Abstract
The pathology of 33 moustached tamarins (Saguinus mystax) previously used in hepatitis A and GB virus studies is reported. Chronic lesions in colon, heart, and kidney were common in the monkeys and appeared not to be due to the experimental exposures. Colitis cystica profunda (CCP), a disease that affects humans and is characterized by the presence of mucin-filled epithelial downgrowths and cysts in the colonic submucosa, was found in 24 of the 33 (72.7%) tamarins. Interstitial myocardial fibrosis was present in 22 (66.6%) animals, and various degrees of membranoproliferative glomerulonephritis occurred in 28 (84.8%) monkeys. In addition, 28 (84.8%) tamarins demonstrated diffuse hepatocellular vacuolation with mild lymphoplasmacytic infiltrates, possibly as a result of the experimental infections, and peliosis hepatis occurred in 7 (21.2%) animals. The etiology of CCP is unknown, and no reliable animal models are available because most cases in animals are reported only sporadically. Myocardial fibrosis in tamarins has not been reported previously, and all current animal models require experimental manipulation of the animal to mimic the human disease. The results from this study suggest that captive S. mystax has high incidence of spontaneous CCP, myocardial fibrosis, and membranoproliferative glomerulonephritis. This species may be a spontaneous animal model for pathogenesis and experimental therapy studies of the analogous human diseases.
Abbreviations: CCP, colitis cystica profunda
The moustached tamarin (Saguinus mystax), a small New World nonhuman primate, is a valuable animal model for viral hepatitis A pathogenesis and experimental vaccine studies.10 The most common reported causes of morbidity and mortality in captive tamarin populations are gastroenteric lesions13 and ‘wasting marmoset syndrome,’ a term commonly used to describe a poorly understood syndrome that affects marmosets and tamarins in captivity and characterized by chronic diarrhea, weight loss, decreased muscle mass, anemia, hypoglycemia, and alopecia.2 Colitis cystica profunda (CCP), a rare but well-recognized nonneoplastic disease affecting the colon in humans, characterized by the presence of mucin-filled cysts with benign epithelial lining in the colonic submucosa,32 occurred in a tamarin colony, was associated with chronic diarrhea, and was suggested to be the cause of wasting marmoset syndrome.15 Macroscopically, CCP often resembles a malignant tumor, but progression to dysplasia or neoplasia has not been reported. In humans, CCP has been associated with a variety of diseases, including adenocarcinoma of the colon, rectal prolapse, and solitary rectal ulcer.32,33 Clinical signs include rectal bleeding and excess straining during defecation and, less frequently, rectal pain and constipation or diarrhea.32 At present, the etiology of CCP remains unknown. Treatment of this condition in humans remains controversial, with surgical resection of the affected tissue the most effective option at present.32,33 Here we describe a high incidence of CCP affecting captive moustached tamarins.
Myocardial fibrosis, a common condition in humans with pressure overload hypertrophic cardiomyopathy,28 has occurred in owl monkeys and chimpanzees.12,16,17,21 In owl monkeys, myocardial fibrosis appears to be a consequence of arterial hypertension and myocardial infarcts.12 In chimpanzees, myocardial fibrosis seems to be the most likely cause for cardiac arrythmias and sudden death in this species.21 Cardiac fibrosis is the main cause of cardiac failure and death in humans with arterial hypertension and dilated cardiomyopathy.28,35 Myocardial fibrosis affected the moustached tamarins in this study; to our knowledge, myocardial fibrosis in tamarins has not been reported previously.
Renal disease is a common finding in captive callitrichids. IgM nephropathy in tamarins has been described.6 Mesangial IgM deposits in young tamarins and glomerulosclerosis without IgM deposits in older tamarins have occurred in the same colony.4 In addition, proliferative glomerulonephritis associated with arteritis in S. labiatus and S. oedipus inoculated with hepatitis A virus has been reported.23 More recently, IgM in conjunction with IgA deposits were reported to be the cause of glomerulopathy in tamarins.5 These studies suggest that the tamarin nephropathy is an immunologic condition. We found a high incidence of membranoproliferative glomerulonephropathy in the tamarins in this study.
