to obtain sufficient bovine colostral and plasma antibody for the study. To obtain serum antibody, the cows initially were immunized intradermally with culture supernatant containing VT2 that had been mixed (1:1) with Freund complete adjuvant (Difco Laboratories, Detroit, MI), after which they were inoculated 13 more times at 7-d intervals with 1 mL of VT2-containing culture supernatant (no adjuvant).
Preparation of colostral whey without antibody.
Colostrum was treated to obtain colostral whey in the same manner as to obtain bovine colostral antibody against VT2.16 Therefore, skim milk was obtained by centrifuging the colostrum at 1600 × g for 15 min to remove butterfat. Skim milk (1 L) then was mixed with 100 mg rennet (MP Biochemicals, Solon, OH), incubated overnight at 22 °C, and centrifuged at 2200 × g for 20 min. Bovine colostral antibody was obtained by filtering the supernatant over a membrane filter (pore size, 22 μm).
Preparation of bovine plasma, bovine serum, and rabbit serum antibodies against VT2.
Blood was collected from the cervical vein of VT2-immunized cows and centrifuged at 1600 × g for preparation of bovine plasma antibody.
Japanese White rabbits were immunized with culture medium containing VT2 suspended in Freund complete adjuvant (Difco Laboratories; 1:1 v/v ratio of medium to adjuvant) and boosted every 7 d for a total of 22 times by using culture supernatant only (no adjuvant). Rabbits were euthanized under pentobarbital anesthesia and exsanguinated. Antisera were obtained by centrifugation at 2200 × g for 20 min.
Measurement of neutralization titer.
Titers of bovine colostral antibody, bovine serum antibody, and rabbit serum antibody were evaluated by neutralization tests according to standard methods using Vero cells.14 Colostral antibody (40 μL) diluted from 2- to 2048-fold was mixed with 40 μL cell culture medium (MEM, Nissui Pharmaceutical, Tokyo) containing VT2. These mixtures were incubated overnight at 37 °C, after which 100 μL Vero cells was added to each antibody sample and cultured for 2 d. The neutralization titer was measured based on the number of dead Vero cells.
Estimation of resistance to intestinal proteases in beagle dogs.
Eight beagle dogs were divided into 2 groups for administration of bovine colostral antibody or rabbit serum antibody. Each dog was fasted for 18 h and received 50 mL bovine colostral antibody or rabbit serum antibody orally. Dogs were euthanized by overdose of pentobarbital under anesthesia at 1.5, 2, 3, or 4 h after administration, and small intestinal fluid was collected. Because the small intestine was empty at the 4-h time point, a sample was not obtained. Small intestinal fluid was passed over a membrane filter (pore size, 0.20 μm) and was stored at –80 °C until further use.
Measurement of activities of bovine colostral and serum antibodies by ELISA.
The concentration of VT2 was adjusted with 0.05 mol/L sodium hydrogen carbonate buffer (pH 9.6) and transferred to 96-well immunoplates (Nunc, Roskilde, Denmark). To all wells, 1% gelatin in 0.05 mol/L sodium hydrogencarbonate buffer (pH 9.6) was added at 200 μL per well to block nonbinding sites. After wells were washed, small intestinal fluid including bovine colostral antibody or rabbit serum antibody was added at 100 μL per well. Antibodies against to bovine immunoglobulin (Monosan, Uden, The Netherlands) were added at 100 μL per well only to wells containing wash samples from small intestine fluid after administration of bovine colostral antibody. Antibodies against rabbit immunoglobulin-coupled peroxidase (MP Biomedicals, Costa Mesa, Canada) were added to all wells at 100 mL per well. After wells were washed, 2,2-aminodi(3-ehtylbenzthiazoline sulphonic acid (Invitrogen, Carlsbad, CA) in citrate buffer (pH 4.2) was added at 100 mL/well, and absorbance at 415 and 492 nm was measured by using an immunoplate reader.
Comparison of resistance of each immunoglobulin class to intestinal proteases in beagle dogs.
Bovine colostral antibody (50 mL) or bovine serum antibody (50 mL) was administered orally to 4 beagle dogs in each group. Beagle dogs were euthanized by overdose of pentobarbital under anesthesia at 1.5, 2, or 3 or 4 h after administration of antibody and the intestines were removed for collection of intestinal fluid. Collection of intestinal fluid at 4 h after administration was attempted but was unsuccessful. Small intestinal fluid was passed over a membrane filter (pore size, 0.20 μm) and stored at –80 °C until further use.
