Abstract
Diarrhea is the gastrointestinal disease most frequently encountered in captive rhesus macaques. The precise pathogenic mechanisms underlying chronic diarrhea in nonhuman primates are not well understood, but a persistent inflammatory component has been implicated strongly. This study evaluated the inflammatory changes in the colon of macaques with diarrhea and assessed the efficacy of a 10-d course of tylosin in a cohort of 21 animals with chronic diarrhea. Stool quality was evaluated daily, and fecal consistency was scored. Colonoscopies were performed; biopsy samples were characterized histologically and assayed for expression of TNFα mRNA. Blood samples collected pre-, mid-, and post-treatment were assayed for C-reactive protein (CRP). The results indicated that 63% of the animals receiving tylosin showed improvement in stool quality, compared with 10% in the sham-treated group. Histologically, 82% of animals in the tylosin-treated group had a reduction in the severity of colonic lesions post-treatment, compared with 40% of animals in the sham group. The amount of TNFα mRNA before treatment did not differ from that afterward in either tylosin- or sham-treated animals. CRP levels serially decreased in tylosin-treated monkeys; the average post-treatment CRP value for tylosin-treated animals was 11.96 ± 3.86 μg/ml compared with 26.48 ± 4.86 μg/ml for sham-treated controls. In conclusion, tylosin significantly improved the fecal consistency score, significantly decreased colonic inflammation, and significantly decreased serum CRP levels post-treatment in rhesus macaques with chronic diarrhea.
Abbreviations: CRP, C-reactive protein; IBD, inflammatory bowel disease
Diarrhea in captive nonhuman primates annually affects as many as 15% of animals in some colonies and can account for approximately 33% of deaths not related to research.3 Similarly at the California National Primate Research Center, chronic or recurrent liquid stool that is refractory to treatment is common and is a leading cause of animal death due to euthanasia because of poor condition and failure to respond to therapy. Clinical management of chronic diarrhea in nonhuman primates is often difficult and unrewarding. Multiple drugs commonly are used for treating chronic diarrhea in nonhuman primates and include tetracycline, metronidazole, and prednisone. These drugs also are used frequently in treating canine chronic enteropathies.7,30 In addition to these drugs, the use of tylosin for the treatment of canine chronic diarrhea is becoming common practice. Tylosin can be effective in treating dogs with chronic or intermittent diarrhea, and this disorder is referred to as tylosin-responsive diarrhea.29,30
Tylosin is an antibiotic of the macrolide class; other drugs in this class include erythromycin, azithromycin, and clarithromycin. Tylosin is produced naturally by the bacterium Streptomyces fradiae, acts to inhibit bacterial protein synthesis by inhibiting the 50S ribosome, and is a bacteriostatic drug.19 Its antimicrobial activity is targeted against aerobic gram-positive organisms, some anaerobic Clostridium spp., some gram-negative bacteria (Helicobacter pylori, Haemophilus spp., Pasteurella spp., Legionella spp.), spirochetes, Cryptosporidium parvum, Chlamydia, and Mycoplasma organisms.21,25 Campylobacter spp., which can be enteropathogenic and are common in nonhuman primates, are sensitive to tylosin,7,14 whereas enteric microorganisms such as Escherichia coli and Salmonella spp. are intrinsically resistant.22 Tylosin is licensed for use as a broad-spectrum antibiotic (injectable or oral) for treatment of bacterial infections in livestock and is a common feed additive in food animal production.21
Many antibiotics have been reported to have beneficial immunomodulatory effects on gut mucosa (for example, metronidazole and ciprofloxacin) and can alleviate chronic inflammation in diseases such as inflammatory bowel disease (IBD) or small intestinal bacterial overgrowth.7 Macrolide antibiotics, including tylosin, have been reported to have a positive treatment effect on canine enteropathies that resemble IBD.30 Further, tylosin has been shown to reduce the severity of colonic lesions in a rat model of colitis induced by 2,4,6-trinitrobenzenesulfonic acid.15 In addition to having antimicrobial properties, tylosin likely has antiinflammatory effects that contribute to its effectiveness in treating diarrhea.15,30
Macrolides are widely used as antibacterial drugs. Clinical and experimental data now indicate that the effects of macrolides are not restricted to direct action on bacteria, but they also involve modulation of host defense mechanisms.12,13 The nonantimicrobial, antiinflammatory properties of macrolides were first identified when patients receiving troleandomycin for the treatment of asthma had reduced need for steroids.26 Most of the additional studies on the immunomodulatory effects of macrolides have been in human patients with diffuse panbronchiolitis or other chronic inflammatory respiratory diseases. The mechanism of the antiinflammatory activity of macrolide antibiotics is unclear. However, these antiinflammatory effects include decreased production of proinflammatory cytokines, such as IL8, IL1, IL6, and TNFα, and reduced neutrophil infiltration.8,12,26
Here we report the results of a study to evaluate the efficacy of tylosin for the treatment of chronic diarrhea in rhesus macaques.
Materials and Methods
Animals.