In addition, tamarins in the present study demonstrated diffuse hepatocellular vacuolation and periportal lymphoplasmacytic infiltrates, possibly as a result of experimental inoculation with hepatitis A virus.19 Interestingly, peliosis hepatis, a rare condition in humans and animals that is characterized by dilatation of hepatic sinusoids and the presence of blood-filled spaces within the liver, was found in some tamarins in the present study. In humans, peliosis hepatis occurs mainly in subjects exposed to toxic substances or estrogens and is often asymptomatic.3 To our knowledge, peliosis hepatis has not been reported to occur in nonhuman primates.
In this retrospective study, we describe S. mystax as a model for the study of CCP in light of the characteristic lesion in tamarins that closely resembles the human condition and the relatively high incidence of spontaneous CCP in this species compared with other animals. In addition, the increased incidences of myocardial fibrosis, membranoproliferative glomerulonephropathy, and peliosis hepatis in these animals suggest that this species could be a potential spontaneous animal model for pathogenesis and experimental therapy studies of the analogous human diseases.
Materials and Methods
Between 1996 and 2004, complete necropsies were performed on 33 S. mystax that died or were euthanized because of untoward clinical signs or poor response to treatment. Clinical records and necropsy reports were not available for all animals; records were available for 15 male and 8 female tamarins. All animals were adults, except for 1 monkey that was 8 mo old at the time of death (Table 1). Because most animals were wild-caught, their exact ages were unknown. Except for 4 animals born in captivity, the monkeys were captured in the Peruvian Amazon basin region by personnel from the Center for Reproduction and Conservation of Nonhuman Primates (Iquitos, Perú) and transferred to the National Institute of Allergy and Infectious Diseases through an agreement with the Pan American Health Organization. The wild-caught animals came in separate shipments, between 1995 and 1998, and were quarantined at the Perrine Primate Center (Perrine, FL) and later at the National Institutes of Health primate quarantine facility (Poolesville, MD). As part of their physical examinations, the animals were screened for intestinal pathogens by use of bacterial cultures and by use of wet mounts and fecal flotation for parasitologic examination. All tamarins were enrolled in IACUC-approved viral hepatitis (hepatitis A virus and hepatitis GB virus type B) studies. However, at least 4 animals were research-naïve at the time of death. The monkeys were housed and cared for according to the Guide for the Care and Use of Laboratory Animals24 and animal welfare regulations. Standard husbandry procedures included feeding commercially formulated chow (New World Primate Diet 5040, Purina Mills, St Louis, MO; Marmoset Diet, ZuPreem, Mission, KS), dietary supplements (bananas, apples, and marshmallows), and water ad libitum. The animals were housed in stainless steel 6.0 ft2 biocontainment cages (Primate Products, Miami, FL) with polyvinyl chloride nesting boxes, ‘nectar logs,’ and other callitrichid environmental enrichment objects. A 12:12-h dark:light photoperiod cycle was observed; room temperature was maintained at 24 ± 2 °C. Animals were pair-housed (male–female) when possible, except while they were on study. At least 15 tamarins were inoculated with hepatitis A virus and 5 with hepatitis GB virus type B, and viral shedding and liver enzymatic activity were followed for 3 to 4 mo. Seven tamarins were on study (from 3 d to 3 mo after infection) when they died; 11 animals were considered to be off study (greater than 4 mo after infection), and 4 (possibly 5, given that the 8-mo-old monkey was too young to be used in viral hepatitis studies) were research-naïve at the time of death. Tissues from all major organs were fixed in 10% neutral buffered formalin, embedded in paraffin, cut at 4 to 5 µm, and stained with hematoxylin and eosin. In addition, selected slides were stained with picrosirius red, Masson trichrome, periodic acid–Schiff, and Warthin–Starry silver stain. The various lesions were graded on an arbitrary scale of 0 to 3+.
