Abstract
With the emergence of the AIDS epidemic over the last 2 decades and the more recent identification of Kaposi sarcoma-associated herpesvirus (KSHV, Human herpesvirus 8), the genera of rhadinoviruses have gained importance as a family of viruses with oncogenic potential. First recognized in New World primates more than 30 y ago, the rhadinoviruses Saimiriine herpesvirus 2 and Ateline herpesvirus 2 have well-described transforming capabilities. Recently several new species-specific rhadinoviruses of Old World primates have been described, including retroperitoneal fibromatosis herpesvirus and rhesus rhadinovirus (Cercopithecine herpesvirus 17). Molecular analysis of these viruses has elucidated several functionally conserved genes and properties shared with KSHV involved in cellular proliferation, transformation, and immune evasion that facilitate the oncogenic potential of these viruses. This review examines the comparative pathobiology of KSHV, discusses the role of macaque rhadinoviruses as models of human disease, and outlines the derivation of specific pathogen-free animals.
Abbreviations: CCL, cellular chemokine ligand; IRF, interferon regulatory factors; KSHV, Kaposi sarcoma-associated herpesvirus; LANA, latent nuclear antigen; MCD, multicentric Castleman disease; MCP1, monocyte chemotactic protein 1; miRNA, microRNA; ORF, open reading frame; PEL, primary effusion lymphoma; RFHV, retroperitoneal fibromatosis herpesvirus; RVV, rhesus rhadinovirus; SaHV2, Saimiriine herpesvirus 2; SPF, specific pathogen-free; SRV2, simian retrovirus type 2; THBS1, thrombospondin
Members of the herpesviridae are enveloped DNA viral agents that can infect a variety of host species, resulting in lifelong infection. The family is divided into Alphaherpesvirinae, Betaherpesvirinae, and Gammaherpesvirinae, according to biologic behavior and phylogenetic relationship. As a group, synthesis of viral DNA occurs in the nucleus, and production of infectious virions is associated with destruction of the cell. Herpesviruses have large complex genomes and often have acquired host genes that allow these viruses to modulate and persist in the face of host immune responses.25,71 This condition (termed ‘latency’) is characteristic of all herpesviral infections of the natural host. Although most members of the herpesviridae are of relatively low virulence in their respective hosts, some lack strict host specificity, and cross-species transmission to an inadvertent host can be associated with severe and fatal disease.
The gammaherpesvirinae subfamily is characterized by in vitro and in vivo infection of lymphoblastoid cells and is further divided into the lymphocryptovirus (γ1 herpesviruses) and rhadinovirus (γ2 herpesviruses) genera. Rhadinoviruses have taken on increased importance with the identification of the novel Kaposi sarcoma-associated herpesvirus (KSHV, Human herpesvirus 8) in association with Kaposi sarcoma, an inflammatory and neoplastic condition seen in many HIV-infected patients with AIDS.20,22 Until the recognition of KSHV more than a decade ago, rhadinovirus infection of primates was thought to be restricted to the New World primate lineages, but subsequent investigation revealed a number of novel species-specific viruses in a variety of Old World primates (Table 1).28 As discussed later, based largely on phylogenetic analysis, it is now believed that the rhadinoviruses are subdivided into 2 distinct groupings (rhadinovirus [RV] 1 and 2).77 This review will examine 2 recently recognized rhadinoviruses of macaques (retroperitoneal fibromatosis virus [RFHV] and rhesus rhadinovirus [RRV, Cercopethecine herpesvirus 17]), focusing on their comparative pathobiology with KSHV, their impact on naturally occurring disease entities, and their roles as animal models of human disease.
Table 1.
Nomenclature of primate rhadinoviruses (RV)
| Group | Abbreviation | Official designationa | Alternative designation | Host | Virus isolated | Genomic sequence available |
| RV1 | ||||||
| HHV8 | Human herpesvirus 8 | KSHV | Homo sapiens | yes | yes | |
| RV1mmu | not available | RFHVmmu | Macaca mulatta | no | no | |
| RV1mne | not available | RFHVmne | Macaca nemestrina | no | no | |
| RV1pan | not available | PtRV1a and | Pan troglodytes | no | no | |
| PtRV1b | ||||||
| RV1gor | not available | GorRV1 | Gorilla gorilla | no | no | |
| RV1agm | not available | ChRV1 | Chlorocebus aethiops | no | no | |
| RV2 | ||||||
| HVS | Saimirine herpesvirus 2 (SaHV2) | none | S. sciureus | yes | yes | |
| HVA | Ateline herpesvirus 2 (AtHV2) | none | Ateles geoffroyi | yes | yes | |
| RV2mmu | Cercopethecine herpesvirus 17 (CeHV17) | RRV | Macaca mulatta | yes | yes | |
| RV2mne | not available | PRV | Macaca nemestrina | yes | no | |
| RV2pan | not available | PtRV2 | Pan troglodytes | no | no | |
| RV2agm | not available | ChRV2 | Chlorocebus aethiops | no | no | |
| RV2pan | not available | PapRV2 | Pan anubis | no | no | |
From the International Committee on Taxonomy of Viruses.
Kaposi Sarcoma-associated Herpesvirus
Etiologic agent.
KSHV is a γ2 herpesvirus first identified in 1994 by use of a representational difference analysis technique.22 Subsequent work and phylogenetic analysis indicates that the virus falls within the RV1 subgrouping of the rhadinovirus genus.1 Like other rhadinoviruses, KSHV has typical herpesvirus morphology consisting of particles 100 to 150 nm in diameter, with a lipid envelope surrounding an electron-dense central core. The capsid has icosahedral geometry comprising 162 hexagonal capsomeres and is surrounded by a protein-filled tegument. The viral genome is 165 to 170 kb in length and encodes approximately 95 genes, 25 of which are unique and not found in other human herpesviruses.1,62 On the basis of available sequence data, several distinct KSHV variants (A through E) have been recognized and differ in their worldwide distribution.40
Diagnosis.
KSHV is difficult to isolate and culture. Although transmission of viral DNA to Raji, BJAB, MoLT4, and owl monkey kidney cells has been demonstrated, no active infection could be recognized, and viral DNA was lost after several passages.61,62 Currently propagation of the virus relies on the KS1, BC3, and BCBC1 transformed cell lines derived from clinical specimens obtained from human patients with primary effusion lymphoma (PEL).6,36 Diagnosis typically has relied on PCR detection of viral nucleic acid in biologic samples. Although viral DNA is detected readily in KS and lymphoproliferative lesions, viral load in the peripheral blood of healthy persons is low, limiting the sensitivity of such molecular techniques. Serologic diagnosis can make use of an immunofluorescent assay involving PEL cell lines or, more recently, an ELISA using a recombinant latent nuclear antigen 1 (LANA1) protein. Although serologic assays are useful for epidemiologic studies, discordant results from different laboratories limit their usefulness in individual patients, and currently there is no ‘gold standard’ against which assays may be compared.53,65 Immunohistochemistry and in situ hybridization has been used to localize KSHV-infected cells in neoplastic and lymphoproliferative disorders and recently has identified infected lymphocytes and epithelial cells within the tonsils of immunologically normal children.21
Epidemiology.
KSHV has long been present in human populations, and the divergence of KSHV variants is thought to have occurred more than 35,000 to 60,000 y ago, in conjunction with migration patterns during the Paleolithic period.40,68 The recognized variants appear to cluster geographically, and whether they differ in their ability to induce disease is unknown. In endemic areas with high seroprevalence rates, such as sub-Saharan Africa and the southern Mediterranean, transmission is thought to occur within families through person-to-person contact. KSHV has been detected in saliva, and molecular evidence suggests that mother-to-child and sibling-to-sibling transmission are common.58 Sexual transmission is thought to play a relatively minor role and may be more important outside of endemic regions.32 A bloodborne route after transfusion and solid organ transplantation has been demonstrated but is uncommon.41,93 In utero transmission has not been demonstrated, and passive immunity likely protects the infant until 1 to 2 y of age.
Disease associations and pathology.