Rhesus macaques in this study were housed at the California National Primate Research Center at the University of California, Davis, an AAALAC-accredited facility, which houses approximately 5000 monkeys. During the period of this study, all project animals were singly housed in indoor cages; they received water ad libitum and commercial monkey chow (Lab Diet, Monkey Diet Jumbo-5037). The ages of the study animals ranged from 8 mo to 6 y. All animals originated at the California National Regional Primate Center and were housed in accordance with the standards of the Guide for the Care and Use of Laboratory Animals.17 An animal care and use protocol for this study was approved by the University of California, Davis Institutional Animal Care and Use Committee.
All animals enrolled in the study were diagnosed with chronic diarrhea. For animals housed indoors, chronic diarrhea was defined as having 45 d of liquid stool in a 90-d period. For animals housed outdoors who were admitted to the veterinary hospital, chronic diarrhea was defined as either 45 d of persistent liquid stool indoors or being admitted for diarrhea 3 or more times in less than a 1-y period. Inclusion criteria for all animals included 3 negative rectal cultures (negative for Salmonella, Shigella, Yersinia, Aeromonas, and Campylobacter jejuni), 1 negative stool sample (negative for Balantidium coli, Entamoeba coli, Entamoeba histolytica, Blastocystis hominis, and Trichuris), a negative immunofluorescent assay for Cryptosporidium spp. and Giardia lamblia, prior treatment with tetracycline (25 mg/kg PO 3 times daily for 10 d), metronidazole (50 mg/kg PO or SC daily for 10 d), dietary fiber supplement (Metamucil, Procter and Gamble, Cincinnati, OH; 1 wafer PO daily for 10 d), an antiparasitic agent (either fenbendazole [Panacur, Intervet, Millsboro, DE] or ivermectin), and a triple-therapy formulation of 20 mg omeprazole, 8 mg prednisone, and 200 mg metronidazole (1 tablet/5 kg PO daily for 10 d). If an animal met all criteria, it was assigned to the project. An additional rectal culture and stool sample were collected immediately prior to initiating the project to confirm that no known bacterial or parasitic infectious agents were present.
A total of 21 macaques with chronic diarrhea were used in this study. The monkeys were assigned randomly to 1 of 2 treatment groups: tylosin-treated (n = 11) and sham-treated (n = 10). Administration of tylosin (20 mg/kg IM daily) or a placebo (sterile water IM daily) was begun 24 h after a baseline colonoscopy procedure and continued for 10 d. The 10-d duration was selected based on clinical evidence in canines, which showed that improvement in fecal consistency should be observed 3 to 5 d after initiating antibiotic therapy.29 In addition, empirical antibiotic treatment for diarrhea typically is administered for 10 d at our facility. After completion of the study and collection of all data, sham animals were given a 10-d course of tylosin to gather additional information and monitor response to treatment. Furthermore, tylosin treatment then was extended for a total of 42 d for all animals who responded to the initial 10-d course. The decision to extend tylosin administration for 6 wk was based on data from small animal medicine that suggests long-term tylosin therapy for the treatment of chronic colitis in canines.30 Animals were followed for 30 d after treatment, and clinical status was evaluated.
Assessment of clinical status.
Throughout the study, 2 animal health technicians, blinded with regard to treatment groups, daily assessed the severity of clinical signs including: alertness (normal or quiet), appetite (good, fair, or poor), hydration (good, fair, or poor), and stool quality. Fecal consistency was evaluated first thing in the morning, prior to cage cleaning. The observed feces therefore represented approximately 12 h of samples. The fecal consistency was described as normal, semisolid, liquid, or a combination thereof; fecal consistency then was assigned a numerical value according to a 7-point scoring system (Table 1).
Table 1.
Fecal consistency scoring system
| Score | Description |
| 1 | Well-formed, normal |
| 1.5 | Normal to semisolida |
| 2 | Semisolid to normal |
| 2.5 | Semisolidb |
| 3 | Semisolid liquid |
| 3.5 | Liquid to semisolid |
| 4 | Liquid |
Normal to semisolid corresponds to more normal stool than semisolid, but both are present; semisolid to normal corresponds to more semisolid stool than normal, and so on.
Semisolid stool is defined as porridge-like or able to be picked up with a fork.
Colonoscopy and biopsy.
All animals were fasted for 36 h prior to colonoscopy. The animals were sedated with ketamine (10 mg/kg IM) and medetomidine (30 μg/kg IM) the morning prior to the procedure to facilitate administration of a gastrointestinal lavage solution (GoLytely, Braintree Laboratories, Braintree, MA) prepared by mixing 68 g of the powder with 1 l citrus-flavored water (Tang, Kraft Foods, Northfield, IL). Initially, 30 ml/kg of the solution was administered slowly via an orogastric tube. The remaining solution was available for the animal to drink via a hanging bottle. If the remaining solution was not consumed, the animal was sedated a second time, approximately 6 h later, to administer a second dose of GoLytely at 30 ml/kg to ensure that a total of 60 ml/kg was ingested.
