Abstract
The simian parvoviruses (SPVs) are in the genus Erythrovirus in the family Parvoviridae and are most closely related to the human virus B19. SPV has been identified in cynomolgus, rhesus, and pigtailed macaques. All of the primate erythroviruses have a predilection for erythroid precursors. Infection, which is common in macaques, is usually clinically silent. Disease from SPV is associated with immunosuppression due to infection with various retroviruses (SIV, simian retrovirus, and simian–human immunodeficiency virus), surgery, drug toxicity studies, and posttransplantation immunosuppressive treatment and therefore is of concern in studies that use parvovirus-positive macaques.
Abbreviations: SHIV, chimeric simian–human immunodeficiency virus; SPV, simian parvovirus; SRV, type D simian retrovirus; SIV, simian immunodeficiency virus
In 1959, a small, nonenveloped virus (rat virus) was isolated from rat tissue cultures.21 Over the next decade, similar viruses were identified as the etiologic agents of severe enteritis in cats and mink and of facial abnormalities in newborn hamsters.44,45 The nonenveloped viruses are among the smallest viruses that infect mammalian cells, with a genome size of approximately 5 kb. They are named parvoviruses, from the Latin parvum, meaning small. The virion has icosahedral symmetry, is composed of 2 or 3 structural proteins (VP1, VP2, and sometimes VP3), and contains a linear single-stranded DNA genome. The viral DNA also encodes 1 or 2 nonstructural proteins (NS1 and NS2).
Currently the family Parvoviridae is divided into 2 subfamilies, the Parvovirinae, which infect vertebrates, and the Densovirinae, invertebrates (Table 1). The Parvovirinae are further divided into 5 genera: Parvovirus, which contains the rodent, feline, canine, and avian parvoviruses; Dependovirus, the adeno-associated viruses; Erythrovirus, the human parvovirus B19 and related viruses; Bocavirus, human and other bocaviruses; and Amdovirus, Aleutian mink disease virus.2 Although many parvoviruses are associated with severe disease, others cause inapparent infections. The adeno-associated viruses are replication-defective parvoviruses, identified as contaminants of adenovirus stocks, and require coinfection with adenovirus or herpesvirus.
Table 1.
Parvovirus taxonomy
| Family | Subfamily | Genus | Type species | Hosts |
| Parvoviridae | Parvovirinae | Parvovirus | Minute virus of mice | Vertebrates |
| Erythrovirus | B19 virus | |||
| Dependovirus | Adeno-associated virus 2 | |||
| Amdovirus | Aleutian mink disease virus | |||
| Bocavirus | Bovine parvovirus | |||
| Densovirinae | Densovirus | Junonia coenia densovirus | Invertebrates | |
| Iteravirus | Bombyx mori densovirus | |||
| Brevidensovirus | Aedes aegypti densovirus | |||
| Pefudensovirus | Periplanta fuliginosa densovirus |
A key feature of replication-competent, autonomous, parvoviruses is the requirement for mitotically active host cells for viral replication. Parvoviruses require a host cell to go through S phase to replicate and lack the ability to initiate host DNA synthesis in resting cells.34 The autonomous parvoviruses are fairly species-specific, although some will grow in cultured cells from other species. In addition, transformation of ordinarily nonpermissive cells can render them permissive for productive infection by rodent parvoviruses.40 However, parvoviruses vary markedly in host range and pathogenicity, as determined primarily by the capsid proteins.10 The most severe clinical effects tend to occur in fetal and newborn animals, including in utero death and congenital lesions.4 In older animals, clinical signs are due to lytic viral replication in target tissues and to the subsequent immune response4.