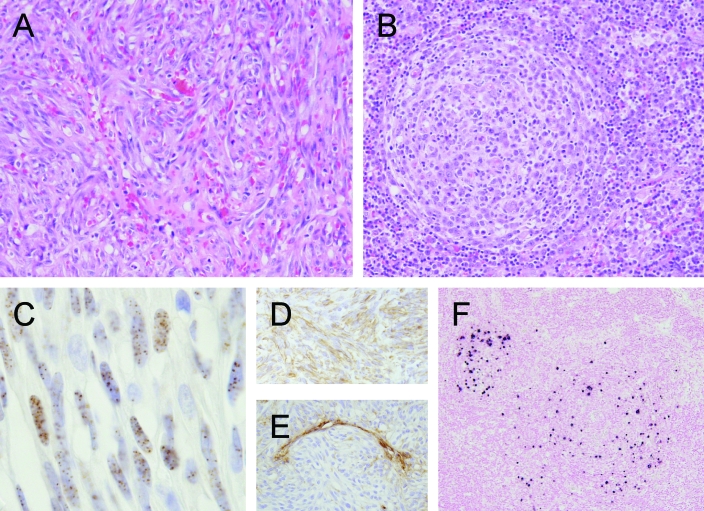
KSHV is detected in all forms of KS and has been linked to PEL (or body-cavity-based lymphomas) and multicentric Castleman disease (MCD). Once common as an AIDS-defining condition in HIV-infected persons, KS is an angioproliferative and inflammatory condition most frequently affecting the skin. Four distinct forms are described based on signalment and clinical presentation: 1) classic KS, typical in elderly men of Mediterranean descent; 2) endemic KS, most frequently in African children; 3) iatrogenic KS, seen in patients after solid-organ transplantation; and 4) AIDS-associated KS, an aggressive form first diagnosed in HIV-infected men in the 1980s.1 Histologically these forms appear identical, and the lesion is composed of a mixture of fibrovascular stroma, infiltrating inflammatory cells, and proliferating spindle cells that form small cleft-like spaces containing erythrocytes (Figure 1). Eosinophilic hyalinized granules are found often in spindle cells, and hemosiderin may be present in macrophages scattered throughout the stroma. Although viral protein and nucleic acid can be demonstrated within spindle cells, the inflammatory component is thought to play a critical role in the production of growth factors and cytokines that promote survival, growth, and dissemination of the tumor.56 The ontogeny of the spindle cell component has been difficult to determine, but based on immunoreactivity to the lymphatic markers LYVE1, D2-40, and VEGFR3, these cells now are believed to demonstrate a lymphoendothelial phenotype and may be derived from latently infected blood endothelial cells, due to alterations in the local cytokine microenvironment.18,19,69 KSHV DNA is detected invariably in all forms of KS, and the neoplastic spindle cells in these lesions are latently infected with KSHV, expressing a limited number of latent viral antigens including KSHV LANA.49 KSHV antigen and nucleic acid may be localized to spindle cells, although viral copy is low (1/cell).1
Figure 1.
Pathology of KSHV infection of humans. (A) Kaposi sarcoma (KS) and (B) multicentric Castleman disease (MCD) are associated with KSHV infection in immunocompromised and normal humans. KS is characterized by bundles of spindeloid cells forming fascicles and irregular channels containing erythrocytes. These spindeloid cells (C) may stain positively for latent nuclear antigen, (D) are vimentin-positive, and (E) often are negative for von Willebrand factor VII. MCD is a lymphoproliferative disorder characterized by generalized follicular lymphadenopathy with accumulation of plasma cells and proliferation of blood vessels within germinal centers (B). (F) Nucleic acid may be localized to the mantle region of affected follicles by means of in situ hybridization.
In addition, KSHV has been associated with several unique lymphoproliferative disorders, including PEL and MCD.1,20,79 PEL is a rare condition of patients with AIDS and presents as a lymphomatous effusion of the pleural, peritoneal, or pericardial cavity, without a demonstrable primary mass and with an immunoblastic or large-cell anaplastic phenotype. High KSHV copy numbers (40 to 80/cell) have been detected in AIDS- and nonAIDS-associated PEL, suggesting a direct etiologic role in viral tumorogenesis.20 A B-cell origin has been demonstrated in light of clonal rearrangement of the immunoglobulin gene, and coinfection with Epstein–Barr virus is common, suggesting that this lymphocryptovirus (γ1 herpesvirus) may play a role as a cofactor in oncogenesis.42,63 Immortalized PEL cell lines have been established in the laboratory and are used to propagate KSHV in vitro.
MCD is a poorly understood lymphoproliferative disorder that has been linked to KSHV infection in AIDS and nonAIDS patients.79 The plasma cell variant is associated with generalized lymphadenopathy and morphologically is characterized by vascular proliferation and accumulation of plasma cells within germinal centers. KSHV DNA is routinely detected in lymph nodes and peripheral blood of affected patients and viral proteins (vIL6 and LANA1) can be demonstrated by immunohistochemistry in the mantle zone surrounding affected germinal centers (Figure 1).14 Of particular interest is the expression of vIL6, a viral homolog of human IL6, that has proliferative, proangiogenic, and proinflammatory activities and may contribute directly to the morphogenesis of MCD. KSHV vIL6 is thought to activate the human IL6 receptor through a gp80-independent fashion, thereby bypassing normal regulatory mechanisms.24,43,85
Clinical disease during primary infection of children has recently been described. Febrile disease and craniocaudal maculopapular rash persisting for 7 to 10 d was recognized in association with seroconversion and detection of KSHV sequences in Egyptian children.4 KSHV also has been localized to epithelial cells in early KS plaque lesions, suggesting that epithelial cells may represent a target during primary infection.21
Molecular basis of KSHV pathogenesis.
Several KSHV genes play important roles in disease pathogenesis by encoding viral proteins that function in cellular signaling, transformation, immune evasion, and modulation of apoptosis. KSHV K1 protein, a transmembrane glycoprotein structurally similar to the B-cell receptor and encoded in the first open reading frame (ORF), is involved in multiple aspects of viral pathogenesis. K1 can initiate signaling in B lymphocytes through an immunoreceptor tyrosine-based activation motif in its cytoplasmic tail, thereby inducing activation and proliferation in B cells.54 In addition, in B lymphocytes, which can harbor KSHV in lifelong latency, K1 has been shown to protect these cells from apoptosis by inhibiting Fas and activating the PI3K–AKT pathway.86 K1 upregulates vascular endothelial growth factor and metalloprotease 9 in endothelial cells, both of which promote angiogenesis to support tumor growth.86 Lastly, K1 has oncogenic potential and can transform rodent fibroblasts in vitro, immortalize marmoset lymphocytes, and induce lymphoma in marmosets.26,55
KSHV ORF73 LANA, sharing functional homology with Epstein–Barr nuclear antigen 1, is expressed in latently infected cells and is essential for maintaining viral episomes and KSHV replication and for restricting transcription of viral proteins during latency as a mechanism of immune evasion.7,46-48,87,91 LANA is capable of prolonging cellular lifespan, increasing cellular proliferation, and inhibiting p53-dependent apoptosis, but LANA cannot transform cells.35,87
In addition, KSHV encodes several homologs of cellular cytokines and chemokines, which are involved in cellular proliferation and immune evasion. KSHV vIL6 (ORFK2) is pleiotropic and can activate numerous cell types by acting directly through either the IL6 receptor (IL6R, CD126) or gp130 or by trans-signaling through soluble IL6R and gp130.25 vIL6 contributes to cellular proliferation by inhibiting IFNα-mediated apoptosis in cells, inducing cellular IL6, promoting B cell proliferation, and inducing cellular angiogenic vascular endothelial growth factor.5,23,25,45 KSHV-encoded vIL6 is also immunomodulatory by inducing cellular chemokine ligand 2 (CCL2; monocyte chemotactic protein 1 [MCP1]), which promotes TH2 responses while inhibiting the antiviral TH1 response.27 In addition to inducing cellular CCL2, KSHV also encodes viral chemokine homologs (vCCL1, vCCL2, and vCCL3) with cellular homology to MIP1α and RANTES that can induce monocyte chemotaxis and signal transduction.64
As another means of immune modulation, KSHV encodes 4 interferon-regulatory factors (vIRF1 through vIRF4), which regulate the transcription of type I IFN genes by interfering with the transcriptional activator p300.64,66 KSHV ORF45 and ORF50-encoded viral proteins specifically inhibit IRF7 replication and transcription activator.92 Another immune modulatory KSHV-encoded protein is vGPCR, a 7-transmembrane receptor protein with sequence homology to IL8 receptor, which binds CC and CXC family chemokines and has potent signaling and transforming capabilities.29
Recently KSHV has been shown to express a total of 17 microRNAs (miRNAs) which are encoded by 12 genes, readily detected in latently infected cells, and involved in posttranscriptional regulation of cellular genes.17,75 Their role is thought to facilitate establishment and maintenance of viral latency by downregulating specific cellular target genes. miRNAs are found in all metazoan eukaryotes, and more than 200 have been found in human cells.9 miRNAs function to suppress cellular gene expression through RNA interference by binding the 3′ untranslated regions of target genes and sequestering them to processing bodies, resulting in translational inhibition or mRNA degradation.9 In cells stably expressing KSHV-encoded miRNAs, 8 genes were downregulated more than 4-fold, including SPP1 (osteopontin), plasticity- related gene 1, thrombospondin (THBS1), S100 calcium-binding protein A2, and integral membrane protein 2A, all of which products are involved in proliferation, immune modulation, angiogenesis, and apoptosis.75 THBS1 is a matricellular protein that functions in cell–cell and cell–matrix adhesion and is downregulated in a number of human cancers. It has potent antiproliferative and antiangiogenic properties mediated through the activation of the latent form of TGFβ. In one study,75 protein levels of THBS1 in KSHV miRNA-transfected cells were downregulated more than 10-fold, causing a decrease in TGFβ activity. In keeping with these findings, THBS1 expression has been shown to be suppressed in KS lesions, supporting the theory that suppression of its antiproliferation and antiangiogenesis activities are important for KSHV oncogenesis.75,81
Retroperitoneal Fibromatosis Herpesvirus
Etiologic agent.