For the colonoscopy, animals were sedated with 10 mg/kg ketamine IM and 30 μg/kg medetomidine IM. Heart rate and oxygen saturation were monitored with a pulse oximeter. The animal was placed in sternal recumbancy. The scope (model EG1840, Pentax Medical Company, Montvale, NJ) was lubricated, inserted into the rectum, and slowly advanced into the colon approximately 8 to 25 cm proximal to the rectum, depending on the animal's size. Oval biopsy forceps were advanced into the scope, and 6 colonic mucosal tissue samples were collected. For each animal, 3 biopsy samples were placed into a 10% buffered formalin for histological evaluation. The remaining 3 biopsy samples were placed in aqueous tissue storage reagent (RNAlater, Ambion, Austin, TX) and stored at –20 °C for subsequent analysis of TNFα mRNA.
A second colonoscopy was performed as described 2 d after the completion of the 10-d experimental period.
Histopathologic evaluation.
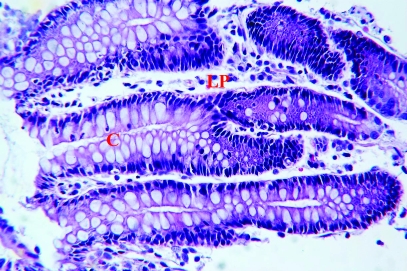
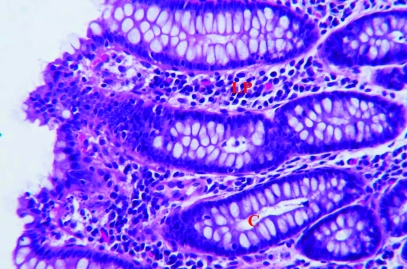
Three of the colonic biopsy samples from each of the 21 rhesus macaques were fixed in 10% neutral buffered formalin, routinely processed for histopathologic examination, sectioned, and stained with hematoxylin and eosin. Histologic examination of all tissues was performed by a single pathologist (RPT), who objectively graded endoscopic specimens and assigned a histologic severity score for each animal. No information regarding treatment type (tylosin or sham) was made available to the pathologist. A histologic diagnosis was assigned and graded as either mild, moderate, or severe colitis. A semi quantitative histologic grading system based on the degree of inflammatory cells infiltrating the lamina propria as well as mucosal epithelial damage was used. Briefly, scores ranging from 0 (normal histology) to 4 (maximum severity of histologic changes) were used to evaluate histologic indices for 1) surface epithelial changes, 2) crypt changes, and 3) lamina propria changes. The total score represents the sum of the individual indices. (Figure 1). Epithelial changes included attenuation, tufting, exocytosis, and ulceration. Crypt changes included the presence of crypt hyperplasia or branching, goblet cell depletion, and crypt abscesses. Lamina propria alterations included the presence of fibrosis or amyloid. Finally, the lamina propria was assessed in terms of overall cellularity and relative proportions of cell types (lymphocytes, plasma cells, macrophages, neutrophils, and eosinophils).
Figure 1.
Representative histology of colon biopsy specimens. (A) Specimen with a histopathologic score of 2 and histologic diagnosis of mild colitis. The epithelium is intact with mild goblet cell depletion and mild lymphocytic lamina propria (LP) cellular infiltrate. (B) Specimen with a histopathologic score of 9 and histologic diagnosis of moderate colitis. The surface epithelium has moderate attenuation and tufting. There is mild goblet cell depletion, mild crypt (C) branching, and mild crypt abscesses. There is a moderate lymphocytic, plasmacytic lamina propria cellular infiltrate. Hematoxylin and eosin stain; magnification, ×375.
TNFα mRNA analysis by real-time PCR.
Total mRNA was isolated from the 3 colon biopsy samples from each of the 21 rhesus macaques. Real-time quantitative PCR was performed as previously described.1 Briefly, samples were tested in duplicate, and amplification reactions for the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase and the target gene were run in parallel on the same plate and were analyzed automatically (ABI 7700 TaqMan Sequence Detector, Applied Biosystems, Foster City, CA).
Relative cytokine mRNA expression levels were calculated from normalized ΔCT (cycle threshold) values. CT values correspond to the cycle number at which the fluorescence due to enrichment of the PCR product reaches significant levels above the background fluorescence (threshold). Results are presented as the CT value for glyceraldehyde-3-phosphate dehydrogenase subtracted from the CT value of the target (cytokine) gene.
C-reactive protein analysis.
C-reactive protein (CRP) is an acute-phase protein in humans and animals that is elevated in serum as a result of injury, infection, or disease. Blood from sedated animals was collected from the femoral vein pre- (day 0 relative to initiation of treatment), mid- (day 5), and post- (day 12) treatment. The blood was separated, and the serum was frozen at –70 °C for future CRP analysis. The serum concentration of CRP was determined by using a commercial enzyme bioassay kit (Monkey CRP ELISA kit, Life Diagnostics, West Chester, PA) according to the manufacturer's protocol, with monkey CRP as a reference standard. Samples were run in duplicate and read at 450 nm, and results were extrapolated from standard curves. In addition, serum from 7 age-matched, healthy animals was analyzed for use as internal controls.
Statistical analysis.
Nonparametric Wilcoxon rank sum tests were used to assess group differences in sham- versus tylosin-treated groups. A 2-sided Fisher exact probability test was used to compare proportions for histologic improvement (Stata Statistical Software, College Station, TX). P values of less than 0.05 were considered significant. Data are expressed as mean ± SEM.
Results
No adverse effects were noted during or after tylosin administration in any of the 21 rhesus macaques studied.