B19, the Erythrovirus of Humans
In 1974,12 the human parvovirus B19 was discovered fortuitously in serum. The B19 genome encodes only 3 proteins of known function: the 2 structural proteins VP1 and VP2 and the nonstructural protein NS1, which has roles in transcriptional activation and cytotoxicity.32
Although no disease was associated with B19 until the 1980s, syndromes of anemia, erythema infectiosum (Fifth disease), arthropathy, and fetal loss are now recognized as being caused by B19.4,19,48 Clinical disease from B19 infection is determined by the hematologic and immunologic status of the host, and primary infections in immunocompetent hosts may be inapparent or have mild nonspecific symptoms. In studies of human infection with B19, rates of infection in the United States, Europe, and Asia are similar, although some remote populations, such as isolated Amazonian tribes, or residents of remote islands, appear to have escaped infection.48 Antibody prevalence gradually decreases with age, varying from 2% to 15% in children 1 to 5 y old, to greater than 85% in persons older than 70 y.2,11,19 Although antibody is prevalent, viremia is rare. Serology, electron microscopy, or PCR analysis can be used to diagnose infection. Transmission occurs through the respiratory tract, by transfusion of blood or blood products, and vertically in utero. Virus can be found in the nasopharynx in experimentally infected humans, followed by viremia, which peaks at 1 to 2 wk after infection.3 Production of antibodies to B19 correlates with the waning of viremia, and persistent infection occurs when antibody production is insufficient or absent, which can be due to immunocompromise for any of a number of reasons.48 Persistent, possibly latent, B19 infection has been recognized in immunocompetent persons.9,23,30,39
B19 has a strong predilection for erythroid precursors, and the subsequent destruction of these cells accounts for the anemias associated with B19 infection and for the name of the genus of which B19 is the prototype, Erythrovirus. The bone marrow in infected patients has decreased numbers of erythroid precursors, along with giant pronormoblasts with intranuclear inclusions and cytoplasmic vacuolization.20,48 Viral particles in the nuclei of infected cells can be demonstrated by using electron microscopy. Globoside, also known as the erythrocyte P antigen, is an important cellular receptor for B19 on erythroid cells.5 Globoside is also present in the placenta and fetal myocardium, some megakaryocytes, and endothelial cells, thus accounting for some of the additional clinical effects of B19. α5β1integrin is a coreceptor that is present on erythroid progenitors and appears to be required for viral entry into the cell.47 Therefore the entry of B19 into erythroid cells requires viral binding to globoside on the host cell surface and then entry mediated by activated β1 integrins. Because mature red blood cells do not carry α5β1integrin, B19 can bind to, but not enter, erythrocytes.47 The autoantigen Ku80 has been implicated as an additional coreceptor for B19, especially with regard to infection of nonerythroid cells.33
In additional to B19, other parvoviruses of humans include a bocavirus,1 identified in the respiratory tract, and PARV4 (and variant PARV5) in plasma.15-17 The bocavirus has been implicated in lower respiratory tract disease.29 PARV4 (and variant PARV5) are different enough from other mammalian parvoviruses that they may be classified as a new genus;15,17 no disease association has been made for these viruses, and they have been found mainly in pooled plasma and in tissues from HIV-infected persons.16,30
Simian Parvoviruses
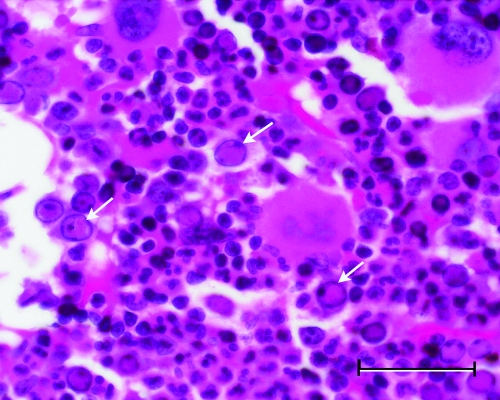
In 1992, severe anemia necessitating euthanasia was recognized in a group of cynomolgus macaques (Macaca fascicularis). Microscopic examination of bone marrow revealed decreased erythroid and myeloid lineages and, in some samples, abnormal erythroid cells with bizarre nuclear forms and intranuclear inclusions morphologically similar to those seen in B19 infection (Figure 1).35 Ultrastructural examination revealed intranuclear viral particles and an occasional viral array characteristic of parvoviruses. The clinically affected macaques had concurrent infection with the immunosuppressive type D simian retrovirus (SRV).22,26,31 Although only macaques dually infected with parvovirus and SRV became anemic,37 infection with SRV alone has been associated with severe anemias in rhesus macaques.28 A second group of anemic cynomolgus macaques with similar histologic characteristics was identified, although the association of anemia with dual infection of SRV and parvovirus was not as absolute.37 The second group of animals was on a safety evaluation study, which may have confounded the findings.