In 1997, novel γ2 herpesvirus sequences were identified by PCR using degenerate primer pools in tissues from macaques that had developed retroperitoneal fibromatosis (RF).73 RF is a fibroproliferative disorder that has occurred in some nonhuman primate facilities in conjunction with simian retrovirus 2 (SRV2) infection and progressive immunodeficiency.37,82,83 Sequencing revealed the presence of 2 viruses: RFHVmmu in Macaca mulatta and RFHVmne in M. nemastrina. Initial identification was based on partial sequences of the DNA polymerase gene, but subsequent analysis of a 4.3-kb fragment of divergent locus B has placed these viruses in the RV1 group, clustering with African primate RV1 rhadinoviruses and KSHV.72,77 Indepth analysis of RFHV has revealed several similarities to KSHV, including the presence of a viral dihydrofolate reductase homolog and a MIR1-like (K3) ORF. Although present, the RFHV vIL6 was only 35% homologous to that of KSHV at the amino acid level. Furthermore, RFHV lacks an ORF10 homolog that is found consistently in variants of KSHV, suggesting that a number of distinct genetic differences exist between the species-specific strains.
Diagnosis.
RFHV has not been isolated or cultured in vitro. Currently no serological assays have been developed to detect antibody responses, and in vivo diagnosis relies on PCR detection of viral nucleic acid in biologic samples. The sensitivity of such assays for detection of latent infection is unknown, but evidence from the KSHV and RV2 viruses suggests that PCR analysis of blood is a relatively insensitive method to detect RV1 viruses in immunocompetent hosts. When RF is present, a number of techniques can be used, including PCR analysis of tissue samples and detection of viral protein by immunohistochemistry. These protocols make use of a cross-reactive monoclonal antibody directed at KSHV LANA and demonstrate large numbers of infected cells in RF tissue.15,16
Epidemiology.
The lack of serologic assays has hampered epidemiologic investigation of RFHV infection. Systematic evaluation of colonies have not been undertaken, and whether RFHV is a naturally occurring infection of macaques or represents inadvertent cross-species transmission of an RV1 agent from a closely related primate is unclear. Historically, not all large macaque breeding colonies demonstrated RF, suggesting that infection may have been limited to certain facilities and that the virus may not be transmitted as readily or spread as widely as the ubiquitous RV2 macaque viruses. In recent years, the number of recognized RF cases has decreased markedly. Whether this decrease is due to changes in the prevalence of RFHV or to elimination of SRV2 through the development of specific pathogen-free (SPF) breeding colonies is not known.
Disease associations and pathology.
Even before the recognition of KSHV and related primate rhadinoviruses, similarities between KS and RF were appreciated. RF was first recognized in several species of macaques (M. mulatta, M. fascicularis, M. fuscata, and M. nemastrina) housed at the Washington National Primate Research Center during the mid 1970s.37,82,83 Animals often presented clinically with chronic weight loss and diarrhea, were infected with SRV2, and had evidence of generalized lymphoid depletion. Grossly, lesions were first noted at the ileocecal junction as small nodules or a diffuse opaque thickening of the mesentery.84 As the lesion progressed, a fibrous mass surrounded and eventually fully encased the small and large intestine, and in some cases, the resultant fibromatosis extended through the inguinal canal and into the pleural and pericardial space. The adhesions that formed likely interfered with normal peristalsis and gastrointestinal function. Rarely spontaneous regression of clinical signs was noted.84
Microscopically RF was characterized by ill-formed bundles of spindeloid cells proliferating below the serosal surface supported by more mature fibrovascular stroma (Figure 2). Multifocally within these masses, lymphocytic and plasmacytic aggregates could be observed. Early lesions appeared to originate from the capsule of mesenteric lymph nodes and spread along the lymphatic vessels. In addition to this proliferative pattern, a sclerotic form has been observed, with fewer and more mature spindeloid cells and a larger component of fibrovascular and collagenous stroma. Oral and subcutaneous forms have been noted rarely, with the latter condition termed ‘subcutaneous fibromatosis.’ Transmission studies using frozen homogenized tissue, performed at the time of collection and later after recognition of RFHV, confirmed the involvement of an infectious agent but were hampered by the inability to isolate the virus.12,37
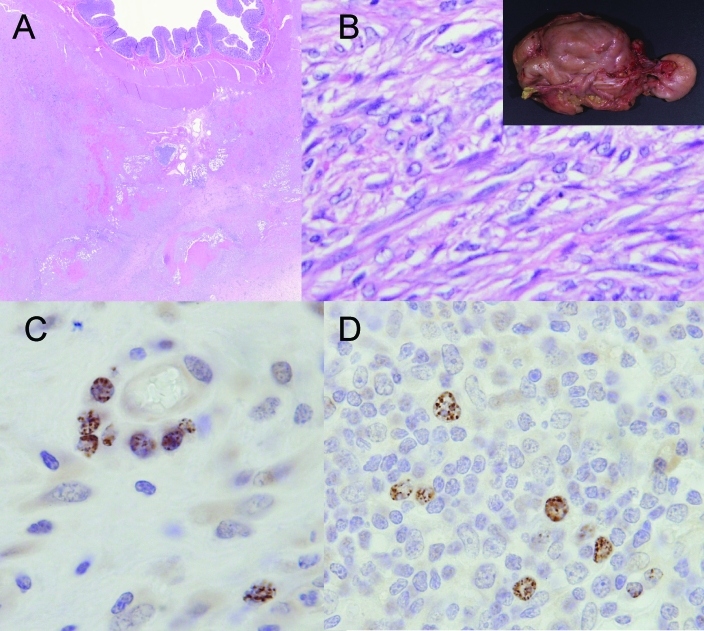
Figure 2.
Pathology of RFHV infection of macaques. (A) Retroperitoneal fibromatosis (RF) occurs in macaques coinfected with SRV2 and RFHV. The lesion shares some similarities with human KS, and virus may be localized to the proliferating spindeloid cells, (B) which form short bundles and fascicles but lack the vascular channels observed in KS. The lesion often arises at the root of the mesentery but may eventually surround and encase the gastrointestinal tract into a single mass (insert). An antiHHV8 LANA rat monoclonal antibody demonstrates characteristic nuclear punctuate staining in (C) an RF lesion and (D) a lymph node with angiofollicular lymphocytic hyperplasia.