Fecal consistency score.
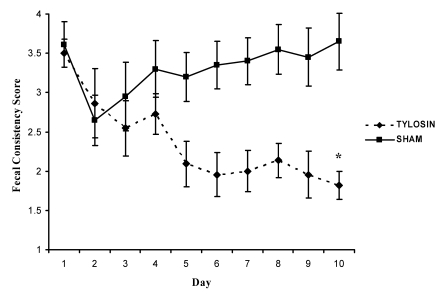
On day 1, the fecal consistency score did not differ significantly between the tylosin- and sham-treated groups (mean ± SEM, 3.5 ± 0.29 and 3.61 ± 0.18, respectively). On day 10, the mean fecal consistency score for tylosin-treated animals showed significant improvement (P = 0.002, Wilcoxon rank-sum test), with a score of 1.82 ± 0.36 compared with 3.65 ± 0.18 for sham-treated animals (Figure 2).
Figure 2.
Daily mean fecal consistency score for tylosin- and sham-treated groups. Over the 10-d course of tylosin, the score gradually decreased, indicating an improvement in stool quality. Day 10 scores differed significantly (P = 0.002, Wilcoxon rank-sum test) between groups. Data are presented as mean ± SE.
To consider the fecal consistency scores of each animal for all 10 d, a median value was determined and then an average was calculated for both the tylosin- and sham-treated groups. The average for each treatment group reflects the full 10 d of fecal consistency scores for each animal, compared with a ‘snapshot’ view of only day 1 or 10 (as just described). The 10-d average fecal consistency score for the tylosin group was 2.15 ± 0.31, compared with 3.55 ± 0.20 for sham-treated animals (P = 0.0039, Wilcoxon rank-sum test). This highly significant difference in the fecal consistency score between tylosin- and sham-treated groups indicates that a 10-d course of tylosin did cause subsidence of diarrhea in monkeys with chronic liquid stool. In addition, 7 of the 11 (64%) animals treated with tylosin had a median fecal consistency score of 2.0 or lower after the 10-d course, compared with only 1 of 10 (10%) animals in the sham-treated group (P = 0.024, Fisher exact probability test).
Colonoscopy.
Pretreatment gross findings on colonoscopy ranged from normal appearance in a few animals to a diffuse, mild to severe inflammation with hyperemia and friable mucosa in the majority of the other animals on study. Pretreatment, 1 animal in the tylosin group showed rare mucosal ulcerations.
Severity of intestinal inflammation.
Morphologic characteristics of the colitis were assessed in biopsy specimens collected before and after treatment. A histologic characteristic of all animals included a diffuse, inflammatory reaction, consisting of a mononuclear cellular infiltration of the colon lamina propria. The mononuclear cell population consisted primarily of lymphocytes and plasma cells. Inflammation typically was limited to the mucosa, with normal muscularis mucosa. A review of the histopathologic diagnoses prior to treatment revealed that 67% of the monkeys had moderate colitis, whereas the remaining 33% of animals had less severe histologic findings. Surface epithelial changes included various degrees of attenuation, tufting, and exocytosis. No surface epithelial ulcerations were present histologically. One animal showed a patchy, blue ‘brush’ border of superficial crypt epithelium. In previous cases at our animal facility, observation of a blue brush border in the superficial crypt epithelium was followed by ultrastructural, immunohistochemical, and molecular studies for colonic Helicobacter spp.; however, such a bacterium was not definitively identified in the current study. Other histologic findings included goblet cell depletion, crypt abscesses, crypt hyperplasia or branching, and lamina propria fibrosis.
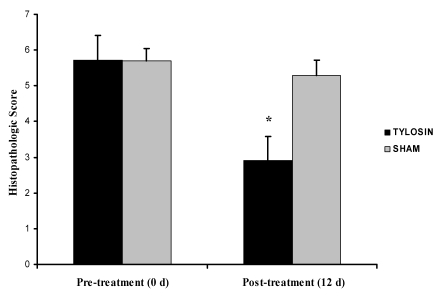
Colon biopsy scores were determined for each animal on the basis of changes in the surface epithelium, crypts, and lamina propria of colonic samples. The pre-treatment mean colonic biopsy scores for tylosin and sham groups were 5.73 ± 0.69 and 5.70 ± 0.68, respectively. The tylosin-treated group had a significant (P = 0.001, Wilcoxon rank sum test) reduction in the mean colonic biopsy score after treatment (2.91 ± 0.34) compared with that for the sham group (5.3 ± 0.42; Figure 3).
Figure 3.
Colonic biopsy scores pre- and post-treatment in tylosin- and sham-treated animals. The histopathologic score post-treatment was decreased in the tylosin-treated group and differed significantly (P = 0.001, Wilcoxon rank-sum test) between groups. Data are presented as mean ± SE.
With regard to each animal's individual colonic biopsy score, 82% of animals in the tylosin group showed a reduction in the severity of colonic lesions after treatment versus 40% of animals in the sham-treated group (P = 0.08, Fisher exact probability test; Table 2).
Table 2.