Measurement of activity of each immunoglobulin class in small intestinal fluid by ELISA.
Horseradish peroxidase-conjugated antibodies against bovine IgA (VMRD, Pullman, WA), bovine IgM (VMRD), and IgG (VMRD) were prepared as described previously19 and used in ELISAs to measure the activity of each immunoglobulin class in the small intestine. Horseradish peroxidase-conjugated (Wako Pure Chemical Industries, Osaka, Japan) antibodies were prepared as described previously.20
VT2 was adjusted with 0.05 mol/L sodium hydrogencarbonate buffer (pH 9.6) and transferred to immunoplates (Nunc). To all wells, 1% gelatin in 0.05 mol/L sodium hydrogencarbonate buffer (pH 9.6) was added at 200 μL per well to block nonbinding sites. After wells were washed, small intestinal fluid collected from beagle dogs was added at 100 μL per well. Horseradish peroxidase-coupled antibody was added at 100 mL per well. After washing, 2,2-aminodi (3-ehtylbenzthiazoline sulphonic acid (Invitrogen) in citrate buffer was added at 100 μL per well. Absorbance at 415 and 492 nm was measured by using an immunoplate reader.
Neutralization efficacy of bovine colostral antibody against VT2 in beagle dogs inoculated with E. coli O157:H7. Comparison between bovine colostral antibody and colostral whey.
To eliminate native enterobacterial flora, beagle dogs were given fradiomycin sulfate (Nippon Kayaku, Tokyo, Japan) at 50 mg/kg daily for 3 d prior to inoculation with E. coli O157:H7. Dogs then were fasted for 18 h and then were inoculated orally through a feeding tube with 5 mL E. coli O157:H7 in physiologic saline (1 × 109 CFU/mL). On the day after inoculation of E. coli, fosfomycin sodium (50 mg/kg; Meiji Seika Kaisha, Tokyo, Japan) was administered. Fecal samples were collected daily after E. coli O157:H7 inoculation, and fecal characteristics were noted. After confirmation of increased VT2 in feces, beagle dogs were divided into 2 groups: 4 dogs received bovine colostral antibody (100 mL each), and 3 dogs received bovine colostral whey without antibody (100 mL each). Titers in feces are shown as geometric means. Unpaired Student t tests (Statview, Hulinks, Tokyo, Japan) were used to compare titers before and on the day after administration of bovine colostral antibody or colostral whey without antibody.
Comparison of bovine colostral antibody, serum antibody, saline.
Nine beagle dogs divided into 3 groups were treated with fradiomycin sulfate (Nippon Kayaku), inoculated with E. coli O157:H7, and given fosfomycin (Meiji Seika Kaisha) as described in the previous paragraph. Each group of 3 beagle dogs was given either bovine colostral antibody, plasma antibody, or saline (dose, 100 mL) after confirmation of increased VT2 in feces. Titers in feces are shown as geometric means. Unpaired Student t tests (Statview, Hulinks) were used to compare titers before and the day after administration of bovine colostral antibody, plasma antibody, or saline.
Results
Neutralization titer of bovine, plasma, and serum antibodies.
The neutralization titer of colostral antibody obtained from the 6-y-old cow against VT2 was 1:32 by neutralization test according to standard methods using Vero cells. The titer of bovine plasma antibody obtained from the same cows was 1:8. The neutralization titer of colostral antibody obtained from the 8-y-old cow against VT2 was 1:128; the titer of bovine serum antibody obtained from the same cows was 1:32. Both bovine colostral whey from nonimmunized cows and rabbit serum antibody lacked neutralization activity against VT2.
Antibody activities of bovine colostral antibody and rabbit serum antibody recovered from small intestines of beagle dogs.