Despite marked differences in the microscopic appearance of RF and KS, the similarities between these 2 disease processes are considerable. Both are mesenchymal proliferative disorders that originate in the context of virally induced immunodeficiency. Moreover, the lesions contain considerable numbers of inflammatory cells, and in vitro evidence suggests an important paracrine role for growth factors produced by these cells in promoting spindle cell proliferation. For these reasons, after the identification of KSHV and confirmation of its role in KS, investigators attempted to define a similar etiology in RF and successfully used degenerate PCR primers to detect novel gammaherpesvirus sequences.73 Subsequent work has demonstrated the close relationship of RFHV to KSHV and has localized viral LANA protein to proliferating spindle cells by immunohistochemistry.15 Finally a quantitative PCR technique demonstrated higher viral copy numbers in RF tissue than in noninfected tissue. The inability to isolate and culture the virus has precluded transmission studies with purified agent to confirm its etiologic role, and a second RV2 rhadinovirus has been detected (albeit at lower copy number) in lesions by PCR.15 Nonetheless, RFHV likely plays a similar role to KSHV in the induction of these mesenchymal proliferative disorders in macaques.
Although most cases of RF have occurred in animals coinfected with SRV2, a recent report documents the presence of rhadinovirus sequences in intestinal stromal tumors in an SIV-infected rhesus macaque.10 The animal was assigned to an experimental study and developed progressive immunodeficiency and multiple masses in the distal colon. RFHVmmu and an RV2 virus were detected in tissue by PCR, and nuclei were positive for LANA antigen by immunohistochemistry (Figure 2C). In addition, an SIV-inoculated rhesus macaque at our institution developed a lymphadenopathy with features of angiofollicular lymphocytic hyperplasia in which typical RFHV LANA-positive cells could be demonstrated by immunohistochemistry (Figure 2D). These findings suggest that the spectrum of mesenchymal lymphoid proliferative disorders in animals infected with macaque rhadinoviruses may be broader than previously recognized and that the development of such lesions may occur independently of SRV2 infection.
Rhesus Rhadinovirus
Etiologic agent.
An RV2 rhadinovirus of rhesus macaques was first isolated during the investigation of a spontaneous disease outbreak at the New England National Regional Primate Center.30 Seroreactivity to Saimiriine herpesvirus 2 (SaHV2; herpesvirus saimiri) antigen was noted in healthy and affected animals, and the presence of a related macaque virus was suspected. Peripheral blood mononuclear cells were cocultured with rhesus primary fibroblasts, and a putative herpesvirus was isolated.30 In these cell cultures, cytopathic effect first becomes evident 8 to 11 d following inoculation with the presence of focal destruction of the cellular monolayer, and the presence of multinucleated syncytial cells and intranuclear inclusions. DNA of purified virions from the initial isolate (H26-95) was digested, cloned, and sequenced, revealing a γ2 herpesvirus related to KSHV. A second independent isolation (17577) was made at the Oregon National Regional Primate Center from bone marrow obtained from an SIV-infected juvenile rhesus macaque.89 Subsequent work has placed this virus within the RV2 grouping, and related viruses have been identified in M. nemastrina and M. fascicularis.3,78 Rhesus rhabdinovirus has been designated Cercopethecine herpesvirus 17 by the International Committee on Taxonomy of Viruses.
The complete sequences of RRV isolates H26-95 and 17795 have been published independently and revealed that the 2 isolates were closely related strains of the same rhesus macaque virus.3,78 RRV H26-95 has 84 open reading frames identified in its 130-kb sequence and shares extensive similarities in genomic organization with KSHV. Several of these ORFs are unique to rhadinoviral genera, including vIL6 homologs and interferon regulating factors, both thought to play a role in viral pathogenesis and persistence. While similarities are extensive, a number of genomic distinctions are apparent suggesting that important differences in biological behavior and pathogenesis may become evident with further study. All KSHV ORFs have at least 1 homolog in RRV H26-95 with the exception of MIR1 (K3), K5, K7, and K12 (kaposin). In addition, KSHV has 3 MIP1 homologs and 4 vIRF elements, compared with 1 MIP1 and 8 vIRFs in RRV. Finally the dihydrofolate reductase gene of RRV shows greater homology to that of SaHV2 and is displaced compared with the location in KSHV. Which, of any of these, account for observed differences in biologic properties between RRV and KSHV is unknown.
Diagnosis.
A number of diagnostic techniques are available to detect RRV infection.28 Because the virus can be grown lytically and to high titer in primary rhesus fibroblasts, whole-virus ELISAs have been developed and can be used to determine serologic status of individuals and colonies.30 As with other herpesviruses, the presence of antibodies indicates life long infection of the animal. A number of other assays have been developed to assist with diagnosis, including viral isolation from peripheral blood mononuclear cells and PCR performed on plasma, whole blood and other biological specimens. Although these assays may provide valuable information, they are likely to be less sensitive than serology, and caution is advised in interpreting negative results.
Epidemiology.
Serologic evidence indicates widespread infection of macaque colonies with RRV or related RV2 viruses.28,30 Typically by 2 y of age, more than 95% of juvenile animals have developed a vigorous IgG antibody response to RRV, indicating that the virus is easily spread before animals reach sexual maturity.30,57 The timing of seroconversion suggests that dam-to-offspring and juvenile-to-juvenile spread are primarily responsible for the high transmission rates. At our institution, we have not noted intrauterine or perinatal transmission of RRV and suspect that the low-level antibodies detected in neonates represent passive maternal immunity, which temporarily protects infants from infection. These responses begin to wane at 6 to 8 mo of age, a period that coincides with the first evidence of natural infection in some animals. The route of natural infection has not been investigated systematically, but evidence from KSHV and the observed high rate of seropositivity are consistent with saliva and an oral route playing a key role. As with other gammaherpesvirinae close contact is probably required for infection, and fomites likely play a minor role. RRV-like sequences have been detected in DNA from recently imported Indonesian macaques suggesting that infection is widespread in feral and wild animals.
Disease associations and pathology.
Experimental inoculation of normal and SIV-infected macaques (M. mulatta and M. nemastrina) with RRV and pigtail macaque rhadinovirus (RV2mne) isolates have been reported.57,89 Animals coinfected with SIV and RRV develop a marked B cell lymphocytosis and associated hypergammaglobulinemia. These cells express the B cell activation marker CD40 but not the Epstein–Barr-associated marker CD23 and, despite generalized B cell activation, antibody responses to rhadinovirus and lentivirus antigens are highly attenuated, compared with those of controls. During these in vivo infections, RRV targets CD20-positive B cells, and virus-induced disruption of normal B cell responses is suspected.57 In addition to evidence of peripheral B cell lymphocytosis, lymph nodes demonstrated changes consistent with angiofollicular lymphoid hyperplasia. Microscopic findings revealed plasmacytic infiltration and vascular hyalinization resembling the plasma cell variant of MCD (Figure 3). An immune-mediated hemolytic anemia, pulmonary arteriopathy, and lymphocytic interstitial pneumonitis were recognized in a subset of animals. Although other additional disease entities were recognized in SIV–RRV-coinfected animals, their relationship to RRV inoculation is less clear.
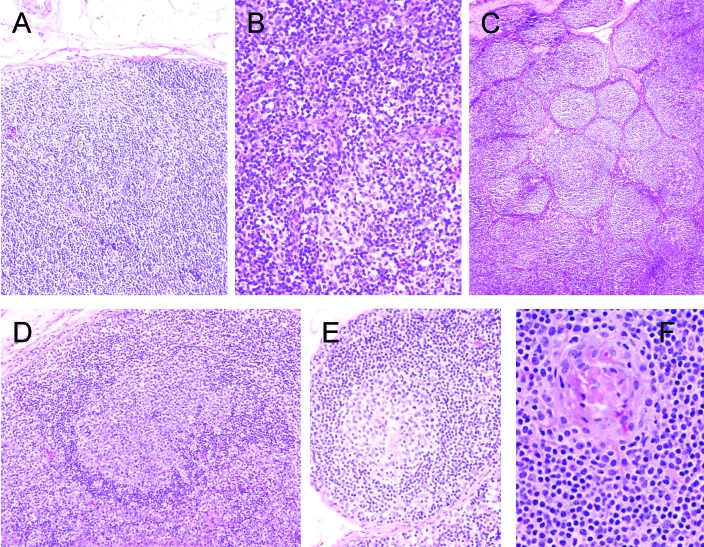
Figure 3.