Colonic histology changes post-treatment in rhesus macaques with chronic diarrhea that received a 10-d course of tylosin (n = 11) or were sham-treated (n = 10)
| Improved | Unchanged | Worsened | |
| Tylosin | 9 (82%) | 1 (9%) | 1 (9%) |
| Sham | 4 (40%) | 2 (20%) | 4 (40%) |
Cytokine gene expression.
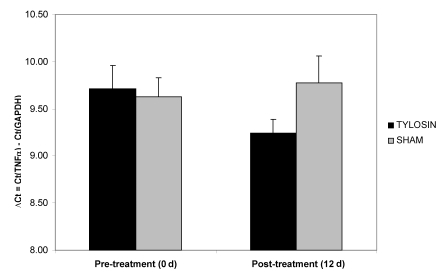
TNFα mRNA was characterized at day 0 and day 12 by means of real-time quantitative RT-PCR in both groups of colitic macaques. TNFα mRNA was detectable in all colonic samples. Pretreatment (day 0) levels of mRNA expression of the cytokine TNFα ranged from 8.62 to 11.54, and were not significantly different between groups. Post-treatment (day 12), the tylosin-treated group showed a nonsignificant (P = 0.103, Wilcoxon rank sum test) decrease in TNFα expression (Figure 4).
Figure 4.
Quantification of TNFα mRNA in colonic biopsies pre- and post-treatment in tylosin and sham treated animals. Numerical values are ΔCt (that is, the cycle threshold of the housekeeping gene [GAPDH] subtracted from that of the TNFα gene). TNFα mRNA levels were decreased after treatment with tylosin, but not significantly compared with those of the sham-treated group. Data are expressed as mean ± SE.
CRP concentrations.
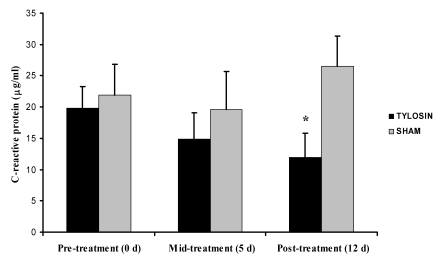
Serum was collected pre- (day 0), mid- (day 5), and post- (day 12) treatment and assayed for CRP, an acute-phase protein synthesized by the liver after tissue damage. The mean CRP value for the 7 normal, control monkeys was 1.25 ± 1.06 μg/ml. Pretreatment CRP values for the 21 colitic monkeys ranged from 0.63 to 44.7 μg/ml; mean CRP on day 0 did not differ between the tylosin- and sham-treated groups (19.78 ± 3.54 and 21.91 ± 4.87 μg/ml, respectively). However CRP levels serially decreased in the tylosin-treated group but not the sham-treated animals. The mean posttreatment CRP value for animals treated with tylosin was 11.96 ± 3.86 μg/ml compared with 26.48 ± 4.86 μg/ml for the sham-treated animals (P = 0.029, Wilcoxon rank sum test; Figure 5). Of the 11 animals treated with tylosin, 9 animals’ (82%) CRP levels decreased from before to after treatment, compared with 3 of 10 sham-treated animals (30%; P = 0.030, Fisher exact probability test). Table 3 summarizes the pre- and posttreatment fecal consistency scores, histopathologic scores, and TNFα and serum C-reactive protein values for each animal.
Figure 5.
Serum C-reactive protein levels for tylosin- and sham-treated groups at 0, 5, and 12 d after initiation of therapy. CRP levels did not differ between groups at either 0 or 5 d, but day 12 levels differed significantly (*, P = 0.029, Wilcoxon rank-sum test) between tylosin- and sham-treated animals. Data are presented as mean ± SE.
Table 3.
Summary of data for individual tylosin-treated (n = 11) and sham-treated (n = 10) animals
| Fecal consistency score |
Histopathologic score |
TNFα (ΔCt) |
C-reactive protein (μg/ml) |
|||||
| Animal no. | day 1 | day 10 | pre-treatment | post- treatment | pre- treatment | post- treatment | pre- treatment | post- treatment |
| Tylosin group | ||||||||
| 32834 | 2.5 | 1 | 3 | 3 | 10.14 | 9.46 | 18.54 | 9.71 |
| 33258 | 4 | 2 | 5 | 1 | 8.89 | 8.80 | 40.12 | 2.75 |
| 34046 | 1 | 1 | 4 | 2 | 8.93 | 9.05 | 2.70 | 1.39 |
| 35638 | 4 | 4 | 7 | 5 | 9.48 | 9.32 | 25.16 | 8.43 |
| 36376 | 4 | 4 | 2 | 3 | 9.15 | 9.61 | 6.74 | 41.24 |
| 36261 | 4 | 1 | 10 | 2 | 9.70 | 9.81 | 22.35 | 29.27 |
| 36551 | 4 | 1 | 7 | 4 | 9.81 | 8.89 | 23.28 | 10.03 |
| 36267 | 3.5 | 2.5 | 6 | 3 | 9.58 | 10.00 | 17.17 | 9.13 |
| 32363 | 4 | 1.5 | 5 | 4 | 11.54 | 9.34 | 30.09 | 16.96 |
| 36147 | 3.5 | 1 | 6 | 2 | 10.73 | 8.82 | 28.02 | 1.97 |
| 36890 | 4 | 1 | 8 | 3 | 8.88 | 8.60 | 3.36 | 0.63 |
| Sham group | ||||||||
| 33250 | 4 | 3 | 8 | 4 | 10.16 | 9.59 | 1.01 | 10.33 |
| 33985 | 3.5 | 4 | 9 | 4 | 9.46 | 8.92 | 35.79 | 8.42 |
| 34801 | 4 | 4 | 7 | 7 | 9.66 | 9.94 | 35.84 | 47.07 |
| 35346 | 4 | 4 | 5 | 7 | 10.03 | 8.78 | 8.40 | 45.87 |
| 36314 | 2.5 | 4 | 3 | 4 | 9.52 | 9.64 | 33.33 | 42.87 |
| 36265 | 4 | 4 | 6 | 4 | 8.65 | 10.21 | 44.70 | 15.65 |
| 36934 | 4 | 2.5 | 2 | 6 | 10.44 | 10.76 | 22.57 | 23.17 |
| 35220 | 1 | 3 | 5 | 5 | 8.62 | 8.42 | 19.22 | 34.94 |
| 37704 | 3 | 4 | 5 | 7 | 9.54 | 11.33 | 0.63 | 26.52 |
| 36855 | 3.5 | 4 | 7 | 5 | 10.21 | 10.12 | 17.58 | 9.95 |
Long-term treatment with tylosin.