Figure 1.
Photomicrograph of bone marrow from an SIV-infected rhesus macaque. Arrows indicate intranuclear parvoviral inclusions in erythroid precursors (hematoxylin and eosin stain; bar, 50 μm).
Simian parvovirus (SPV) has been cloned and sequenced and was determined to have 50% overall homology with B19 and little sequence similarity to other parvoviruses. 6 Similar viruses have been identified in anemic rhesus (Macaca mulatta) and pigtailed (Macaca nemestrina) macaques experimentally infected with the chimeric immunodeficiency virus SHIV.14,18 Sequence analysis revealed more than 70% homology among the 3 nonhuman primate parvoviruses and 50% to 60% similarity to B19, and the primate parvoviruses are evolutionarily linked.27 Like B19, SPV encodes a single structural protein, NS1, and 2 capsid proteins, VP1 and VP2.6,25 Because the nonhuman primate parvoviruses also have a predilection for erythroid precursors and use globoside as a receptor,7 they have been added to the genus Erythrovirus.13
Experimental infection of cynomolgus macaques by intravenous or intranasal inoculation with SPV produced transient viremia, which waned after seroconversion, and clinically silent transient anemia and reticulocytopenia, with typical changes in bone marrow erythroid line cells.36 Although the macaques in the cited report were seropositive for SRV as well, no signs of immunosuppression were apparent during the SPV inoculation study. Experimental SPV infection in pregnant cynomolgus macaques resulted in hydrops fetalis and fetal death.38,46 Epidemiologic studies suggest that SPV transmission is usually horizontal, probably through the respiratory tract or by means of fomites, as for B19 infection in humans.19,37,41
Little has been published regarding the prevalence of SPV in macaques, but in one study,8 approximately 50% of cynomolgus macaques and 35% of rhesus macaques had antibodies against VP2. In another colony of rhesus macaques, use of a commercial human B19 enzyme immunoassay that uses VP1 as antigen identified fewer than 6% of animals younger than 4 y as positive; this rate increased to just greater than 19% of macaques 14 to 19 y old.24 In immunocompetent animals, primary infection typically is clinically silent; animals with anti-SPV antibody are resistant to reinfection.8
Since the original reports of parvoviruses in 3 species of macaques, parvovirus infection with associated anemia has been identified in cynomolgus macaques in conjunction with SRV,42 in an SHIV-infected pigtailed macaque,14 and in an SIV-infected rhesus macaque.43 SPV viremia was detected in 11 of 22 cynomolgus macaques that received heterotopic heart transplants;41 5 animals were euthanized because of severe anemia. Among 7 SPV-negative animals that received hearts from SPV-seropositive animals, 5 became viremic, 3 of which died with anemia. Further, 2 of the 3 that died also received bone marrow from the donor. The 2 animals that did not become viremic also did not seroconvert to SPV. Most of the recipients received some form of immunosuppressive therapy to prevent transplant rejection, and their SRV status was not determined, thereby complicating interpretation of this study. To summarize the work to date, disease from SPV is associated with immunosuppression due to infection with retroviruses (SRV, SIV, and SHIV) or as a sequela of surgery, use in drug toxicity studies, or immunosuppressive therapy to prevent transplant rejection.
Zoonotic Potential of Simian Parvoviruses
Do simian parvoviruses have zoonotic potential? In one study, SPV VP2 immunoblotting of sera from animal handlers appeared to indicate a correlation between exposure to SPV-positive macaques and the presence of SPV antibody.7 However, sera from humans with no known exposure to macaques were occasionally positive in the same immunoblot assay, so the possibility of crossreactivity to B19 has not been excluded. In addition, SPV can replicate, albeit not robustly, in human bone marrow in vitro.7 Therefore, although SPV may be able to infect humans, definitive studies have yet to be published.