Table 1.
Summary of histologic findings and clinical or gross diagnosis in 33 captive moustached tamarins
| Severity of lesionc |
||||||||
| Agea | Sex | Statusb | CCP | MF | CGN | HV | Other microscopic lesions | Clinical or gross diagnosis |
| 8 mo | male | naive | – | – | – | – | no significant findings | no gross lesions |
| 2 y | male | naive | – | – | + | ++ | no significant findings | neurologic signs |
| 4 y | male | HAV | + | ++ | +++ | + | no significant findings | hematoma |
| unknown | male | HAV | +++ | ++ | +++ | +++ | pneumonia | unknown |
| unknown | male | HAV | – | +++ | +++ | +++ | no significant findings | cardiomegaly |
| unknown | male | HAV | +++ | ++ | + | ++ | no significant findings | congestive heart failure |
| unknown | male | HAV | +++ | +++ | ++ | – | no significant findings | congestive heart failure |
| unknown | male | HAV | +++ | + | +++ | +++ | peliosis hepatis, renal infarct | congestive heart failure |
| unknown | male | HAV | – | ++ | +++ | ++ | peliosis hepatis, renal infarct | congestive heart failure |
| unknown | male | HAV | ++ | – | +++ | – | no significant findings | no gross lesions |
| unknown | male | HAV | - | - | + | + | no significant findings | cardiomegaly |
| unknown | male | GBV-B | ++ | + | ++ | – | lung nematodes | congestive heart failure |
| unknown | male | GBV-B | + | + | ++ | ++ | peliosis hepatis | congestive heart failure |
| unknown | male | GBV-B | + | – | + | – | no significant findings | hematoma |
| unknown | male | GBV-B | ++ | +++ | ++ | +++ | no significant findings | unknown |
| unknown | female | HAV | – | +++ | + | + | pneumonia | congestive heart failure |
| unknown | female | HAV | – | +++ | ++ | + | no significant findings | no gross lesions |
| unknown | female | HAV | + | + | + | + | no significant findings | liver failure |
| unknown | female | HAV | ++ | ++ | ++ | +++ | visceral larva migrans | congestive heart failure |
| unknown | female | GBV-B | + | +++ | ++ | ++ | no significant findings | cardiomegaly |
| unknown | female | naive | + | – | +++ | ++ | no significant findings | renal disease |
| unknown | female | naive | +++ | ++ | +++ | ++ | renal infarct | hepatopathy |
| unknown | female | naive | +++ | – | ++ | ++ | peliosis hepatis | cardiopathy, hepatopathy |
| unknown | unknown | HAV | + | – | + | ++ | no significant findings | liver failure |
| 2 y | unknown | HAV | + | – | – | + | no significant findings | hematoma |
| unknown | unknown | unknown | – | – | +++ | ++ | peliosis hepatis | unknown |
| unknown | unknown | unknown | – | +++ | – | +++ | no significant findings | unknown |
| unknown | unknown | unknown | + | ++ | +++ | +++ | peliosis hepatis | unknown |
| unknown | unknown | unknown | +++ | ++ | ++ | ++ | peliosis hepatis | unknown |
| unknown | unknown | unknown | +++ | + | ++ | +++ | no significant findings | unknown |
| unknown | unknown | unknown | + | – | – | + | pneumonia | unknown |
| unknown | unknown | unknown | + | +++ | – | ++ | pneumonia | unknown |
| unknown | unknown | unknown | +++ | +++ | ++ | ++ | no significant findings | unknown |
CCP, colitis cystica profunda; CGN, membranoproliferative or chronic glomerulonephritis; HV, hepatocellular vacuolation or swelling; MF, myocardial fibrosis
Animals with known ages were captive-born; others with unknown ages were wild-caught as adults.
