Abstract
Mucopolysaccharidosis type IIIB (MPS IIIB; Sanfilippo syndrome type B) is a metabolic disorder with devastating clinical characteristics starting in early childhood and leading to premature death. A knockout mouse strain was developed that models this disease. Mice of the strain B6.129S6- Naglutm1Efn/J are invaluable for investigating pathogenesis and possible treatment modalities. However, the mouse strain also exhibits some objectionable phenotypic features. One such feature, urinary retention, not only is atypical of human MPS IIIB but often leads to early termination of experiments due to animal welfare concerns. The aim of this study was to investigate abnormalities associated with the urinary retention. Necropsies were performed on 9-mo-old mice; urinalysis, hematology and blood chemistry parameters were evaluated, and urogenital specimens were microscopically examined. Histopathologic examinations of urinary tract specimens proved illuminating regarding pathology in the urinary tract. A large mononuclear cell infiltrate was discovered in mutant mice of both sexes, more pronounced in females compared with male mice. The infiltrate comprises of large rounded or polygonal cells with generous variably vacuolated, granular eosinophilic cytoplasm and small round vesicular nuclei. These cells were present throughout and expand the interstitium of the lower urinary tract. Either this results in extrinsic compression of the lumen of the urethra, eventually leading to obstructive uropathy, bladder hyperdistension, and urinary retention or possibly interferes with the neurogenic component of micturition needs to be further investigated. The novel finding of an unexpected mononuclear cell infiltrate in the urinary tract in the knockout mice B6.129S6- Naglutm1Efn/J is reported.
Abbreviations: BSA, bovine serum albumin; BUN, blood urea nitrogen; MPS III B, mucopolysaccharidosis type III B
Mucopolysaccharidosis type IIIB, also called Sanfilippo syndrome type B, is a metabolic disorder with devastating clinical characteristics in humans. The onset of the disease is usually between 2 and 4 y of age; clinical symptoms, including hyperactivity, aggressive behavior, hearing and vision defects, mental retardation, and mild somatic changes progress rapidly and are followed by premature death, generally in the second decade of life.3,24 The pathogenesis of MPS IIIB can be described as a lysosomal storage disorder resulting from failure to degrade the lysosomal glycosaminoglycan heparan sulfate due to absence of the enzyme α-N-acetylglucosaminidase (Naglu). This inherited disorder is elicited by mutations in the Naglu gene, which is located on chromosome 17q21.3 More than 85 mutations have been identified so far.
The B6.129S6- Naglutm1Efn/J mouse strain was developed through targeted mutation by disruption of exon 6 of the Naglu gene.20 These mice are invaluable to continued investigations of pathogenesis of MPS IIIB, possible clinical interventions, and an eventual cure for this devastating disease. However, in addition to a number of desirable characteristics, B6.129S6- Naglutm1Efn/J mice exhibit several objectionable phenotypic features.12 One such adverse feature is urinary retention, leading to a grossly enlarged urinary bladder, potential hydronephrosis, and uremia. This specific abnormality of the genetically engineered mice not only is atypical of human MPS IIIB but due to its effect on animal welfare may lead to early termination of experiments.
Urinary retention leading to overdistension of the urinary bladder is a consequence of altered micturition due to urinary incontinence, dysuria, or a combination of both. Micturition is normally a conscious, voluntary act, whereas urinary incontinence is distinguished by the loss of the voluntary management of urination. Dysuria, however, relates to difficult and painful voiding of urine.19 Both disorders of micturition can cause moderate to severe distension of the bladder either as an acute or chronic condition. Dysuria may be elicited by any obstructive uropathy, and urinary incontinence can be classified as neurogenic or nonneurogenic.1
Bladder distension in multiple strains of mice has been described as a sequela of the toxicity of various substances,17,25,32 in studies of urinary tract malformations,26 after infection,6 and a characteristic of various transgenic mouse strains with various pathogenic pathways, such as mice with a mutated preprotachykinin gene, hypersensitive serotonin 3A receptor mutant mice, and mice lacking the muscarinic receptors M2 and M3.4,16,23 In addition, urinary retention with bladder enlargement comprises a part of the mouse urologic syndrome, which develops spontaneously in aged mice15,28 and has only been reported to occur in male mice. The pathogenesis of mouse urologic syndrome is unclear.
In our study colony of B6.129S6- Naglutm1Efn/J mice, most exhibited moderate to severe enlargement of the urinary bladder in a sex-independent fashion, whereas control (wildtype) mice had a normal or, in a few cases only, slightly enlarged bladder.12 As mentioned previously, bladder enlargement was the most common cause of euthanasia for animal-welfare reasons, thereby decreasing the data collection time.
The aim of the present study was to investigate the clinical and pathologic correlations underlying anomalous bladder enlargement in a mouse model of MPS IIIB. In addition, we attempted to determine whether the observed urinary retention was dependent on the genetic mutation or background strain used, to facilitate interpretation of results from treatment development studies, interpretation which might otherwise be difficult or impossible.
Materials and Methods
All described procedures were approved by the Institutional Animal Care and Use Committee and conducted in compliance with the Guide for the Care and Use of Laboratory Animals14 in an AAALAC-accredited facility.