In total, 18 of the 21 macaques received long-term tylosin treatment, and feces remained normal throughout the 6-wk treatment period. One month (30 d) after tylosin treatment was discontinued, 11 (61%) of the animals continued to have normal stool, whereas the remaining 7 (39%) had relapse of diarrhea.
Patient follow-up.
At 1 y after study completion, 6 of the 21 (28.5%) study animals no longer have liquid stool and are actively participating in the colony. The remaining 15 animals are no longer alive due to culling, but 2 of the 15 animals were assigned to a study and were sent to necropsy for project purposes. For the group of animals for which diarrhea reappeared, the latency until liquid stool returned ranged from 6 to 158 d.
Discussion
Diarrhea in nonhuman primates remains a serious clinical problem in primate colonies. Diarrhea can lead to dehydration, weight loss, poor body condition, metabolic abnormalities, and even death. Although many animals recover from diarrhea spontaneously or after specific treatment, others develop chronic diarrhea. Chronic diarrhea is a major economic impact in terms of diagnostics and treatment, and often the animals are deemed unsuitable for use in research or breeding.16 Therefore, determining the etiology of and effective treatments for chronic diarrhea in nonhuman primates is highly desirable.
The current study represents the first trial assessing the therapeutic efficacy of tylosin on chronic diarrhea in a nonhuman primate. Our results suggest that parenteral administration of tylosin provided therapeutic benefit, as assessed by improved fecal consistency and reduced severity of colonic lesions.
The exact mechanism of tylosin responsive diarrhea is unknown. Explanations for improvement in clinical signs in tylosin-treated animals include the possibility that tylosin-sensitive bacteria reside in the gastrointestinal tract. These organisms likely are common residents that perpetuate intestinal inflammation. The action of tylosin could be mediated by a decrease in total bacterial counts or by suppression of a specific bacterial species. In addition, reportedly only 40% of the bacterial strains present in the intestinal flora of humans is culturable, 27 and this scenario likely holds for macaques as well; therefore, tylosin may be affecting bacterial species not yet characterized or isolated. Another explanation for the favorable response may lie in tylosin's ability to interfere with bacterial adhesion to gut mucosa.25,30 The first step in the pathogenic process is the attachment and adherence of the bacterial organisms to the host, and macrolide agents are known to impair the adherence of gram-negative and gram-positive bacteria to the mucosa.25
The immunomodulatory properties of tylosin might explain the positive response seen in this cohort of animals. Tylosin can exert therapeutic effects independent of its antibacterial activity. Macrolides have been reported to alter immune and inflammatory responses in the host both in vivo and in vitro; in humans, macrolide antibiotics (such as erythromycin) have immunomodulatory effects on leukocytes, especially macrophages.5,25 Tylosin suppresses transcription of the inducible nitric oxide synthase and cyclooxygenase 2 and decreases the production of TNFα, IL1β, and IL6 in lipopolysaccharide-induced mononuclear cells.5 Other research has demonstrated similar inhibitory effects on these cytokines with the use of clarithromycin and azithromycin in human macrophages and monocytes.8,12
TNFα is an important cytokine involved in systemic inflammation and the acute-phase response. Elevated levels of TNFα have consistently been found in humans with IBD and in 1 study of macaques with chronic colitis.4,6,20,23,24 In human IBD, the inflammatory properties of TNFα lead to the activation of macrophages, priming of neutrophils, and increased epithelial permeability.2 Other animal species with spontaneous or induced mucosal inflammation also have demonstrated the importance of TNFα. Trinitrobenzene sulfonic acid-induced chronic mucosal inflammation in mice results in a Crohn's disease-like illness; Crohn's disease is 1 of the 2 chronic, idiopathic disorders that fall under the broad classification of IBD. Mononuclear cells from the lamina propria of these mice generate concentrations of TNFα mRNA that are 10- to 30-fold greater than those produced by cells from control mice.18 In addition, increased fecal TNFα concentrations have been demonstrated in cottontop tamarins with active colitis.28 The cottontop tamarin is a spontaneous model of IBD that shows similarities to ulcerative colitis in humans.