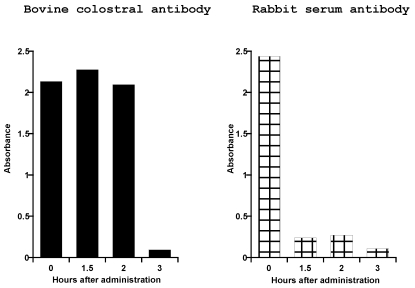
The activity of the bovine colostral antibody did not change for the first 2 h after administration (Figure 1). At 3 h after administration, activity was decreased to approximately 4% of the baseline level. In contrast, the activity of rabbit serum antibody decreased immediately after administration, giving values of 10% of baseline levels at both 1.5 and 2 h after administration. The activities of both bovine colostral antibody and rabbit serum antibody were approximately 4% of baseline values at 3 h after administration.
Figure 1.
Activities of bovine colostral and rabbit serum antibodies recovered from the small intestine. Beagle dogs each received 50 mL bovine colostral antibody or rabbit serum antibody orally. Samples of fluid from the small intestine were collected at 1.5, 2, and 3 h after administration of antibody. Antibody activities are given in terms of absorbance, as measured by ELISA. The time 0 value represents the antibody activity before administration.
Activities of IgA, IgG, and IgM antibodies in small intestinal fluid recovered from beagle dogs.
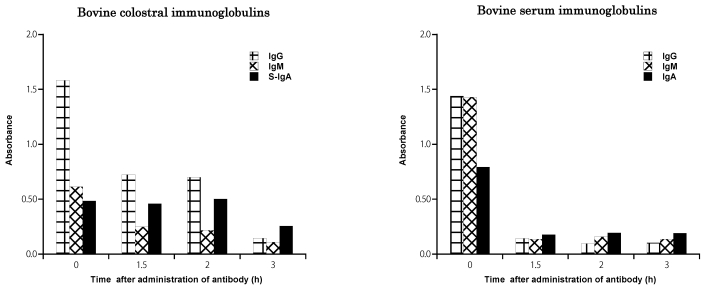
At 2 h after administration of bovine colostral antibody, the activity of IgG antibody in small intestinal fluid harvested from beagle dogs (Figure 2) was decreased by approximately 60%, activity of IgM antibody was decreased by approximately 40%, but activity of secretory IgA was essentially unchanged. At 3 h after administration, the activity of secretory IgA antibody had decreased by 50%, whereas those of IgG and IgM antibodies were 10% and 17% of baseline values, respectively. At 1.5 h after administration of bovine serum antibodies, the activities of IgG and IgM both were approximately 20% of control levels, whereas IgA activity was approximately 50% of the baseline value.
Figure 2.
Class-specific activities of immunoglobulins [IgG, IgM, and secretory IgA (s-IgA)] recovered from the small intestine of beagle dogs after administration of bovine colostral antibody or serum antibody. Samples of fluid from the small intestine were collected at 1.5, 2, and 3 h after administration of antibody. Antibody activities are given in terms of absorbance, as measured by ELISA. The time 0 value represents the antibody activity before administration.
Neutralization efficacies of bovine colostral antibody and whey in beagle dogs inoculated with E. coli O157:H7.
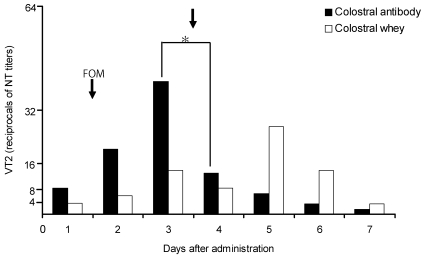
Beagle dogs received oral bovine colostral antibody when the amount of VT2 in feces was 1:40.7, and the amount decreased significantly (P < 0.05) to 1:12.6 on the day after antibody administration (Figure 3). The titer of VT2 in feces was 1:13.5 on the day when bovine colostral whey (no antibody) was given, 1:7.9 on day 2, and 1:26.9 at 5 d after inoculation of E. coli O157:H7.
Figure 3.
Changes in fecal VT2 levels after administration of bovine colostral antibody or whey in beagle dogs inoculated with Escherichia coli O157:H7. Fosfomycin (50 mg/kg) was administered on the day after inoculation. Vertical arrows indicate the administration of 100 mL bovine colostral antibody or whey. The reciprocals of neutralizing test (NT) titers to VT2 are plotted on the ordinate. *, P < 0.05 (unpaired Student t test) compared with day 3 value.
Neutralization efficacies of bovine colostral and plasma antibodies in beagle dogs inoculated with E. coli O157:H7.