Pathology of RRV infection of macaques. (A) Experimental inoculation and natural infection initially is characterized by parafollicular lymphocytic hyperplasia, (B) accompanied by marked vascular hypertrophy and hyperplasia. (C) Florid follicular hyperplasia can occur after this phase, followed by (D) follicular dissolution or (E, F) follicles containing proliferative and hyalinized vessels. The lesions show similarities to plasma cell variant of MCD.
Experimental inoculation of immunologically normal animals resulted in infection, a vigorous antibody response, and a modest B cell lymphocytosis.57,89 Although animals remained clinically normal throughout the study period, peripheral lymphadenopathy was noted after inoculation of RRV H26-95. Lymph node changes progressed through marked paracortical lymphocytic hyperplasia with vascular hypertrophy and hyperplasia at 2 wk to florid follicular hyperplasia between 4 and 12 wk. In this latter phase hyalinized germinal centers with increased vascularity were often evident and mantle and interfollicular zones were infiltrated by plasma cells.
Evidence suggesting naturally occurring disease associated with RRV infection is more limited, and in immunologically normal animals, the virus is likely of low virulence.30 In juvenile macaques we have seen similar alterations in lymph node morphology as after experimental inoculation. Florid follicular hyperplasia with plasmacytic infiltration and vascular hyalinization coincided with evidence of seroconversion in 8- to 10-mo-old colony-housed rhesus macaques. No other clinical signs were associated with this lymphadenopathy, and changes resolved over a 2-mo period. A quantitative PCR technique has been developed to examine viral load in peripheral blood mononuclear cells of normal and SIV-infected rhesus macaques. This technique detected peripheral viremia in only 6.7% of normal animals.74 In SIV-inoculated animals, the incidence of PCR positivity appeared to increase with duration of infection, suggesting reactivation of latent virus with progressive immunodeficiency, but PCR positivity was not associated with various lymphomas diagnosed at necropsy. RRV 17577 was isolated from a lymphocryptovirus-negative SIV-associated multicentric lymphoproliferative disease, suggesting that the virus may play a role in the spectrum of this disorder.89
Other Primate Rhadinoviruses
Rhadinovirus infections occur in a number of other primate species. Of these SaHV2 and Ateline herpesvirus 2 are arguably the best studied viruses and were first recognized more than 30 y ago.59,60 Squirrel monkeys are the natural host of SaHV2 and similar to that with other rhadinoviruses, infection of squirrel monkeys is nearly 100% by 1 to 2 y of age. In the natural host, disease has not been described; however, cross-species transmission to several other neotropical primate species, including tamarins, owl monkeys, and marmosets, results in lymphomagenesis and a rapidly progressive disease course.44 The model has proven useful in elucidating the molecular basis of herpesvirus oncogenesis and the role of viral determinants in this process.34
Since the recognition of KSHV and the macaque rhadinoviruses, several other Old World primate species have been identified to harbor their own KSHV-like gammaherpesviruses. KSHV-homologous rhadinoviruses have been identified in African green monkeys, baboons, drills and mandrills.39,52,88 Two RV1 viruses closely related to KSHV have been identified in chimpanzees, with higher seropositivity rates detected in animals experimentally infected with HIV.38,50,51 In many cases, identification of these viruses has been based on PCR amplification of sequences from normal animals, and disease associations have not been recognized. Moreover, viral isolation and cultivation has not been possible, and assignment of the viruses to 1 of the 2 established rhadinovirus lineages is based on limited sequence data. Interestingly, although both HIV1 and a KSHV-like rhadinovirus have been detected in chimpanzees, KS-like proliferative disorders have not been recognized in this species.
Models of KSHV Infection and Pathogenesis
The recognition of KSHV as an important human pathogen has prompted intense interest in the development of animal models to investigate aspects of disease pathogenesis. Rhesus macaques have been inoculated with KSHV-infected PEL cells, and although low-level viral persistence could be detected by PCR, seroconversion and adverse disease outcome did not occur.70 Long-term infection of NOD/SCID mice with KSHV has been described.13,67,90 In this model, latency is established, and the virus appears to target specific murine cell types, including B cells, macrophages, and dendritic cells.2 Alternative surrogate murine model systems have been developed and include the use of murine gammaherpesviruses MHV68 and MHV72.80 Compared with MHV68 and MHV72, the RV1 viruses RFHVmmu and RFHVmne are more closely related to KSHV, but these strains have not been isolated and cultivated in vitro and thus are more difficult to work with in laboratory animal models.
In contrast to the RV1 macaque viruses, RV2 viruses have been isolated and are easily cultured in vitro. As discussed earlier, experimental inoculation with 2 closely related RV2 strains have been conducted in immunocompetent and immunodeficient animals and have helped define the spectrum of disease with these agents.57,89 Morphologic similarities to MCD in lymphoid tissue after inoculation have been described in rhesus macaques, and this finding has set the stage for understanding the molecular basis of rhadinovirus oncogenesis. Several recent technical advances with the RV2 virus models likely will enhance their use as animal models and include the development of tools such as systems to genetically manipulate viruses, recombinant viruses with tags such as green fluorescent protein that can be used to track and quantify levels of viral replication, and reagents to assess immunologic responses.11,31 Two groups recently have developed systems to genetically manipulate infectious clones of RV2 viruses.11,33 These systems allow the creation of recombinants, which can be used to study the impact of individual genes and viral determinants on disease pathogenesis and are critical to the aim of developing these viruses as potential vector systems. Further, as with KSHV, regions encoding 11 distinct miRNAs have recently been cloned from RV2mmu.76 Although these RV2mmu miRNAs do not share sequence homology with the KSHV miRNAs identified to date, they are located in a single cluster and reside in the same genomic region as the KSHV miRNAs.76 The RV2mmu miRNAs likely share functional similarities with the KSHV miRNAs in the role of downregulating host factors to allow for establishment and persistence of viral latency. Future studies on these miRNAs will elucidate target cellular genes and mechanisms common to KSHV. Such work is currently difficult or impossible to perform with KSHV or other RV1 viruses because of the lack of permissive cell lines that allow propagation of the virus.
Derivation of Rhadinovirus-free Animals and Colonies
The importance of primate rhadinovirus models leads to the frequent need to study animals that have not previously been infected with the virus. This requirement may be problematic, because most colonies have a high rate of seroreactivity to RV2 viruses. Seroprevalence studies30,57indicate that transmission often occurs between 6 to 12 mo of age and that separation of juvenile animals at this time from the breeding colony with establishment of small peer groups will often break the transmission cycle. Although earlier separation decreases the likelihood of transmission, establishment of peer groups at 6 to 12 mo facilitates normal social development. After their establishment, peer groups should be tested serologically every month for 4 mo. If no animals seroconvert, larger groups can then be formed, with testing continued on a quarterly basis.
SPF breeding colonies of rhesus macaques that are free of RV2 and several other ubiquitous viruses (including cytomegalovirus, rhesus lymphocryptovirus, SV40, and simian foamy virus) have been established by separating infant macaques from dams on the day of birth, combined with hand-rearing and quarterly testing with both serologic and molecular techniques.8,30,57 Although development of expanded SPF breeding programs is useful for scientific programs, these programs are not without substantial costs and potential risks. Such animals must have complete physical separation from the source colony, including separate infrastructure and personnel, to prevent inadvertent transmission of agents. In addition to the cost of the initial derivation of the colony, there is the continued cost of testing and duplication of facilities, equipment, and housing.
The establishment of expanded SPF programs is associated with various risks, as well. Development of a separate colony may introduce further genetic subdivision within a facility's breeding program. Although this potential can be managed in the short term through careful selection of founder animals in the expanded program, genetic drift may lead to substantial differences as colonies grow and age. Additional risks may be posed by breaks in SPF status. Although these ubiquitous agents are largely nonpathogenic in the immunocompetent host, infection most commonly occurs at a young age, and whether virulence differs if infection is delayed until animals are substantially older or pregnant is unknown. Finally, these ubiquitous viral agents fundamentally affect cellular immune responses within the host, and their removal may have unknown effects on the ontogeny and senescence of the normal immune system.