In the present study, TNFα expression did not differ significantly between tylosin- and sham-treated macaques on either day 0 or 12. Cytokine expression did not correlate with the severity of enteric symptoms. Why TNFα expression did not significantly decrease with treatment is unclear. Perhaps the duration of treatment was too brief, or perhaps TNFα expression was not increased in this cohort of animals initially. Animals in this study had chronic diarrhea lasting for months to years, and TNFα decreases with disease duration in pediatric patients with Crohn's disease.6 In addition, TNFα might have continued to decrease post-treatment, such that biopsy samples taken beyond 12 d might have shown reduced cytokine expression.
Because TNFα does not appear to have been a key mediator in colonic inflammation in our animals, a more extensive cytokine evaluation is desirable. By identifying cytokines that are altered in this colitis model, we could better clarify their role in the pathogenesis of enteric infections and offer insight regarding new treatment modalities. In addition, a research avenue to consider is extensive evaluation of fecal bacterial populations by using molecular techniques. By evaluating the changes and shifts in the organisms in the feces of tylosin-treated animals before and during antibiotic therapy, it might be possible to identify a causative agent.
Treatment with tylosin significantly decreased CRP levels in this cohort of rhesus macaques with chronic diarrhea. CRP is an acute-phase protein in humans and animals that is elevated in serum as a result of injury, infection, or disease.9,10 CRP levels are nonspecific markers of inflammation. These proteins typically are involved in the regulation of the host's early response to an agent causing inflammation and of immune system activity in general. During inflammatory disease, hepatic synthesis of CRP is upregulated dramatically and may increase 100-fold or more during the acute-phase response.11
In small animal medicine, CRP is not only used as a marker of systemic inflammation but also of the severity of intestinal disease in particular. CRP is elevated in dogs with IBD, and serum levels of this protein correlate well with clinical severity indices.9 The greatest clinical utility of this assay is likely to be in the monitoring of response to treatment. It is reasonable to expect that effective therapy will be associated with a decrease in the patient's serum CRP. In the present study, CRP levels clearly demonstrate the efficacy of tylosin treatment.
In summary, this study indicates that the macrolide drug tylosin is efficacious in improving stool quality in macaques with chronic colitis and in decreasing inflammation in the distal colon. Our results do not reveal whether the effect of tylosin was mediated by a decrease in total bacterial counts, by suppression of a specific bacterial species, or by the agent's immunomodulatory effects. Most of the animals that initially responded to tylosin had normalized stool 30 d after treatment ended. These data are clinically important, considering that all animals in the study were previously nonresponsive to typical empirical antibiotic therapy.
Acknowledgments
The authors would like to thank Keith Mansfield at the New England Primate Research Center for his initial suggestion regarding the use of tylosin for treatment of chronic diarrhea in macaques; Miles Christensen and Allyn Marsh for their willingness to evaluate stool quality for many days; Kristina Abel and the Virology and Immunology Laboratory for their technical assistance in analyzing TNFα; and Shelley Lenz for her valuable comments on an earlier draft.
References
- 1.Abel K, Pahar B, Van Rompay KKA, Fritts L, Sin C, Schmidt K, Colon R, McChesney M, Marthas ML. 2006. Rapid virus dissemination in infant macaques after oral simian immunodeficiency virus exposure in the presence of local innate immune responses. J Virol 80:6357–6367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blam ME, Stein R, Lichtenstein G. 2001. Integrating anti-tumor necrosis factor therapy in inflammatory bowel disease: current and future prospectives. Am J Gastroenterol 96:1977–1997 [DOI] [PubMed] [Google Scholar]
- 3.Brady A, Morton D. Gastrointestinal system: approach to diarrhea diagnosis and treatment. In: Bennett A, Henrickson, editors Nonhuman primates in biomedical research: diseases San Diego: Academic Press; [Google Scholar]
- 4.Braegger CP, Nicholls S, Murch SH, MacDonald TT, Stephens S. 1992. Tumour necrosis factor α in stool as a marker of intestinal inflammation. Lancet 339:89–91 [DOI] [PubMed] [Google Scholar]
- 5.Cao XY, Dong M, Shen JZ, Wu BB, Wu CM, Du XD, Wang Z, Qi YT, Li BY. 2006. Tilmicosin and tylosin have antiinflammatory properties via modulation of COX-2 and iNOS gene expression and production of cytokines in LPS-induced macrophages and monocytes. Int J Antimicrob Agents 27:431–438 [DOI] [PubMed] [Google Scholar]