All animals were group-housed in shoebox-style cages with static microisolation tops under climate-controlled conditions on a 12:12-h light:dark cycle, fed a commercial diet (Harlan Teklad Global Diet 2018, Harlan Laboratories, Indianapolis, IN), and provided tap water ad libitum. The animal facility maintains a SPF status based on a sentinel system including colony representatives and quarantine of animals received from nonapproved vendors or other institutions, respectively. Sentinel mice, housed in the same rooms as the study animals, were evaluated for ectoparasites, endoparasites, Sendai virus, mouse hepatitis virus, Mycoplasma pulmonis, pneumonia virus of mice, minute virus of mice, mouse parvovirus, Theiler disease virus, reovirus 3, lymphocytic choriomeningitis virus, mouse adenovirus types 1 and 2, Ectromelia virus, mouse rotavirus, and polyoma virus. The test results verified a negative status throughout the study period.
All 28 animals used in the study were obtained from our established colony of Naglu mice developed from 3 heterozygous breeding pairs of the JAX GEMM strain B6.129S6-Naglutm1Efn (The Jackson Laboratory, Bar Harbor, ME). Phenotyping of animals was performed by enzyme assay (see next section) by using tail biopsy (10 mg tissue) at 10 to 15 d of age and based on characteristic levels of Naglu enzyme activity.20 Homozygous mutant mice had no Naglu activity. Heterozygous mice demonstrated half the normal enzyme activity but were not used in this study. Wildtype mice were used as controls. All mice were evaluated at 9 mo of age because of an experienced high morbidity and mortality at subsequent ages in the authors' laboratory and welfare-related restrictions in previous studies involving this strain. The animals underwent gross necropsy examination, hematology and blood chemistry evaluation, urine volume assessment, urinalysis, and histopathology.
Enzyme assay.
Naglu enzyme activity was assayed by using fluorogenic substrate as described previously.20 Briefly, mouse tissue cell homogenates (25 µL) were incubated for 1 h at 37 °C with an equal volume of 0.2 mM 4-methylumbelliferyl α-N-acetylglucosaminide (Calbiochem) in 0.1 M sodium acetate buffer (pH 4.3) containing 0.5 mg/mL bovine serum albumin. The fluorescence of 4-methylumbelliferone released was measured after adding 1.0 mL glycine buffer (pH 10.5). One unit of activity corresponded to the hydrolysis of 1 nmol substrate per hour. Enzyme activity was determined fluorometrically using a microplate reader (Bio-Tech Instruments) by using 365 µm filters for excitation and 450 µm for emission.
Necropsy.
At the time of euthanasia, all mice were deeply anesthetized with 80 mg/kg pentobarbital intraperitoneally. The thorax was opened and blood collected by cardiocentesis. Final exsanguinations were performed with transcardial perfusion, first with PBS followed by 10% paraformaldehyde.
Urine was collected through cystocentesis and the amount measured. Bladder volume was classified as normal (0.0 to 0.25 mL), slightly increased (0.26 to 0.45 mL), moderately increased (0.46 to 0.80 mL), and severely increased (greater than 0.8 mL).
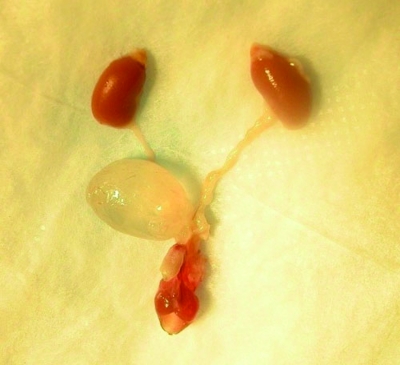
All 28 mice were inspected for gross abnormalities. The urinary tract was dissected intact (Figure 1). After removal, the bladder was distended by injection with 10% formalin solution. The amount of formalin administered into the urinary bladder was dependent on the amount of urine collected and therefore the size of the bladder. Care was taken to avoid further distension of the bladder while instilling sufficient formalin to avoid flaccidity of the organ. The intact urinary tract was transferred to 10% formalin. After fixation, the tissues were cut uniformly. The kidneys were cut midsagittally so that the cortex, medulla, and pelvis could easily be distinguished. Transverse sections of the ureter, each proximal close to the kidney and distal just before the ureter enters the bladder at the trigone, were collected. The bladder was bisected at the midline and both halves embedded, with open edges down. In addition, the urethra also transversely sectioned in proximal, middle, and distal parts.
Figure 1.
Dissected intact urinary tract of a male B6.129S6- Naglutm1Efn/J mutant mouse. Note the distension of the urinary bladder.
Hematology and serum chemistry.
Blood was collected from 10 wildtype and 5 homozygous mice for hematology and blood chemistry. The blood samples were evaluated at a commercial laboratory specialized in handling veterinary specimens (Antech Diagnostics, Tampa, FL). A full hematology evaluation including hemoglobin, packed cell volume, red blood cell count, white blood cell count, and a white blood cell differential were performed. The serum chemistry parameters blood urea nitrogen and creatinine were measured. The serum was separated by centrifugation after clotting within 30 min of blood collection.
Urinalysis.
Urinalysis was performed in 8 wildtype and 9 mutant mice. Urine was collected and stored at 4 °C for no longer than 2 h before urinalysis was performed. Color, transparency, and odor were assessed. Nitrate, pH, protein, glucose, urobilinogen, bilirubin, and ketones were evaluated by using a urine test strip (Chemstrip with SG, Roche Diagnostics, Florence, SC). The remaining urine was centrifuged for 5 min, the supernatant discarded, and the resuspended unstained sediment examined microscopically at ×100 and ×400 for bacteria, crystals, red blood cells, white blood cells, epithelial cells, and any abnormal material. Urine volume was measured in all 12 wildtype and 16 mutant mice.