Summary
Recent molecular and serologic evidence suggests widespread infection of Old World primates with rhadinoviruses. These agents are grouped into 2 distinct lineages (RV1 and RV2) and, because they show functional and sequence similarity to KSHV, are important animal models of KSHV-mediated disease. In particular, the RV2 viruses RRV and RV2mne provide the most opportunities for extensive molecular analysis because they can be cultivated in vitro. Future work will address many of the remaining questions on rhadinovirus-induced neoplastic diseases, and primate models likely will play an important role in these studies.
Acknowledgments
Acknowledgment
Supported by NIH grants RR00168 and RR16020.
References
- 1.Ablashi DV, Chatlynne LG, Whitman JE, Jr, Cesarman E. 2002. Spectrum of Kaposi's sarcoma-associated herpesvirus, or human herpesvirus 8, diseases. Clin Microbiol Rev 15:439–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adang LA, Parsons CH, Kedes DH. 2006. Asynchronous progression through the lytic cascade and variations in intracellular viral loads revealed by high-throughput single-cell analysis of Kaposi's sarcoma-associated herpesvirus infection. J Virol 80:10073–10082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander L, Denekamp L, Knapp A, Auerbach MR, Damania B, Desrosiers RC. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J Virol 74:3388–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreoni M, Sarmati L, Nicastri E, El Sawaf G, El Zalabani M, Uccella I, Bugarini R, Parisi SG, Rezza G. 2002. Primary human herpesvirus 8 infection in immunocompetent children. J Am Med Assoc 287:1295–1300 [DOI] [PubMed] [Google Scholar]
- 5.Aoki Y, Jaffe ES, Chang Y, Jones K, Teruya-Feldstein J, Moore PS, Tosato G. 1999. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood 12:4034–4043 [PubMed] [Google Scholar]
- 6.Arvanitakis L, Mesri EA, Nador RG, Said JW, Asch AS, Knowles DM, Cesarman E. 1996. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein–Barr virus. Blood 7:2648–2654 [PubMed] [Google Scholar]
- 7.Ballestas ME, Chatis PA, Kaye KM. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641–644 [DOI] [PubMed] [Google Scholar]
- 8.Barry PA, Strelow L. 2008. Development of breeding populations of rhesus macaques (Macaca mulatta) that are specific pathogen-free for rhesus cytomegalovirus. Comp Med 58:43–46 [PMC free article] [PubMed] [Google Scholar]
- 9.Bartel DP. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297 [DOI] [PubMed] [Google Scholar]
- 10.Bielefeldt-Ohmann H, Barouch DH, Bakke AM, Bruce AG, Durning M, Grant R, Letvin NL, Ryan JT, Schmidt A, Thouless ME, Rose TM. 2005. Intestinal stromal tumors in a simian immunodeficiency virus-infected, simian retrovirus 2-negative rhesus macaque (Macaca mulatta). Vet Pathol 42:391–396 [DOI] [PubMed] [Google Scholar]
- 11.Bilello JP, Morgan JS, Damania B, Lang SM, Desrosiers RC. 2006. A genetic system for rhesus monkey rhadinovirus: use of recombinant virus to quantitate antibody-mediated neutralization. J Virol 80:1549–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosch ML, Harper E, Schmidt A, Strand KB, Thormahlen S, Thouless ME, Wang Y. 1999. Activation in vivo of retroperitoneal fibromatosis-associated herpesvirus, a simian homologue of human herpesvirus 8. J Gen Virol 80(Pt 2):467–475 [DOI] [PubMed] [Google Scholar]
- 13.Boshoff C, Gao SJ, Healy LE, Matthews S, Thomas AJ, Coignet L, Warnke RA, Strauchen JA, Matutes E, Kamel OW, Moore PS, Weiss RA, Chang Y. 1998. Establishing a KSHV+ cell line (BCP1) from peripheral blood and characterizing its growth in Nod/SCID mice. Blood 5:1671–1679 [PubMed] [Google Scholar]
- 14.Brousset P, Cesarman E, Meggetto F, Lamant L, Delsol G. 2001. Colocalization of the viral interleukin 6 with latent nuclear antigen 1 of human herpesvirus 8 in endothelial spindle cells of Kaposi's sarcoma and lymphoid cells of multicentric Castleman's disease. Hum Pathol 32:95–100 [DOI] [PubMed] [Google Scholar]
- 15.Bruce AG, Bakke AM, Bielefeldt-Ohmann H, Ryan JT, Thouless ME, Tsai CC, Rose TM. 2006. High levels of retroperitoneal fibromatosis (RF)-associated herpesvirus in RF lesions in macaques are associated with ORF73 LANA expression in spindleoid tumour cells. J Gen Virol 87(Pt 12):3529–3538 [DOI] [PubMed] [Google Scholar]
- 16.Burnside KL, Ryan JT, Bielefeldt-Ohmann H, Bruce AG, Thouless ME, Tsai CC, Rose TM. 2006. RFHVMn ORF73 is structurally related to the KSHV ORF73 latency-associated nuclear antigen (LANA) and is expressed in retroperitoneal fibromatosis (RF) tumor cells. Virology 354:103–115 [DOI] [PubMed] [Google Scholar]
- 17.Cai X, Lu S, Zhang Z, Gonzalez CM, Damania B, Cullen BR. 2005. Kaposi's sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc Natl Acad Sci USA 102:5570–5575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carroll PA, Brazeau E, Lagunoff M. 2004. Kaposi's sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation. Virology 328:7–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll PA, Kenerson HL, Yeung RS, Lagunoff M. 2006. Latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors. J Virol 80:10802–10812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity–based lymphomas. N Engl J Med 332:1186–1191 [DOI] [PubMed] [Google Scholar]
- 21.Chagas CA, Endo LH, Sakano E, Pinto GA, Brousset P, Vassallo J. 2006. Detection of herpesvirus type 8 (HHV8) in children's tonsils and adenoids by immunohistochemistry and in situ hybridization. Int J Pediatr Otorhinolaryngol 70:65–72 [DOI] [PubMed] [Google Scholar]
- 22.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869 [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee M, Osborne J, Bestetti G, Chang Y, Moore PS. 2002. Viral IL6-induced cell proliferation and immune evasion of interferon activity. Science 298:1432–1435 [DOI] [PubMed] [Google Scholar]
- 24.Chen D, Nicholas J. 2006. Structural requirements for gp80 independence of human herpesvirus 8 interleukin 6 (vIL6) and evidence for gp80 stabilization of gp130 signaling complexes induced by vIL6. J Virol 80:9811–9821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coscoy L. 2007. Immune evasion by Kaposi's sarcoma-associated herpesvirus. Nat Rev Immunol 7:391–401 [DOI] [PubMed] [Google Scholar]
- 26.Damania B. 2007. DNA tumor viruses and human cancer. Trends Microbiol 15:38–44 [DOI] [PubMed] [Google Scholar]
- 27.Damania B. 2004. Modulation of cell signaling pathways by Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8). Cell Biochem Biophys 3:305–322 [DOI] [PubMed] [Google Scholar]
- 28.Damania B, Desrosiers RC. 2001. Simian homologues of human herpesvirus 8. Philos Trans R Soc Lond B Biol Sci 1408:535–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damania B, Jeong JH, Bowser BS, DeWire SM, Staudt MR, Dittmer DP. 2004. Comparison of the Rta/Orf50 transactivator proteins of γ2 herpesviruses. J Virol 78:5491–5499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desrosiers RC, Sasseville VG, Czajak SC, Zhang X, Mansfield KG, Kaur A, Johnson RP, Lackner AA, Jung JU. 1997. A herpesvirus of rhesus monkeys related to the human Kaposi's sarcoma-associated herpesvirus. J Virol 12:9764–9769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeWire SM, Money ES, Krall SP, Damania B. 2003. Rhesus monkey rhadinovirus (RRV): construction of a RRV–GFP recombinant virus and development of assays to assess viral replication. Virology 312:122–134 [DOI] [PubMed] [Google Scholar]