- 6.Dionne S, Hiscott J, D'Agata I, Duhaime A, Seidman EG. 1997. Quantitative PCR analysis of TNFα and IL1β mRNA levels in pediatric IBD mucosal biopsies. Dig Dis Sci 42:1557–1566 [DOI] [PubMed] [Google Scholar]
- 7.Hall E. 2002. Rational selection of gastrointestinal drugs for cats and dogs. In Pract 24:242–249 [Google Scholar]
- 8.Ianaro A, Ialenti A, Maffia P, Sautebin L, Rombolà L, Carnuccio R, Iuvone T, D'Acquisto F, Rosa MD. 2000. Antiinflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther 292:156–163 [PubMed] [Google Scholar]
- 9.Jergens AE, Schreiner CA, Frank DE, Niyo Y, Ahrens FE, Eckersall PD, Benson TJ, Evans R. 2003. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med 17:291–297 [DOI] [PubMed] [Google Scholar]
- 10.Jinbo T, Ami Y, Suzaki Y, Kobune F, Ro S, Naiki M, Iguchi K, Yamamoto S. 1999. Concentrations of C-reactive protein in normal monkeys (Macaca irus) and in monkeys inoculated with Bordetella bronchiseptica R-5 and Measles Virus. Vet Res Commun 23:265–274 [DOI] [PubMed] [Google Scholar]
- 11.Jinbo T, Hayashi S, Iguchi K, Shimizu M, Matsumoto T, Naiki M, Yamamoto S. 1998. Development of monkey C-reactive protein (CRP) assay methods. Vet Immunol Immunopathol 61:195–202 [DOI] [PubMed] [Google Scholar]
- 12.Khan AA, Slifer TR, Araujo FG, Remington JS. 1999Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int J Antimicrob Agents 11:121–132 [DOI] [PubMed] [Google Scholar]
- 13.Labro MT. 1998. Antiinflammatory activity of macrolides: a new therapeutic potential?. J Antimicrob Chemother 41:37–46 [DOI] [PubMed] [Google Scholar]
- 14.Marks SL, Kather E. 2003. Bacterial-associated diarrhea in the dog: a critical appraisal. Vet Clin North Am Small Anim Pract 33:1029–1060 [DOI] [PubMed] [Google Scholar]
- 15.Menozzi A, Pozzolia C, Polia E, Lazzarettib M, Cantonia A, Grandia D, Giovanninic E, Coruzzia G. 2005. Effect of the macrolide antibacterial drug, tylosin, on TNBS-induced colitis in the rat. Pharmacology 74:135–142 [DOI] [PubMed] [Google Scholar]
- 16.Munoz-Zanzi CA, Thurmond M, Hird D, Lerche N. 1999. Effect of weaning time and associated management practices on postweaning chronic diarrhea in captive rhesus monkeys (Macaca mulatta). Lab Anim Sci 49:617–621 [PubMed] [Google Scholar]
- 17.National Research Council 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 18.Neurath MF, Fuss I, Pasparakis M, Alexopoulou L, Haralambous S, Meyer zum Buschenfelde KH, Strober W, Kollias G. 1997. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur J Immunol 27:1743–1750 [DOI] [PubMed] [Google Scholar]
- 19.Noli C, Boothe D. 1999. Macrolides and lincosamides. Vet Dermatol 10:217–223 [DOI] [PubMed] [Google Scholar]
- 20.Papadakis KA, Targan SR. 2000. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med 51:289–298 [DOI] [PubMed] [Google Scholar]
- 21.Plumb D. 2002. Veterinary drug handbook. Ames (IA): Iowa State Press [Google Scholar]
- 22.Poole TL, Genovese KJ, Knape KD, Callaway TR, Bischoff KM, Nisbet DJ. 2003. Effect of subtherapeutic concentrations of tylosin on the inhibitory stringency of a mixed anaerobe continuous-flow culture of chicken microflora against Escherichia coli O 157:H 7. J Appl Microbiol 94:73–79 [DOI] [PubMed] [Google Scholar]
- 23.Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. 1993. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol 94:174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sestak K, Merritt CK, Borda J, Saylor E, Schwamberger SR, Cogswell F, Didier ES, Didier PJ, Plauche G, Bohm RP, Aye PP, Alexa P, Ward RL, Lackner AA. 2003. Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus macaques. Infect Immun 71:4079–4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shryock TR, Mortensen JE, Baumholtz M. 1998. The effects of macrolides on the expression of bacterial virulence mechanisms. J Antimicrob Chemother 41:505–512 [DOI] [PubMed] [Google Scholar]
- 26.Tamaoki J, Katdota J, Takizawa H. 2004. Clinical implications of the immunomodulatory effects of macrolides. Am J Med 117:5S–11S [DOI] [PubMed] [Google Scholar]
- 27.Tannock GW. The normal microflora: an introduction. In: Tannock GW, editor Medical importance of normal microflora London: Kluwer Academic Publishers [Google Scholar]
- 28.Watkins PE, Warren BF, Stephens S, Ward P, Foulkes R. 1997. Treatment of ulcerative colitis in the cottontop tamarin using antibody to tumour necrosis factor α. Gut 40:628–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westermarck E, Frias R, Skrzypczak T. 2005. Effect of diet and tylosin on chronic diarrhea in beagles. J Vet Intern Med 19:822–827 [DOI] [PubMed] [Google Scholar]
- 30.Westermarck E, Skrzypczak T, Harmoinen J, Steiner JM, Ruaux CG, Williams DA, Eerola E, Sundback P, Rinkinen M. 2005. Tylosin-responsive chronic diarrhea in dogs. J Vet Intern Med 19:177–186 [DOI] [PubMed] [Google Scholar]