Experimentally infected with hepatitis A virus (HAV), hepatitis GB virus type B (GBV-B), or research-naive
Severity: –,no lesions; +, mild; ++, moderate; +++, marked
Results
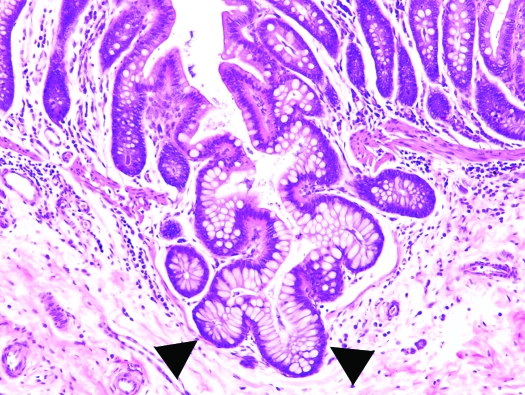
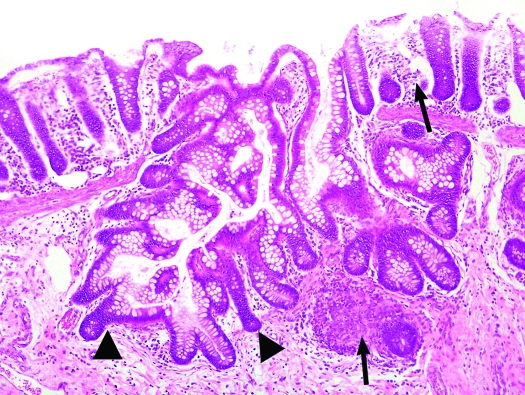
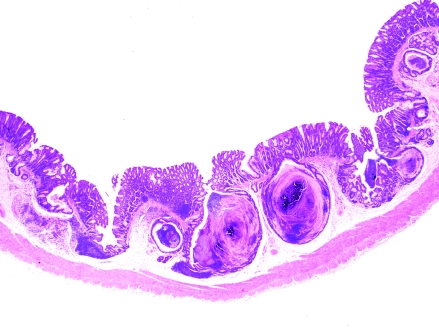
Of the 33 tamarins, 12 showed clinical signs or those at necropsy (or both) of congestive heart failure or cardiomegaly. At necropsy, 4 tamarins were suspect for hepatopathy or hepatic failure, 3 showed subcutaneous hematomas after phlebotomy, 1 was diagnosed grossly with chronic renal disease, 1 showed neurologic signs before death, and 3 animals lacked noteworthy findings at necropsy. Microscopically, we found several common lesions among the tamarins (Tables 1 and 2). Specifically, 24 of the 33 tamarins (72.7%) had variable degrees of diffuse CCP, characterized by mucus-filled cysts in the colonic and cecal mucosa, submucosa, and muscularis propria; these cysts frequently were lined by demonstrable epithelium. The lesions did not appear to have a predilection for a particular section of the large intestine, as is often the case in the diffuse form of CCP, in which lesions can be found from the cecum all the way to the rectum.32 Fifteen tamarins had mild to moderate lesions, whereas 9 animals showed marked lesions. Initial or mild stages of the disease (grade, 1+) were characterized by minimal proliferation of mature colonic glands and herniation through breaks in the muscularis mucosae, with no or mild diffuse lymphoplasmacytic infiltration in the submucosa and/or lamina propria (Figure 1). Cases of moderate CCP (grade, 2+) were characterized by herniation of colonic mucosal glands, with mild cystic dilatation and accumulation of mucus and sloughed cells (Figure 2). Marked lesions (grade, 3+) were characterized by large cysts containing mucus, cellular debris, and bacterial colonies that bulged over the mucosal epithelium, in some instances rupturing toward the intestinal lumen (Figure 3). No neoplastic changes or dysplasia were noted. In some cases, lymphoid hyperplasia in the lamina propria was present. Lesions compatible with CCP were observed in experimental as well as in research-naïve animals and affected both sexes. In those cases for which detailed records were available, CCP was present in 3 of 4 (75%) research-naïve animals, 10 of 13 (77%) research males, 3 of 5 (60%) research females, and in all 3 (100%) research-naïve females (these are the same 3 of 4 research-naïve animals described above, the only research-naïve male didn't have CCP). In addition, CCP was present in 2 of the 4 (50%) tamarins born in captivity.