Histopathology.
Necropsy was performed on all 12 wildtype and 16 mutant mice and tissues collected were stored in 10% formalin, but 12 control and 10 mutant mice were evaluated microscopically. Paraffin blocks, 5-µm tissue sections, and staining with hematoxylin and eosin, Alcian blue at pH 1.0 and 2.5, and periodic acid Schiff with and without diastase were produced in a commercial laboratory. In addition, immunoperoxidase reaction for S100 protein and immunohistochemistry with murine antibody CD68 were performed. The slides were evaluated by light microscopy, during which the tissues were screened for any abnormalities, especially inflammatory changes throughout the entire urinary tract, vacuolization of the renal tubules, and attenuation of the urinary bladder wall. In addition, specific areas of the specimen were assessed by using electron microscopy.
Statistics.
Data are represented as mean ± SEM. Groups were compared by using the Mann–Whitney test (Instat, version 3.0, GraphPad, San Diego, CA).
Results
Necropsy.
During necropsy of B6.129S6- Naglutm1Efn/J mutant and wildtype mice, all gross abnormalities were noted. A recurrent observation was moderate splenomegaly in 8 mutant (3 male, 5 female) mice. The penile body was missing in 1 mouse but no other abnormalities of the urinary tract were detected in this animal; the urinary bladder exhibited a normal size. One female mutant mouse revealed rectal prolapse combined with dilatation of the distal urethra and moderate splenomegaly. A few renal retention cysts were present in both kidneys of a female wildtype mouse. Three different mutant (1 male, 2 female) mice had mucosal hyperemia of the bladder wall with vasodilatation and eventual hemorrhage. The bladders of all 3 of these mice were moderately to severely distended, and 1 of the female mice had grossly visible blood in the urine. Two female mutant mice exhibited dilated uterine horns.
In general, the urinary bladders of all mutant mice were moderate to severely enlarged, and bladder walls appeared stretched and flaccid. In contrast, the bladders of wildtype mice were normal in size and wall thickness.
Hematology and serum chemistry.
All evaluated blood samples of 5 mutant and 10 wildtype mice showed unremarkable results in evaluated hematology and serum chemistry parameters. Table 1 shows those data considered to be blood biomarkers for any obstructive uropathy.
Table 1.
Blood parameters relevant to obstructive uropathy
| Animal | Sex | Genotype | PCV | WBC | BUN | Creatinine |
| 228 | M | +/+ | 44.2 | 2.4 | 17 | 0.1 |
| 229 | M | +/+ | 42.0 | 3.0 | 22 | 0.1 |
| 230 | M | +/+ | 39.8 | 12.0 | 21 | 0.1 |
| 231 | M | +/+ | 38.2 | 3.6 | 27 | 0.1 |
| 232 | M | +/+ | 46.1a | 2.5 | 28 | 0.1 |
| 233 | M | +/+ | 35.6 | 2.4 | 24 | 0.1 |
| 236 | F | −/− | 33.8 | 2.2 | 26 | 0.1 |
| 238 | F | +/+ | 42.0 | 1.5 | 25 | 0.1 |
| 239 | F | +/+ | 44.7 | 1.7 | 33 | 0.3 |
| 240 | F | +/+ | 45.3a | 1.1 | 19 | 0.1 |
| 241 | F | +/+ | 42.6 | 2.0 | 28 | 0.1 |
| 244 | M | −/− | 42.2 | 1.3 | 26 | 0.1 |
| 245 | M | −/− | 40.2 | 3.9 | 28 | 0.1 |
| 250 | F | −/− | 43.4 | 2.6 | 29 | 0.1 |
| 252 | F | −/− | 39.4 | 2.8 | 26 | 0.1 |
Value that is increased beyond reference range [packed cell volume (PCV), 38.5% to 45.%; white blood cell count (WBC), 3.0 to 14.2 × 103 cells/μL; blood urea nitrogen (BUN), 19 to 34 mg/dL; creatinine, 0.5 to 0.8 mg/dL].31
Urine volume.
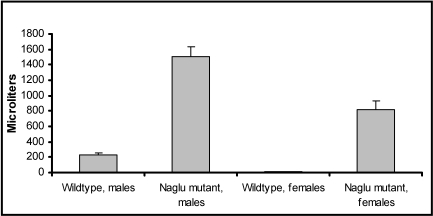
All wildtype mice had normal urine volumes and therefore normal bladder sizes. Wildtype and mutant mouse populations differed significantly, as did male and female mutant mice (Figure 2). Whereas all 6 male mutant mice had severely increased bladder volumes (greater than 0.8 mL urine), only 3 of the 10 mutant female mice showed severely increased bladder volumes; in addition, 6 had moderately (0.46 to 0.8 mL) and 1 slightly (0.26 to 0.45 mL) increased bladder volumes. However, all mutant mice showed significantly distended bladders. Bladders of male mutant mice contained 1.508 ± 1.203 mL urine compared with 0.225 ± 33.5 mL in wildtype male mice. Female mutant mice had 0.820 ± 108.9 mL urine stored in the bladder compared with 0.015 ± 3.33 mL in wildtype female mice. The range of urine volume at necropsy in mutant mice was 1.15 to 1.9 mL in male mice and 0.45 to 1.3 mL in female mice. Urine volume differed significantly between male (P = 0.002) and female (P = 0.0007) wildtype and mutant mice, as did that between mutant male and mutant female mice (P = 0.0057).
Figure 2.