- 32.Engels EA, Atkinson JO, Graubard BI, McQuillan GM, Gamache C, Mbisa G, Cohn S, Whitby D, Goedert JJ. 2007. Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. J Infect Dis 196:199–207 [DOI] [PubMed] [Google Scholar]
- 33.Estep RD, Powers MF, Yen BK, Li H, Wong SW. 2007. Construction of an infectious rhesus rhadinovirus bacterial artificial chromosome for the analysis of Kaposi's sarcoma-associated herpesvirus-related disease development. J Virol 81:2957–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fickenscher H, Fleckenstein B. 2001. Herpesvirus saimiri. Philos Trans R Soc Lond B Biol Sci 1408:545–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friborg J, Jr, Kong W, Hottiger MO, Nabel GJ. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 6764:889–894 [DOI] [PubMed] [Google Scholar]
- 36.Gaidano G, Cechova K, Chang Y, Moore PS, Knowles DM, la-Favera R. 1996. Establishment of AIDS-related lymphoma cell lines from lymphomatous effusions. Leukemia 7:1237–1240 [PubMed] [Google Scholar]
- 37.Giddens WE, Jr, Tsai CC, Morton WR, Ochs HD, Knitter GH, Blakley GA. 1985. Retroperitoneal fibromatosis and acquired immunodeficiency syndrome in macaques. Pathologic observations and transmission studies. Am J Pathol 2:253–263 [PMC free article] [PubMed] [Google Scholar]
- 38.Greensill J, Sheldon JA, Murthy KK, Bessonette JS, Beer BE, Schulz TF. 2000. A chimpanzee rhadinovirus sequence related to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8: increased detection after HIV1 infection in the absence of disease. AIDS 14:F129–F135 [DOI] [PubMed] [Google Scholar]
- 39.Greensill J, Sheldon JA, Renwick NM, Beer BE, Norley S, Goudsmit J, Schulz TF. 2000. Two distinct γ2 herpesviruses in African green monkeys: a second γ2 herpesvirus lineage among Old World primates? J Virol 74:1572–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayward GS, Zong JC. 2007. Modern evolutionary history of the human KSHV genome. Curr Top Microbiol Immunol 312:1–42 [DOI] [PubMed] [Google Scholar]
- 41.Hladik W, Dollard SC, Mermin J, Fowlkes AL, Downing R, Amin MM, Banage F, Nzaro E, Kataaha P, Dondero TJ, Pellett PE, Lackritz EM. 2006. Transmission of human herpesvirus 8 by blood transfusion. N Engl J Med 355:1331–1338 [DOI] [PubMed] [Google Scholar]
- 42.Horenstein MG, Nador RG, Chadburn A, Hyjek EM, Inghirami G, Knowles DM, Cesarman E. 1997. Epstein-Barr virus latent gene expression in primary effusion lymphomas containing Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8. Blood 3:1186–1191 [PubMed] [Google Scholar]
- 43.Hu F, Nicholas J. 2006. Signal transduction by human herpesvirus 8 viral interleukin 6 (vIL6) is modulated by the nonsignaling gp80 subunit of the IL6 receptor complex and is distinct from signaling induced by human IL6. J Virol 80:10874–10878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunt RD, Melendez LV, King NW, Gilmore CE, Daniel MD, Williamson ME, Jones TC. 1970. Morphology of a disease with features of malignant lymphoma in marmosets and owl monkeys inoculated with Herpesvirus saimiri. J Natl Cancer Inst 2:447–465 [PubMed] [Google Scholar]
- 45.Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. 1999. Involvement of interleukin 10 (IL10) and viral IL6 in the spontaneous growth of Kaposi's sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood 8:2871–2879 [PubMed] [Google Scholar]
- 46.Kelley-Clarke B, Ballestas ME, Komatsu T, Kaye KM. 2007. Kaposi's sarcoma herpesvirus C-terminal LANA concentrates at pericentromeric and peritelomeric regions of a subset of mitotic chromosomes. Virology 357:149–157 [DOI] [PubMed] [Google Scholar]
- 47.Kelley-Clarke B, Ballestas ME, Srinivasan V, Barbera AJ, Komatsu T, Harris TA, Kazanjian M, Kaye KM. 2007. Determination of Kaposi's sarcoma-associated herpesvirus C-terminal latency-associated nuclear antigen residues mediating chromosome association and DNA binding. J Virol 81:4348–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Komatsu T, Barbera AJ, Ballestas ME, Kaye KM. 2001. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen. Viral Immunol 14:311–317 [DOI] [PubMed] [Google Scholar]
- 49.Krishnan HH, Naranatt PP, Smith MS, Zeng L, Bloomer C, Chandran B. 2004. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J Virol 78:3601–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lacoste V, Mauclere P, Dubreuil G, Lewis J, Georges-Courbot MC, Gessain A. 2000. KSHV-like herpesviruses in chimps and gorillas. Nature 6801:151–152 [DOI] [PubMed] [Google Scholar]
- 51.Lacoste V, Mauclere P, Dubreuil G, Lewis J, Georges-Courbot MC, Gessain A. 2001. A novel gamma 2-herpesvirus of the Rhadinovirus 2 lineage in chimpanzees. Genome Res 11:1511–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lacoste V, Mauclere P, Dubreuil G, Lewis J, Georges-Courbot MC, Rigoulet J, Petit T, Gessain A. 2000. Simian homologues of human gamma-2 and betaherpesviruses in mandrill and drill monkeys. J Virol 74:11993–11999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laney AS, Peters JS, Manzi SM, Kingsley LA, Chang Y, Moore PS. 2006. Use of a multiantigen detection algorithm for diagnosis of Kaposi's sarcoma-associated herpesvirus infection. J Clin Microbiol 44:3734–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee H, Guo J, Li M, Choi JK, DeMaria M, Rosenzweig M, Jung JU. 1998. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi's sarcoma-associated herpesvirus. Mol Cell Biol 9:5219–5228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee H, Veazey R, Williams K, Li M, Guo J, Neipel F, Fleckenstein B, Lackner A, Desrosiers RC, Jung JU. 1998. Deregulation of cell growth by the K1 gene of Kaposi's sarcoma-associated herpesvirus. Nat Med 4:435–440 [DOI] [PubMed] [Google Scholar]
- 56.Liu C, Okruzhnov Y, Li H, Nicholas J. 2001. Human herpesvirus 8 (HHV8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent antiapoptotic effects. J Virol 75:10933–10940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mansfield KG, Westmoreland SV, DeBakker CD, Czajak S, Lackner AA, Desrosiers RC. 1999. Experimental infection of rhesus and pig-tailed macaques with macaque rhadinoviruses. J Virol 12:10320–10328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mbulaiteye S, Marshall V, Bagni RK, Wang CD, Mbisa G, Bakaki PM, Owor AM, Ndugwa CM, Engels EA, Katongole-Mbidde E, Biggar RJ, Whitby D. 2006. Molecular evidence for mother-to-child transmission of Kaposi sarcoma-associated herpesvirus in Uganda and K1 gene evolution within the host. J Infect Dis 193:1250–1257 [DOI] [PubMed] [Google Scholar]
- 59.Melendez LV, Daniel MD, Garcia FG, Fraser CE, Hunt RD, King NW. 1969. Herpesvirus saimiri. I. Further characterization studies of a new virus from the squirrel monkey. Lab Anim Care 3:372–377 [PubMed] [Google Scholar]
- 60.Melendez LV, Hunt RD, King NW, Barahona HH, Daniel MD, Fraser CE, Garcia FG. 1972. Herpesvirus ateles, a new lymphoma virus of monkeys. Nat New Biol 58:182–184 [DOI] [PubMed] [Google Scholar]
- 61.Mesri EA, Cesarman E, Arvanitakis L, Rafii S, Moore MA, Posnett DN, Knowles DM, Asch AS. 1996. Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J Exp Med 183:2385–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore PS, Gao SJ, Dominguez G, Cesarman E, Lungu O, Knowles DM, Garber R, Pellett PE, McGeoch DJ, Chang Y. 1996. Primary characterization of a herpesvirus agent associated with Kaposi's sarcomae. J Virol 1:549–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Sald J, Knowles DM. 1996. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood 2:645–656 [PubMed] [Google Scholar]