Summary
The simian parvoviruses are closely related to the human erythrovirus B19. Infection is common in macaques, at an estimated rate of 20% to 50%. Primary infection is usually clinically silent, with perhaps a transient anemia and reticulocytopenia, due to the viruses' predilection for erythroid precursors. The epidemiology of SPV is not well established; extrapolation from what is known about B19 warrants concern for persistent or latent infections and possible recrudescence during immunosuppression.
Disease from SPV is associated with immunosuppression and therefore poses a concern in studies that use parvovirus-positive macaques. Although vertical transmission has been demonstrated experimentally, seroconversion increases gradually with age, such that continued testing and exclusion likely will yield parvovirus-negative animals. Serology indicates past exposure to SPV; subsequent PCR or culture analysis is needed to identify animals with active infection.
References
- 1.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA 102:12891–12896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson LJ, Tsou C, Parker RA, Chorba TL, Wulff H, Tattersall P, Mortimer PP. 1986. Detection of antibodies and antigens of human parvovirus B19 by enzyme-linked immunosorbent assay. J Clin Microbiol 24:522–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson MJ, Higgins PG, Davis LR, Willman JS, Jones SE, Kidd IM, Pattison JR, Tyrrell DA. 1985. Experimental parvoviral infection in humans. J Infect Dis 152:257–265 [DOI] [PubMed] [Google Scholar]
- 4.Bloom ME, Young NS. 2001. Parvoviruses. Knipe DM, Howley PM. Fields virology. Philadelphia: Lippincott Williams and Wilkins; p 2361–2379 [Google Scholar]
- 5.Brown KE, Anderson SM, Young NS. 1993. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science 262:114–117 [DOI] [PubMed] [Google Scholar]
- 6.Brown KE, Green SW, O'Sullivan MG, Young NS. 1995. Cloning and sequencing of the simian parvovirus genome. Virology 210:314–322 [DOI] [PubMed] [Google Scholar]
- 7.Brown KE, Liu Z, Gallinella G, Wong S, Mills IP, O'Sullivan MG. 2004. Simian parvovirus infection: a potential zoonosis. J Infect Dis 190:1900–1907 [DOI] [PubMed] [Google Scholar]
- 8.Brown KE, Young NS. 1997. The simian parvoviruses. Rev Med Virol 7:211–218 [DOI] [PubMed] [Google Scholar]
- 9.Candotti D, Etiz N, Parsyan A, Allain JP. 2004. Identification and characterization of persistent human erythrovirus infection in blood donor samples. J Virol 78:12169–12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman MS, Rossmann MG. 1993. Structure, sequence, and function correlations among parvoviruses. Virology 194:491–508 [DOI] [PubMed] [Google Scholar]
- 11.Cohen BJ, Buckley MM. 1988. The prevalence of antibody to human parvovirus B19 in England and Wales. J Med Microbiol 25:151–153 [DOI] [PubMed] [Google Scholar]
- 12.Cossart YE, Field AM, Cant B, Widdows D. 1975. Parvovirus-like particles in human sera. Lancet 305:72–73 [DOI] [PubMed] [Google Scholar]
- 13.Fauguet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA. 2005. Virus taxonomy, VIIIth report of the ICTV. London: Elsevier Academic Press [Google Scholar]
- 14.Foresman L, Narayan O, Pinson D. 1999. Progressive anemia in a pig-tail macaque with AIDS. Contemp Top Lab Anim Sci 38:20–22 [PubMed] [Google Scholar]
- 15.Fryer JF, Delwart E, Bernardin F, Tuke PW, Lukashov VV, Baylis SA. 2007. Analysis of two human parvovirus PARV4 genotypes identified in human plasma for fractionation. J Gen Virol 88:2162–2167 [DOI] [PubMed] [Google Scholar]