Comparison of urine volume at 9 mo of age between wildtype and B6.129S6- Naglutm1Efn/J mutant mice. All mutant mice had significant increases in urine volume, and male mice were affected even more than female mice (male, P = 0.002; female, P = 0.0007)
Urinalysis.
Urine transparency and odor were normal in all mutant and wildtype animals. The urine color was light yellow in wildtype mice, whereas the urine in mutants was often dark yellow to brown, probably due to prolonged presence in the bladder. The mean pH was 5.0 in mutant mice and 6.0 in wildtype mice. The evaluations of the protein content of the urine were either zero or trace, both considered normal in mice; no genotype or sex bias in urine protein content was discernable. Test strip readings of nitrite, glucose, urobilinogen, and bilirubin were negative for all evaluated animals. Microscopic examination of the urine sediment did not reveal anything unusual; no crystals and only infrequent casts, epithelial cells, and bacteria were present in the urine. The numbers of red blood cells and leukocytes exceeded 5 per high-power field in 1 mutant female mouse each; in all other examined mice, these values were within normal limits (Table 2).
Table 2.
Urinalysis results according to commercial urine test strips and microscopy of urinary sediment
| Animal | Sex | Genotype | pH | Protein | Hemoglobin/red blood cells | White blood cells | casts | cells |
| 229 | M | +/+ | 6.0 | 0 | 0/0–1 | 0–3 | 1–5 fgc | 0 |
| 230 | M | +/+ | 6.0 | trace | ++/0–1 | 0–1 | 0–2 hc 0–3 fgc | 0 |
| 231 | M | +/+ | 6.0 | trace | trace/0–1 | 0–1 | 0–1 fgc | 0 |
| 232 | M | +/+ | 6.0 | trace | 0/0–1 | 0–1 | >3 fgc | 0 |
| 233 | M | +/+ | 6.0 | trace | +/0 | — | — | — |
| 236 | F | −/− | 5.0 | trace | +++/0 | — | — | — |
| 239 | F | +/+ | 6.0 | trace | +/0–1 | 0–1 | 0–1 hc 0–3 fgc | 0–3 ec |
| 244 | M | −/− | 5.0 | trace | 0/0 | 0–2 | 0–1 fgc | 0 |
| 245 | M | −/− | 5.0 | trace | 0/0–2 | 0–2 | 0–2 fgc | 0 |
| 250 | F | −/− | 5.0 | trace | +++/>5 | 0–3 | 0–2 fgc | 0–3 squ >5 rc |
| 252 | F | −/− | 5.0 | 0 | 0/0–5 | 0–5 | 0–2 fgc | 0–1squ 0–3 rc |
| 253 | F | −/− | 5.0 | 0 | 0/0 | 0 | 0 | 0–1 ec |
| 254 | F | −/− | 5.0 | trace | 0/0 | >5 | 0 | 0–3 ec |
| 255 | F | −/− | 5.0 | trace | 0/0 | 0–1 | 0–1 fgc | 0 |
| 257 | M | −/− | 5.0 | trace | 0/0–4 | 0–1 | 0–2 fgc | 0 |
| 259 | M | +/+ | 6.0 | trace | 0/0 | 0 | 0 | 0 |
| 261 | M | +/+ | 6.0 | trace | 0/0 | 0 | 0–1 fgc | 0 |
—, not evaluated; ec, epithelial cast; fgc, fine granular cast; hc, hyaline cast; rc, renal cell; squ, squamous cell
Histopathology.
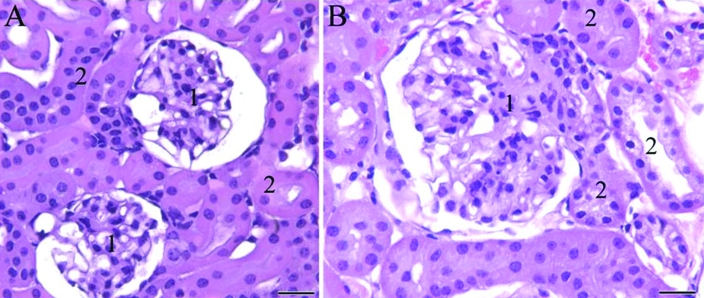
The most striking histologic finding was that of a mononuclear cell infiltrate in the lower urinary tract of mutant mice. The infiltrate preferentially involved female mice but was apparent in both sexes. The cells were found as single or cluster of cells in the lamina propria of the lower urinary tract and sometimes were within renal glomerular epithelium and tubules (Figure 3).
Figure 3.
Photomicrograph of kidney sections of female mice. (A) Wild-type mouse. (B) B6.129S6- Naglutm1Efn/J mutant mouse. Note the large, distended glomerulus (1) with increased mesangium as well as the cells containing vacuolar or granular cytoplasm within the glomerular epithelium and adjacent renal tubules (2) in (B) compared with the normal-appearing renal tissue of the wildtype mouse (A). Hematoxylin and eosin staining; scale bar, 50 μm.
Mild focal chronic interstitial renal inflammation was seen in some animals in each group independent of sex and genotype. One female wildtype mouse had a few dilated simple renal cysts. All male mice had moderate to major cytoplasmic vacuolization of tubular cells, without difference between wildtype and mutant animals. In contrast female mice showed very minor vacuolization that was present in 2 of 7 homozygotes but not in any wildtype mice. Cells with vacuolated and granular cytoplasm within glomerular epithelium and sometimes tubules were seen in some mutant mice of both sexes. However, these were single cells rather than a compact cell infiltrate, as seen in other tissues.