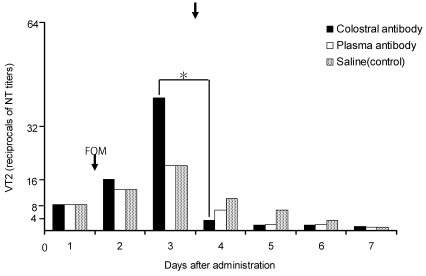
The amount of VT2 in the feces of beagle dogs decreased significantly (P < 0.05) from 1:40.8 to 1:3.2 on the day after administration of bovine colostral antibody and reached 1:1.3 at 7 d after inoculation of bacteria (Figure 4). In addition, the amount of VT2 in feces decreased on the day after administration of bovine plasma antibody or saline (from 1:20.0 to 1:6.3 and from 1:20.0 to 1:10.0, respectively) and continued to decrease gradually thereafter.
Figure 4.
Changes in fecal VT2 levels after administration of bovine colostral or plasma antibody or saline in beagle dogs inoculated with Escherichia coli O157:H7. Fosfomycin (50 mg/kg) was administered on the day after inoculation. Vertical arrow indicates the administration of 100 mL of bovine colostral antibody, bovine plasma antibody, or saline, respectively. The reciprocals of neutralizing test (NT) titers to VT2 are plotted on the ordinate. *, P < 0.05 (unpaired Student t test) compared with day 3 value.
Discussion
We previously obtained a bovine colostral antibody against from VT2-immunized cows and confirmed its neutralization efficacy against VT2 in mice given VT2 or inoculated with E. coli O157:H7 producing VT2.15 If this bovine colostral antibody is to be administered orally to patients infected with E. coli O157:H7, it must be resistant to protease degradation in the digestive tract. We thus investigated the resistance of this bovine colostral antibody to intestinal proteases in beagle dogs, which were presumed to secrete proteases more abundantly than do mice and therefore suitable for extrapolation of results to humans.
Because beagle dogs inoculated with live E. coli O157:H7 (1 × 109 CFU/ml) without pretreatment with antibiotics lacked symptoms such as diarrhea (data not shown), we were unable to use untreated healthy dogs in the current study. beagle dogs with normal enterobacterial flora may not be sensitive to E. coli O157:H7. The dogs used in the current study were pretreated with fradiomycin sulfate to alter their indigenous enterobacterial flora and had slight to severe diarrhea after inoculation with E. coli O157:H7. Aminoglycoside antibiotics, such as fradiomycin sulfate, are poorly absorbed and largely pass through the intestine.8,9 We allowed 1 d after administration for excretion of fradiomycin sulfate from the intestine prior before inoculating the dogs with E. coli O157:H7. Therefore, we feel that treatment with fradiomycin sulfate did not influence the elimination of E. coli O157:H7.
The bovine colostral antibody to VT2 remained relatively unchanged for 2 h after administration, whereas that of the rabbit serum antibody was decreased significantly at 1.5 h after administration. In addition, the activity level of secretory IgA colostral antibody remained near baseline at 2 h after administration and that of IgG antiVT2 was approximately 60% of baseline at the same time point. These results suggest that bovine colostral antibody is more resistant to proteases than is serum antibody in vivo.
Secretory IgA is more resistant to pepsin than is serum IgA, because the presence of secretory component (SC) protects against proteolysis in vitro.17,18,24,27-30 Less than 15% of secretory IgA was digested during an in vitro experiment with papain.29 In the present study, secretory IgA activity in the beagle dog intestine remained for at least 2 h after administration of bovine colostral antibody, similar to its resistance in vitro.1,3,26,28 In contrast, IgG1 was digested completely in vitro,29 but activity levels remained at approximately 60% of pretreatment values in the present study. These results suggest that both secretory IgA and IgG antibodies contribute to the resistance of bovine colostral antibody to intestinal proteases. Therefore, the bovine colostral antibody against VT2 appears to be resistant to intestinal proteases and able to maintain its neutralizing activity against VT2 in the digestive tract.