- 16.Fryer JF, Delwart E, Hecht FM, Bernardin F, Jones MS, Shah N, Baylis SA. 2007. Frequent detection of the parvoviruses, PARV4 and PARV5, in plasma from blood donors and symptomatic individuals. Transfusion 47:1054–1061 [DOI] [PubMed] [Google Scholar]
- 17.Fryer JF, Kapoor A, Minor PD, Delwart E, Baylis SA. 2006. Novel parvovirus and related variant in human plasma. Emerg Infect Dis 12:151–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green SW, Malkovska I, O'Sullivan MG, Brown KE. 2000. Rhesus and pig-tailed macaque parvoviruses: identification of two new members of the erythrovirus genus in monkeys. Virology 269:105–112 [DOI] [PubMed] [Google Scholar]
- 19.Heegaard ED, Brown KE. 2002. Human parvovirus B19. Clin Microbiol Rev 15:485–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ICTVdB—The Universal Virus Database [Internet] Version 4 New York: Columbia University; 2002 [updated 2007 July 17; cited 2006]. Available from: http://www.ncbi.nlm.nih.gov/ICTVdb/ [Google Scholar]
- 21.Kilham L, Olivier LJ. 1959. A latent virus of rats isolated in tissue culture. Virology 7:428–437 [DOI] [PubMed] [Google Scholar]
- 22.Kwang HS, Pedersen NC, Lerche NW, Osborn KG, Marx PA, Gardner MB. 1987. Viremia, antigenemia, and serum antibodies in rhesus macaques infected with simian retrovirus type 1 and their relationship to disease course. Lab Invest 56:591–597 [PubMed] [Google Scholar]
- 23.Lefrere JJ, Servant-Delmas A, Candotti D, Mariotti M, Thomas I, Brossard Y, Lefrere F, Girot R, Allain JP, Laperche S. 2005. Persistent B19 infection in immunocompetent individuals: implications for transfusion safety. Blood 106:2890–2895 [DOI] [PubMed] [Google Scholar]
- 24.Lerche NW.2006. Personal communication.
- 25.Liu Z, Qiu J, Cheng F, Chu Y, Yoto Y, O'Sullivan MG, Brown KE, Pintel DJ. 2004. Comparison of the transcription profile of simian parvovirus with that of the human erythrovirus B19 reveals a number of unique features. J Virol 78:12929–12939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowenstine LJ. 1993. Type D retrovirus infection, macaques. Jones TC, Mohr U, Hunt RD. Nonhuman primates I. Washington (DC): Springer–Verlag; p 20–32 [Google Scholar]
- 27.Lukashov VV, Goudsmit J. 2001. Evolutionary relationships among parvoviruses: virus-host coevolution among autonomous primate parvoviruses and links between adeno-associated and avian parvoviruses. J Virol 75:2729–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacKenzie M, Lowenstine L, Lalchandani R, Lerche N, Osborn K, Spinner A, Bleviss M, Hendrickson R, Gardner M. 1986. Hematologic abnormalities in simian acquired immune deficiency syndrome. Lab Anim Sci 36:14–19 [PubMed] [Google Scholar]
- 29.Manning A, Russell V, Eastick K, Leadbetter GH, Hallam N, Templeton K, Simmonds P. 2006. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J Infect Dis 194:1283–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manning A, Willey SJ, Bell JE, Simmonds P. 2007. Comparison of tissue distribution, persistence, and molecular epidemiology of parvovirus B19 and novel human parvoviruses PARV4 and human bocavirus. J Infect Dis 195:1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maul DH, Lerche NW, Osborn KG, Marx PA, Zaiss C, Spinner A, Kluge JD, MacKenzie MR, Lowenstine LJ, Bryant ML, Blakeslee JR, Henrickson RV, Gardner MB. 1986. Pathogenesis of simian AIDS in rhesus macaques inoculated with the SRV1 strain of type D retrovirus. Am J Vet Res 47:863–868 [PubMed] [Google Scholar]