In most mice, the ureters were free of abnormalities. Some of the mutant mice had an isolated infiltrate, mostly single cells. In 3 (2 of which were mutants) of the 12 male mice evaluated, mild to moderate lymphoid cuffing of the ureter was revealed; a single female mutant mouse exhibited the same abnormality.
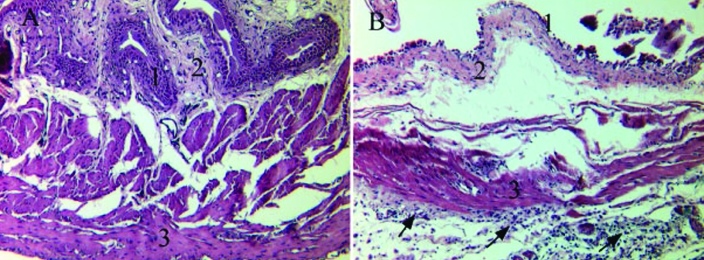
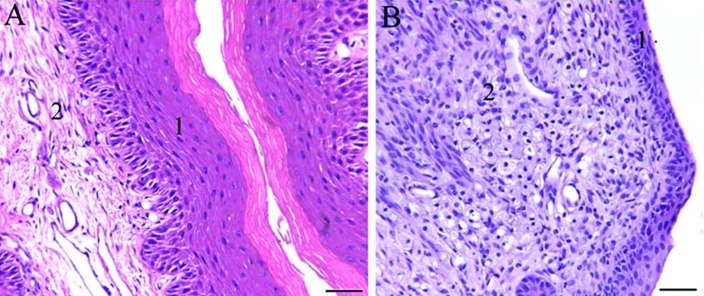
The bladder was the most affected organ, insofar that it exhibited the most obvious anomalies in mutant mice. One female wildtype mouse presented with focal submucosal fibrosis. Otherwise the urinary bladder was mainly normal in wildtype mice of both sexes, whereas it was attenuated in all mutant mice, more severely in male mice but also moderately in female animals. Changes in the bladder wall included submucosal edema and congestion, mild inflammation, expansion and thinning of the lamina propria by edema, and infiltration of single cells (Figure 4). The mononuclear cell infiltrate was less prominent in the trigone area than in other areas of the bladder.
Figure 4.
Urinary bladder at the trigone area of female mice. (A) Wild-type mouse. (B) B6.129S6- Naglutm1Efn/J mutant mouse. 1, Mucosa; 2, lamina propria; 3, muscularis (detrusor muscle). Note the mononuclear infiltrate composed of single foamy granular cells (arrows), the loss of normal architecture of the bladder wall, submucosal edema, and denuded mucosa in (B) compared with the normal morphology of the urinary bladder of the wildtype mouse (A). Hematoxylin and eosin staining; scale bar, 100 μm.
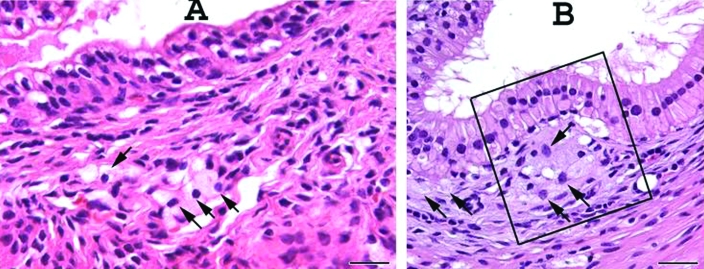
No abnormalities were discovered in the urethral tissues of wildtype mice. In contrast, mutant mice had moderate to severe mononuclear cell infiltrates in the urethra. In both sexes, the infiltrate appeared denser in more proximal areas of the urethra. In female mice, submucosal mononuclear infiltrate was abundant in the stroma surrounding the urethra (Figure 5), which led to distension of the glands of the endocervix due to compression of the ducts by the foamy cells. In male mice, the mononuclear cell infiltrate was largely in the lamina propria of the lower urinary tract, prostate, and seminal vesicles (Figure 6).
Figure 5.
Proximal urethral–vaginal submucosa. (A) Wildtype mouse. (B) B6.129S6- Naglutm1Efn/J mutant mouse. 1, Squamous epithelium of the vagina; 2, submucosa. Note the abundant amount of submucosal mononuclear infiltrate composed of large foamy granular cells which attenuate the squamous mucosa in (B) compared with the normal histology in (A). Hematoxylin and eosin staining; scale bar, 100 μm.
Figure 6.
Urogenital tract structures in prostatic region of a B6.129S6- Naglutm1Efn/J mutant mouse. (A) Subtle mononuclear cell infiltrate (arrows) in the lamina propria of an intraprostatic duct. (B) Subepithelial accumulation of mononuclear cell infiltrate (arrows), which elevates the epithelium toward the lumen of the ductus deferens (boxed area). Note that the infiltrate within the lamina propria is less prominent than that in the urogenital structures of female mutant mice (Figure 5). Male mice had only single granular cells or small clusters of granular cells compared with the abundant infiltrate in female mice. Scale bar, 25 µm.
In 2 female mutant mice the uterus was enlarged. Genital tissue was collected for histopathology, which revealed the same mononuclear cell infiltrate in the uterine and fallopian tube walls and ovaries as was present in the urinary tract.