- 64.Nakano K, Isegawa Y, Zou P, Tadagaki K, Inagi R, Yamanishi K. 2003. Kaposi's sarcoma-associated herpesvirus (KSHV)-encoded vMIP1 and vMIP2 induce signal transduction and chemotaxis in monocytic cells. Arch Virol 148:871–890 [DOI] [PubMed] [Google Scholar]
- 65.Nascimento MC, de Souza V, Sumita LM, Freire W, Munoz F, Kim J, Pannuti CS, Mayaud P. 2007. Comparative study of Kaposi's sarcoma-associated herpesvirus serological assays using clinically and serologically defined reference standards and latent class analysis. J Clin Microbiol 3:715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neipel F, Albrecht JC, Ensser A, Huang YQ, Li JJ, Friedman-Kien AE, Fleckenstein B. 1997. Human herpesvirus 8 encodes a homolog of interleukin 6. J Virol 1:839–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parsons CH, Adang LA, Overdevest J, O'Connor CM, Taylor JR, Jr, Camerini D, Kedes DH. 2006. KSHV targets multiple leukocyte lineages during long-term productive infection in NOD/SCID mice. J Clin Invest 116:1963–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poole LJ, Zong JC, Ciufo DM, Alcendor DJ, Cannon JS, Ambinder R, Orenstein JM, Reitz MS, Hayward GS. 1999. Comparison of genetic variability at multiple loci across the genomes of the major subtypes of Kaposi's sarcoma-associated herpesvirus reveals evidence for recombination and for two distinct types of open reading frame K15 alleles at the righthand end. J Virol 8:6646–6660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pyakurel P, Pak F, Mwakigonja AR, Kaaya E, Heiden T, Biberfeld P. 2006. Lymphatic and vascular origin of Kaposi's sarcoma spindle cells during tumor development. Int J Cancer 119:1262–1267 [DOI] [PubMed] [Google Scholar]
- 70.Renne R, Dittmer D, Kedes D, Schmidt K, Desrosiers RC, Luciw PA, Ganem D. 2004. Experimental transmission of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV8) to SIV-positive and SIV-negative rhesus macaques. J Med Primatol 33:1–9 [DOI] [PubMed] [Google Scholar]
- 71.Rezaee SA, Cunningham C, Davison AJ, Blackbourn DJ. 2006. Kaposi's sarcoma-associated herpesvirus immune modulation: an overview. J Gen Virol 87(Pt 7):1781–1804 [DOI] [PubMed] [Google Scholar]
- 72.Rose TM, Ryan JT, Schultz ER, Raden BW, Tsai CC. 2003. Analysis of 4.3 kilobases of divergent locus B of macaque retroperitoneal fibromatosis-associated herpesvirus reveals a close similarity in gene sequence and genome organization to Kaposi's sarcoma-associated herpesvirus. J Virol 77:5084–5097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rose TM, Strand KB, Schultz ER, Schaefer G, Rankin GW, Jr, Thouless ME, Tsai CC, Bosch ML. 1997. Identification of two homologs of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in retroperitoneal fibromatosis of different macaque species. J Virol 5:4138–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ruff K, Baskin GB, Simpson L, Murphey-Corb M, Levy LS. 2003. Rhesus rhadinovirus infection in healthy and SIV-infected macaques at Tulane National Primate Research Center. J Med Primatol 32:1–6 [DOI] [PubMed] [Google Scholar]
- 75.Samols MA, Skalsky RL, Maldonado AM, Riva A, Lopez MC, Baker HV, Renne R. 2007. Identification of cellular genes targeted by KSHV-encoded microRNAs. PLoS Pathog 3:e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schafer A, Cai X, Bilello JP, Desrosiers RC, Cullen BR. 2007. Cloning and analysis of microRNAs encoded by the primate gammaherpesvirus rhesus monkey rhadinovirus. Virology 364:21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schultz ER, Rankin GW, Jr, Blanc MP, Raden BW, Tsai CC, Rose TM. 2000. Characterization of two divergent lineages of macaque rhadinoviruses related to Kaposi's sarcoma-associated herpesvirus. J Virol 74:4919–4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Searles RP, Bergquam EP, Axthelm MK, Wong SW. 1999. Sequence and genomic analysis of a rhesus macaque rhadinovirus with similarity to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J Virol 4:3040–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay MF, Clauvel JP, Raphael M, Degos L. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 4:1276–1280 [PubMed] [Google Scholar]
- 80.Stevenson PG, Efstathiou S. 2005. Immune mechanisms in murine gammaherpesvirus 68 infection. Viral Immunol 18:445–456 [DOI] [PubMed] [Google Scholar]
- 81.Taraboletti G, Benelli R, Borsotti P, Rusnati M, Presta M, Giavazzi R, Ruco L, Albini A. 1999. Thrombospondin 1 inhibits Kaposi's sarcoma (KS) cell and HIV1 Tat-induced angiogenesis and is poorly expressed in KS lesions. J Pathol 188:76–81 [DOI] [PubMed] [Google Scholar]
- 82.Tsai CC, Giddens WE, Jr, Morton WR, Rosenkranz SL, Ochs HD, Benveniste RE. 1985. Retroperitoneal fibromatosis and acquired immunodeficiency syndrome in macaques: epidemiologic studies. Lab Anim Sci 5:460–464 [PubMed] [Google Scholar]
- 83.Tsai CC, Giddens WE, Jr, Ochs HD, Morton WR, Knitter GH, Blakley GA, Benveniste RE. 1986. Retroperitoneal fibromatosis and acquired immunodeficiency syndrome in macaques: clinical and immunologic studies. Lab Anim Sci 2:119–125 [PubMed] [Google Scholar]
- 84.Tsai CC. 1993. Fibromatosis in macaques infected with type D retroviruses. p 48–57 [Google Scholar]
- 85.Wan X, Wang H, Nicholas J. 1999. Human herpesvirus 8 interleukin 6 (vIL6) signals through gp130 but has structural and receptor-binding properties distinct from those of human IL6. J Virol 10:8268–8278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang L, Wakisaka N, Tomlinson CC, DeWire SM, Krall S, Pagano JS, Damania B. 2004. The Kaposi's sarcoma-associated herpesvirus (KSHV/HHV8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res 64:2774–2781 [DOI] [PubMed] [Google Scholar]
- 87.Watanabe T, Sugaya M, Atkins AM, Aquilino EA, Yang A, Borris DL, Brady J, Blauvelt A. 2003. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen prolongs the lifespan of primary human umbilical vein endothelial cells. J Virol 77:6188–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whitby D, Stossel A, Gamache C, Papin J, Bosch M, Smith A, Kedes DH, White G, Kennedy R, Dittmer DP. 2003. Novel Kaposi's sarcoma-associated herpesvirus homolog in baboons. J Virol 77:8159–8165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong SW, Bergquam EP, Swanson RM, Lee FW, Shiigi SM, Avery NA, Fanton JW, Axthelm MK. 1999. Induction of B cell hyperplasia in simian immunodeficiency virus-infected rhesus macaques with the simian homologue of Kaposi's sarcoma-associated herpesvirus. J Exp Med 190:827–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu W, Rochford R, Toomey L, Harrington W, Jr, Feuer G. 2005. Inhibition of HHV8/KSHV-infected primary effusion lymphomas in NOD/SCID mice by azidothymidine and interferon α. Leuk Res 29:545–555 [DOI] [PubMed] [Google Scholar]
- 91.You RI, Chen MC, Wang HW, Chou YC, Lin CH, Hsieh SL. 2006. Inhibition of lymphotoxin β receptor-mediated cell death by survivin–DeltaEx3. Cancer Res 66:3051–3061 [DOI] [PubMed] [Google Scholar]
- 92.Zhu FX, King SM, Smith EJ, Levy DE, Yuan Y. 2002. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF7 phosphorylation and nuclear accumulation. Proc Natl Acad Sci USA 99:5573–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zmonarski SC, Boratynska M, Puziewicz-Zmonarska A, Kazimierczak K, Klinger M. 2005. Kaposi's sarcoma in renal transplant recipients. Ann Transplant 2:59–65 [PubMed] [Google Scholar]