The amount of VT2 in the feces of our beagle dogs decreased significantly on the day after administration of bovine colostral antibody. In contrast, the amount of VT2 in feces was slightly decreased on the day after administration of colostral whey (no antibody) and actually was increased at 2 d after administration. Due to commensal flora, E. coli O157:H7 does not flourish in the mouse intestine.19 This finding suggests that VT2 is not neutralized by colostral whey and that E. coli O157:H7 is able to survive and reproduce. Colostrum from nonimmunized cows with antibodies against numerous pathogens showed efficacy in mice infected with E. coli O157:H7.6,16 Our results suggest that the colostral antibody we obtained from cows immunized with VT2 is more effective at neutralizing VT2 than were the bovine reagents produced previously.6,16
The amount of VT2 in feces decreased significantly the day after administration of bovine colostral antibody but not after plasma antibody or saline. This result suggests that bovine plasma antibody was digested by proteases, as was that in rabbit serum, and therefore decreased more rapidly than did bovine colostral antibody in the beagle dog intestine. Therefore, plasma antibody likely would not neutralize VT2 effectively because of rapid degradation by proteases. In contrast, colostral antibody (which includes secretory IgA and IgG components) was resistant to protease degradation and showed sufficient neutralization efficacy against VT2 in the intestine.
Bovine colostrum containing antibodies has already been used to treat human rotavirus infection.2,5,7 However, that reagent was not evaluated in regard to degradation in the small intestine. We therefore investigated the resistance to proteases in the small intestine and efficacy against VT2 of the present bovine colostral antibody. Our results indicate that colostrum obtained from pathogen-immunized cows may be useful for neutralizing toxins and pathogens. Bovine colostral antibody against VT2, which contains IgG and secretory IgA, showed resistance to proteases in the small intestine of beagle dogs, thus suggesting the possibility of resistance to proteases in the human intestine. Treatment with both fosfomycin sodium and bovine colostral antibody improves the survival of mice infected with E. coli O157:H7.15 In the future, combination therapy with antibiotics and bovine colostral antibody against VT2 may prevent serious complications, such as hemolytic uremic syndrome, of EHEC infection.
Acknowledgments
This research was supported by the Promotion and Mutual Aid Corporation for Private Schools of Japan, Grant-in-Aid for Matching Fund Subsidy for Private Universities and a project grant (Start-Up Support for the Matching Fund Subsidy for Private Universities, 2007-2008) awarded by the Azabu University Research Services Division.
References
- 1.Brown WR, Newcomb RW, Ishizuka K. 1970. Proteolytic degradation of exocrine and serum immunoglobulins. J Clin Invest 49:1374–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brussow H, Hilpert H, Walther I, Sidoti J, Mietens C, Bachmann P. 1987. Bovine milk immunoglobulins for passive immunity to infantile rotavirus gastroenteritis. J Clin Microbiol 25:982–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler JE. 1969. Bovine immunoglobulins: a review. J Dairy Sci 2:1895–1909. [Google Scholar]
- 4.Cleary TG. 2004. The role of Shigatoxin-producing Escherichia coli in hemorrhagic colitis and hemolytic uremic syndrome. Semin Pediatr Infect Dis 15:260–265 [DOI] [PubMed] [Google Scholar]
- 5.Ebina T, Ohta M, Kanamaru Y, Yamamoto-Osumi Y, Baba K. 1992. Passive immunization of suckling mice and infants with bovine colostrum containing antibodies to human rotavirus. J Med Virol 38:117–123 [DOI] [PubMed] [Google Scholar]
- 6.Funatogawa K, Ide T, Kirikae F, Saruta K, Nakano M, Kirikae T. 2002. Use of immunoglobulin-enriched bovine colostrum against oral challenge with enterohaemorrhagic Escherichia coli O157:H7 in mice. Microbiol Immunol 46:761–766 [DOI] [PubMed] [Google Scholar]
- 7.Hilpert H, Brussow H, Mietens C, Sidoti J, Lerner L, Werchau H. 1987. Use of bovine milk concentrate containing antibody to rotavirus to treat rotavirus gastroenteritis in infants. J Infect Dis 156:158–166 [DOI] [PubMed] [Google Scholar]
- 8.Hombach J, Hoyer H, Berkop-Schnurch A. 2007. Thiolated chitosans: development and in vitro evaluation of an oral tobramycin sulphate delivery system. Eur J Pharm Sci 33:1–8 [DOI] [PubMed] [Google Scholar]
- 9.Horspool LJ, Taylor DJ, McKellar QA. 1994. Plasma disposition of amikacin and interactions with gastrointestinal microflora in Equidae following intravenous and oral administration. J Vet Pharmacol Ther 17:291–298 [DOI] [PubMed] [Google Scholar]
- 10.Infection Disease Surveillance Center. [Internet]. Surveillance data table. 2005. Category III., Enterohemorrhagic Escherichia coli infection. Available from: http://idsc.nih.go.jp/disease/ehec/index.html.