Multiple stains, immunohistochemistry, and electron microscopy were performed to differentiate the infiltrate further. Staining with Alcian blue at pH 1.0 and 2.5 and murine antibody CD68 and the immunoperoxidase reaction for S100 protein yielded negative results. In addition, the cells did not stain for periodic acid Schiff either with or without diastase.
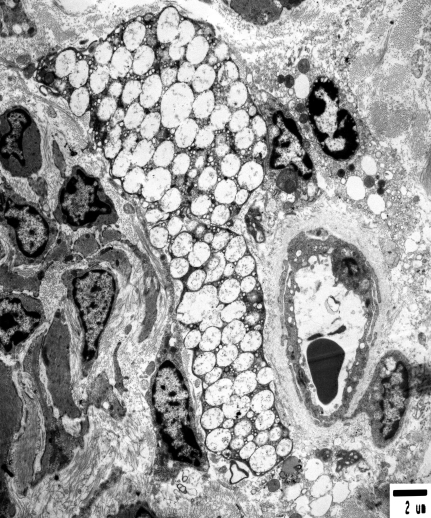
Electron microscopy revealed aggregates of electron-lucent vacuoles of varying size in numerous cells. These aggregates typically replaced much of the cytoplasm, such as in fibroblasts. In other cells, the aggregates are smaller and restricted to focal areas (for example, in endothelial and smooth muscle cells). Most of these vacuoles were delimited from the cytoplasm by membranes, but no separation between coalescing vacuoles was noted (Figure 7). The enlarged lysosomes with curved lamellar lipid leaflets or fluffy material that are typical of Sanfilippo inclusions were not evident.
Figure 7.
Electron microscopy of vaginal tissue of a B6.129S6- Naglutm1Efn/J mutant mouse. Note the fibroblast-like cell filled with electron-lucent vacuoles in the center of the picture. Scale bar, 2 µm.
Discussion
Urinary retention eventually leading to a cascade of events such as hydronephrosis, chronic renal failure, and uremia is a serious limitation of the murine model of MPS IIIB and often necessitates early termination of experiments.12 Furthermore, urinary retention is not a feature of the disease in humans, making the mouse strain B6.129S6- Naglutm1Efn a less than ideal model. The present study therefore was designed to elucidate the cause for the retention of urine and subsequent distention of the urinary bladder in these mutant mice. This clarification is important not only in regard to expectations when using the model but also to the animal welfare aspect of establishing humane endpoints. Our experience of high morbidity and mortality among mice older than 9 mo has been substantiated by others.5,20 One of these studies5 reported that Naglu mice usually died at 7 to 10 mo of age with a distended bladder among other pathologies.
The normal daily amount of urine produced by a mouse is 0.5 to 1 mL, with only 1 to 2 drops excreted at a time.13 A recent study9 reported that the void urine volume of C57Bl/6 mice was 143 ± 28.0 µL in female mice and 140 ± 23.0 µL in male mice. In the present study, urine volume at necropsy in mutant mice was 1.15 to 1.9 mL in male mice and 0.45 to 1.3 mL in female mice. Even allowing for residual volumes, the bladder capacity was significantly greater in mutant mice, suggesting urine retention.
Urine retention is caused by either bladder dysfunction, which can be neurogenic or myogenic, or inappropriate outlet resistance due to physical or functional obstruction.18 To investigate, we evaluated several diagnostic parameters for obstructive uropathy including packed cell volume, peripheral blood neutrophil count, blood urea nitrogen, serum creatinine, hematuria, pyruria, bacteruria, crystalluria, and increased urinary protein.2 These measures were either within normal limits or if a value was beyond the reference range, was not sufficiently abnormal to account for the urine retention in the Naglu mice.7,22,30
Histopathologic assessment comprised the entire urinary tract including accessory sex glands of both sexes in wildtype and mutant mice. Although all animals showed mild focal chronic interstitial inflammation independent of group, sex, and genotype, the typical mononuclear cell infiltrate was present only in both sexes of mutant mice. To our knowledge, no detailed descriptions of this type of infiltrate or this pattern of distribution throughout the genitourinary tract of mice of both sexes are available. One study20 described the phenotype of this strain of mice and reported vacuolation in many cells including macrophages, epithelial cells, and neurons, in which the vacuoles contained fine granular material characteristic of glycosaminoglycan accumulation. We were unable to confirm this result by electron microscopy or specific staining techniques such as Alcian blue and CD68 antibody, a macrophage marker. Alcian blue is a specific staining technique to demonstrate sulfated mucosubstances8,29 and commonly is used to stain heparan sulfate, the substance accumulated in MPS IIIB. In addition, tubular and glomerular cells of the kidney are the first epithelial cells to show vacuolation and fine granular deposits in the vacuole.20 In the present study, however, kidney tissue was less severely affected than were tissues of the lower urinary tract. The moderate to severe cytoplasmic vacuolation of tubular cells seen in both wildtype and mutant male mice is more consistent with reports of tubular vacuolation that were more numerous and larger in male mice21 or noted only in male mice.28 This finding is considered a feature unique to the renal histology of mice rather than an abnormality. The histiomorphology of the infiltrate appears comparable to a benign neoplasm, a granular cell tumor. This neoplasm is fairly common in humans33 and has been described as incidental finding in female mice.10 However, PAS and S100 stains, which were both negative in the present study, failed to confirm this finding. In particular, S100 protein is a stable antigen that can be localized in the cells of granular cell tumors,27 and PAS is commonly used to demonstrate polysaccharides.8
We noted bladder distension in both male and female mutant mice, and distension was more severe in male than in female mice. During tissue sampling, we paid particular attention to avoid inadequate distension or over inflation of the bladder, which can lead to artifacts and misinterpretation as edema, emphysema, and cysts in the bladder wall.11 These artifacts were especially important to exclude because attenuated bladder walls included, besides deposition of single cells of infiltrate mild inflammation, submucosal edema and congestion leading to expansion and thinning of the lamina propria. This finding, however, indicates a secondary condition, whereas the primary problem was a mononuclear cell infiltrate that was present throughout the entire urinary tract but most pronounced in the lower part. Clusters of foamy cells collected particularly in the lamina propria of the lower urinary tract, that is, the soft tissue surrounding the urethra including adjacent structures such as prostate in male mice and glands of the endocervix in female mice. This effect might contribute to compression and extramural mechanical obstruction of the urethra, although hematologic, serum, and urine markers of obstructive uropathy were unspecific, and the few changes present were independent of genotype. One possible explanation is that the criteria we chose for interpreting urinary tract disease in mice were extrapolated from veterinary textbooks22 that pertain to larger mammalian species, because reference values for mice are sparse or unavailable. This lack complicates interpretation of mouse diagnostic samples. Another possible mechanism of urinary retention is that the mononuclear cell infiltrate may interfere with the neuromuscular function of the bladder sphincter, although foamy cells were not detected within either the smooth muscle layer of the bladder or in the ganglion cells. The results suggest that urinary retention does not relate to the background C57BL/6 strain, because bladder volume and histopathology were normal in wildtype mice.12 The mononuclear cell infiltrate was found only in mutant mice.