- 32.Moffatt S, Yaegashi N, Tada K, Tanaka N, Sugamura K. 1998. Human parvovirus B19 nonstructural (NS1) protein induces apoptosis in erythroid lineage cells. J Virol 72:3018–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munakata Y, Saito-Ito T, Kumura-Ishii K, Huang J, Kodera T, Ishii T, Hirabayashi Y, Koyanagi Y, Sasaki T. 2005. Ku80 autoantigen as a cellular coreceptor for human parvovirus B19 infection. Blood 106:3449–3456 [DOI] [PubMed] [Google Scholar]
- 34.Muzyczka N, Berns KI. 2001. Parvoviridae: the viruses and their replication. Knipe DM, Howley PM. Fields virology. Philadelphia: Lippincott Williams and Wilkins; p 2327–2359 [Google Scholar]
- 35.O'Sullivan MG, Anderson DC, Fikes JD, Bain FT, Carlson CS, Green SW, Young NS, Brown KE. 1994. Identification of a novel simian parvovirus in cynomolgus monkeys with severe anemia. J Clin Invest 93:1571–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Sullivan MG, Anderson DK, Goodrich JA, Tulli H, Green SW, Young NS, Brown KE. 1997. Experimental infection of cynomolgus monkeys with simian parvovirus. J Virol 71:4517–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Sullivan MG, Anderson DK, Lund JE, Brown WP, Green SW, Young NS, Brown KE. 1996. Clinical and epidemiological features of simian parvovirus infection in cynomolgus macaques with severe anemia. Lab Anim Sci 46:291–297 [PubMed] [Google Scholar]
- 38.O'Sullivan MG, Veille JC, Block WA, Turner AR, Young NS, Brown KE. Hydrops fetalis induced by simian parvovirus. Presented at the 17th Annual Meeting of the American Society for Virology; 2004 July 11-15; Vancouver, British Columbia, Canada [Google Scholar]
- 39.Parsyan A, Candotti D. 2007. Human erythrovirus B19 and blood transfusion—an update. Transfus Med 17:263–278 [DOI] [PubMed] [Google Scholar]
- 40.Rommelaere J, Cornelis JJ. 1991. Antineoplastic activity of parvoviruses. J Virol Methods 33:233–251 [DOI] [PubMed] [Google Scholar]
- 41.Schroder C, Pfeiffer S, Wu G, Azimzadeh A, Aber A, Pierson RN, O'Sullivan MG. 2006. Simian parvovirus infection in cynomolgus monkey heart transplant recipients causes death related to severe anemia. Transplantation 81:1165–1170 [DOI] [PubMed] [Google Scholar]
- 42.Schroder MA, Fisk SK, Lerche NW. 2006. Eradication of simian retrovirus type D from a colony of cynomolgus, rhesus, and stump-tailed macaques by using serial testing and removal. Contemp Top Lab Anim Sci 39:16–23 [PubMed] [Google Scholar]
- 43.Simon MA. 1999 Unpublished observations. [Google Scholar]
- 44.Toolan HW. 1960. Experimental production of mongoloid hamsters. Science 131:1446–1448 [DOI] [PubMed] [Google Scholar]
- 45.Toolan HW, Dalldore G, Barclay M, Chandra S, Moore AE. 1960. An unidentified, filtrable agent isolated from transplanted human tumors. Proc Natl Acad Sci USA 46:1256–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vashisht K, Faaberg KS, Aber AL, Brown KE, O'Sullivan MG. 2004. Splice junction map of simian parvovirus transcripts. J Virol 78:10911–10919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weigel-Kelley KA, Yoder MC, Srivastava A. 2003. α5β1 integrin as a cellular coreceptor for human parvovirus B19: requirement of functional activation of β1 integrin for viral entry. Blood 102:3927–3933 [DOI] [PubMed] [Google Scholar]
- 48.Young NS, Brown KE. 2004. Parvovirus B19. N Engl J Med 350:586–597 [DOI] [PubMed] [Google Scholar]