- 11.Japanese Association for Laboratory Animal Science. 1987. Guidelines for animal experimentation. Tokyo (Japan). Available from: http://wwwsoc.nii.ac.jp/jalas/english/en_policy.html.
- 12.Karch H, Tarr PI, Bielaszewska M. 2005. Enterohaemorrhagic Escherichia coli in human medicine. Int J Med Microbiol 295:405–418 [DOI] [PubMed] [Google Scholar]
- 13.Karmali MA. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev 2:15–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konowalchuk J, Sepeirs JI, Stavric S. 1977. Vero response to a cytotoxin of Escherichia coli. Infect Immun 18:775–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuribayashi T, Seita T, Fukuyama M, Furuhata K, Honda M, Matsumoto M, Seguchi H, Yamamoto S. 2006. Neutralizing activity of bovine colostral antibody against verotoxin derived from enterohemorrhagic Escherichia coli O157:H7 in mice. J Infect Chemother 12:251–256 [DOI] [PubMed] [Google Scholar]
- 16.Lissner R, Schmidit H, Karch H. 1996. A standard immunoglobulin preparation produced from bovine colostra shows antibody reactivity and neutralization activity against Shiga-like toxins and EHEC-hemolysin of Escherichia coli O157:H7. Infection 24:378–383 [DOI] [PubMed] [Google Scholar]
- 17.Mach JP, Pahud JJ. 1971. Secretory IgA, a major immunoglobulin in most bovine external secretions. J Immunol 106:552–563 [PubMed] [Google Scholar]
- 18.Mcnabb PC, Tomasi TB. 1981. Host defense mechanisms at mucosal surfaces. Annu Rev Microbiol 35:477–496 [DOI] [PubMed] [Google Scholar]
- 19.Michino H, Araki K, Minami S, Takaya S, Sakai N, Miyazaki M, Ono A, Yazagawa H. 1999. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am J Epidemiol 150:787–796 [DOI] [PubMed] [Google Scholar]
- 20.Nakane PK, Kawaoi A. 1974. Peroxidasae-labelled antibody. A new method of conjugation. J Histochem Cytochem 22:1084–1091 [DOI] [PubMed] [Google Scholar]
- 21.Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newby TJ, Bourne FJ. 1976. Relative resistance of bovine and porcine immunoglobulins to proteolysis. Immunol Commun 5:631–635 [DOI] [PubMed] [Google Scholar]
- 23.Paton JC, Paton AW. 1998. Pathogenesis and diagnosis Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev 11:450–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plaut AG. 1972. A review of secretory immune mechanisms. Am J Clin Nutr 25:1344–1350 [DOI] [PubMed] [Google Scholar]
- 25.Tarr PI, Gordon CA, Chandler WL. 2005. Shigatoxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365:1073–1086 [DOI] [PubMed] [Google Scholar]
- 26.Tax A, Korngold L. 1971. Comparison of the effect of elasatase on human secretory IgA and serum IgA. J Immunol 107:1189–1191 [PubMed] [Google Scholar]
- 27.Tomasi TB, Grey HM. 1972. Structure and function of immunoglobulin A. Prog Allergy 16:81-213–133 [PubMed] [Google Scholar]
- 28.Tomasi TB Jr, Tan EM, Solomon A, Prendergast RA. 1965. Characteristics of an immune system common to certain external secretions. J Exp Med 121:101–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Underdown BJ, Dorrington KJ. 1974. Studies on the structural and conformational basis for the relative resistance of serum and secretory immunoglobulin A to proteolysis. J Immunol 112:949–959 [PubMed] [Google Scholar]
- 30.Yurchak AM, Bulter JE, Tomasi TB. 1971. Fluorescent localization of immunoglobulins in the tissue of the cow. J Dairy Sci 54:1324–1325 [PubMed] [Google Scholar]
- 31.Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. 2000. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med 342:1930–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]