Histopathologic examinations of urinary tract specimens yielded an interesting finding in mutant mice of the strain B6.129S6- Naglutm1Efn/J. Both male and female mutant mice demonstrated mononuclear cell infiltrates comprising large rounded or polygonal cells with generous variably vacuolated, granular eosinophilic cytoplasm and small round vesicular nuclei. These cells were present throughout the interstitium of the lower urinary tract. This space-occupying lesion results in either extrinsic compression of the lumen of the urethra, eventually leading to obstructive uropathy, bladder hyperdistension, and urinary retention or may interfere with the neurogenic component of micturition. Further investigation is warranted. Although this study was unable to confirm a causative relationship between the infiltrate and the urinary retention, the novel discovery of a mononuclear cell infiltrate in this knockout mouse strain remains an important finding.
Acknowledgments
This work was supported by The Children's Medical Research Foundation. We especially want to thank Susan and Mr Bradford Wilson for their marvelous support. We are grateful for the invaluable services and support by the staff of the Division of Comparative Medicine at the University of South Florida.
References
- 1.Barsanti JA. 1993. Urinary incontinence. : Lorenz MD, Cornelius LM, Small animal medical diagnosis. 2nd ed. Philadelphia (PA): JB Lippincott Company [Google Scholar]
- 2.Barsanti JA. 1993. Urine analysis. : Lorenz MD, Cornelius LM, Small animal medical diagnosis. 2nd ed. Philadelphia (PA): JB Lippincott Company [Google Scholar]
- 3.Beesley CE, Young EP, Vellodi A, Winchester BG. 1998. Identification of 12 novel mutations in the α-N-acetylglucosaminidase gene in 14 patients with Sanfilippo syndrome type B (mucopolysaccharidosis type IIIB). J Med Genet 35:910–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya A, Dang H, Zhu QM, Schnegelsberg B, Rozengurt N, Cain G, Prantil R, Vorp DA, Guy N, Julius D, Ford AP, Lester HA, Cockayne DA. 2004. Uropathic observations in mice expressing a constitutively active point mutation in the 5HT3A receptor subunit. J Neurosci 24:5537–5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhaumik M, Muller VJ, Rozaklis T, Johnson L, Dobrenis K, Bhattacharyya R, Wurzelmann S, Finamore P, Hopwood JJ, Walkley SU, Stanley P. 1999. A mouse model for mucopolysaccharidosis type III A (Sanfilippo syndrome). Glycobiology 9:1389–1396 [DOI] [PubMed] [Google Scholar]
- 6.Boczko J, Tar M, Melman A, Jelicks LA, Wittner M, Factor SM, Zhao D, Hafron J, Weiss LM, Tanowitz HB, Christ GJ. 2005. Trypanosoma cruzi infection-induced changes in the innervation, structure, and function of the murine bladder. J Urol 173:1784–1788 [DOI] [PubMed] [Google Scholar]
- 7.Brayton C. 2006. Spontaneous diseases in commonly used mouse strains. : Fox JG, Barthold S, Davisson M, Newcomer C, Quimby F, Smith A, The mouse in biomedical research, 2nd ed. New York (NY): Academic Press [Google Scholar]
- 8.Carson F. 1997. Histotechnology: a self-instructional text. Chicago (IL): American Society of Clinical Pathologists Press [Google Scholar]
- 9.Cornelissen LL, Misajet B, Brooks DP, Hicks A. 2008. Influence of genetic background and gender on bladder function in the mouse. Auton Neurosci 140:53–58 [DOI] [PubMed] [Google Scholar]
- 10.Davis BJ, Dixon D, Herbert RA. 1999. Ovary, oviduct, uterus, cervix, and vagina. : Maronpot RR, Boorman GA, Gaul BW, Pathology of the mouse. Vienna (IL): Cache River Press [Google Scholar]
- 11.Gaillard ET. 1999. Ureter, urinary bladder, and urethra. : Maronpot RR, Boorman GA, Gaul BW, Pathology of the mouse. Vienna (IL): Cache River Press [Google Scholar]
- 12.Gografe SI, Garbuzova-Davis S, Willing AE, Haas K, Chamizo W, Sanberg PR. 2003. Mouse model of Sanfilippo syndrome type B: relation of phenotypic features to background strain. Comp Med 53:622–632 [PubMed] [Google Scholar]
- 13.Hoyt RF, Jr, Hawkins JV, St Clair MB, Kennett MB. 2006. Mouse physiology. : Fox JG, Barthold S, Davisson M, Newcomer C, Quimby F, Smith A, The mouse in biomedical research, 2nd ed. New York (NY): Academic Press [Google Scholar]
- 14.Institute of Laboratory Animal Resources (US) 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press; [DOI] [PubMed] [Google Scholar]
- 15.Jacoby RO, Fox JG, Davisson M. 2002. Biology and diseases of mice, p 35–120 In: Fox JG, Anderson LC, Loew FM, Quimby FW, editors Laboratory animal medicine, 2nd ed. New York (NY): Academic Press [Google Scholar]
- 16.Kiss S, Yoshiyama M, Cao YQ, Basbaum AI, de Groat WC, Lecci A, Maggi CA, Birder LA. 2001. Impaired response to chemical irritation of the urinary tract in mice with disruption of the preprotachykinin gene. Neurosci Lett 313:57–60 [DOI] [PubMed] [Google Scholar]
- 17.Kuroda H, Kohrogi T, Uchida N, Imai I, Terada N, Matsumoto K, Kitamura Y. 1985. Urinary retention induced by estrogen injections in mice: an analytical model. J Urol 134:1268–1270 [DOI] [PubMed] [Google Scholar]
- 18.Lane IF. 2005. Urinary obstruction and functional urine retention. : Ettinger SJ, Feldman EC, Textbook of veterinary internal medicine. 5th ed. Philadelphia (PA): WB Saunders [Google Scholar]
- 19.Lees GS. 2005. Incontinence, enuresis, dysuria, and nocturia. : Ettinger SJ, Feldman EC, Textbook of veterinary internal medicine. 5th ed. Philadelphia (PA): WB Saunders [Google Scholar]
- 20.Li HH, Yu WH, Rozengurt N, Zhao HZ, Lyons KM, Anagnostaras S, Fanselow MS, Suzuki K, Vanier MT, Neufeld EF. 1999. Mouse model of Sanfilippo syndrome type B produced by targeted disruption of the gene encoding α-N-acetylglucosaminidase. Proc Natl Acad Sci USA 96: 14505–14510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liebelt AG. 1998. Unique features of anantomy, histology, and ultrastructure, kidney, mouse, p 37–57 : Jones TC, Hard GC, Mohr U, Monographs on pathology of laboratory animals. Urinary system, 2nd ed. Berlin (Germany): Springer [Google Scholar]
- 22.Lorenz MD. 1993. Abnormal micturition: dysuria, pollakiuria, and stranguria. : Lorenz MD, Cornelius LM, Small animal medical diagnosis. 2nd ed. Philadelphia (PA): JB Lippincott Company [Google Scholar]
- 23.Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi S, Manabe T, Taketo MM. 2002. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci 22:10627–10632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muenzer J. 2000. Mucopolysaccharidoses. : Behrman R, editor Nelson textbook of pediatrics. 16th ed. Philadelphia (PA): WB Saunders [Google Scholar]
- 25.Mumtaz MM, Farooqui MY, Ghanayem BI, Rajaraman S, Frankenberg L, Ahmed AE. 1991. Studies on the mechanism of urotoxic effects of N,N′-dimethylaminopropionitrile in rats and mice. 1. Biochemical and morphologic characterization of the injury and its relationship to metabolism. J Toxicol Environ Health 33:1–17 [DOI] [PubMed] [Google Scholar]
- 26.Murawski IJ, Gupta IR. 2006. Vesicoureteric reflux and renal malformations: a developmental problem. Clin Genet 69:105–117 [DOI] [PubMed] [Google Scholar]
- 27.Nadji M, Morales A. 1986. Immunoperoxidase techniques: a practical approach to tumor diagnosis. Chicago (IL): American Society of Clinical Pathologists Press [Google Scholar]
- 28.Percy DH, Barthold SW. 2001. Pathology of laboratory rodents and rabbits. Ames (IA): Iowa State University Press [Google Scholar]
- 29.Schumacher U, Adam E. 1994. Standardization of staining in glycosaminoglycan histochemistry: alcian blue, its analogues, and diamine methods. Biotech Histochem 69:18–24 [DOI] [PubMed] [Google Scholar]
- 30.Sodeman WA, Sodeman TM. 1985. Sodeman's pathologic physiology: mechanisms of disease. Philadelphia (PA): WB Saunders [Google Scholar]
- 31.Suckow MA, Danneman P, Brayton C. 2001. The laboratory mouse. Boca Raton (FL): CRC Press [Google Scholar]
- 32.Webster RG, Brain KL, Wilson RH, Grem JL, Vincent A. 2005. Oxaliplatin induces hyperexcitability at motor and autonomic neuromuscular junctions through effects on voltage-gated sodium channels. Br J Pharmacol 146:1027–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss S, Goldblum J. 2008. Enzinger and Weiss's soft tissue tumors. Philadelphia (PA): Mosby Elsevier [Google Scholar]