Table 2.
Incidence of colonic, cardiac, renal, and hepatic lesions in 33 captive moustached tamarins
| Severity of lesion |
|||||
| Organ | Lesion | mild | moderate | marked | Total (%) |
| Colon | CCP | 11 | 4 | 9 | 24 (72.7) |
| Heart | MF | 5 | 8 | 9 | 22 (66.6) |
| Kidney | CGN | 7 | 11 | 10 | 28 (84.8) |
| Liver | HV | 7 | 13 | 8 | 28 (84.8) |
CCP, colitis cystica profunda; CGN, membranoproliferative or chronic glomerulonephritis ; HV, hepatocellular vacuolation; MF, myocardial fibrosis;
Figure 1.
Colon, Saguinus mystax. Note the branching dilated glands extending through the disrupted muscularis mucosae and into the submucosa (arrowhead). Hematoxylin and eosin stain; magnification, ×200.
Figure 2.
Colon, S. mystax. More extensive branching of colonic crypts, extending through the disrupted muscularis mucosae into the submucosa (arrowheads) and inflammatory cell infiltrates within the lamina propria and submucosa (arrows). Magnification, ×100.
Figure 3.
Colon, S. mystax. Low magnification showing various degrees of herniation of colonic mucosal glands, with marked cystic dilatation and accumulation of mucus and sloughed cells. Hematoxylin and eosin stain; magnification, ×12.5.
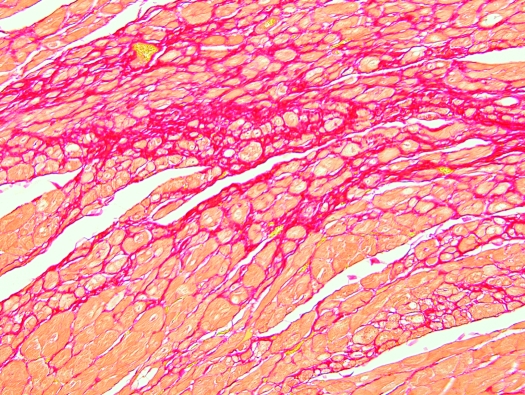
Lesions common to multiple tamarins also were found in the heart, kidney, and liver, but there was no clear association between the presence of CCP and these lesions. Interstitial myocardial fibrosis was present in 22 animals (66.6%), membranoproliferative glomerulonephritis in 28 tamarins (84.8%), and hepatic lesions composed mainly of diffuse hepatocellular vacuolation or swelling with mild lymphoplasmacytic infiltrates in 28 animals (84.8%). Multifocally, cardiac myocytes were separated, expanded, and replaced by mild to moderate amounts of dense mature fibrous connective tissue that stained red with picrosirius red stain for collagen (Figure 4) and blue with Masson trichrome stain. Fibrosis affected the left ventricle, interventricular septum, right ventricle, and, to a lesser degree, the atria. Discrete myocardial wavy fibers were present in a few cases, with the exception of 1 animal that showed marked angulations of cardiac myocytes (Figure 5). Myocardial fibrosis occurred in 1 of 4 (25%) research-naïve tamarins, 10 of 13 (77%) research males, all 5 (100%) research females, and 1 of the 4 (25%) captive-born tamarins. Cardiac fibrosis (grade, 1+ to 3+) was graded based on increasing amounts of collagenous connective tissue separating or replacing cardiac myocytes in slides stained with hematoxylin and eosin and confirmed by specific collagen stains. Higher-grade fibrosis often was associated with myocyte degeneration and cytoplasmic vacuolization.
Figure 4.
Heart, S. mystax. Cardiac myocytes separated, expanded, and replaced by mild to moderate amounts of dense mature fibrous connective tissue (red). Picrosirius red stain; magnification, ×100.
Figure 5.
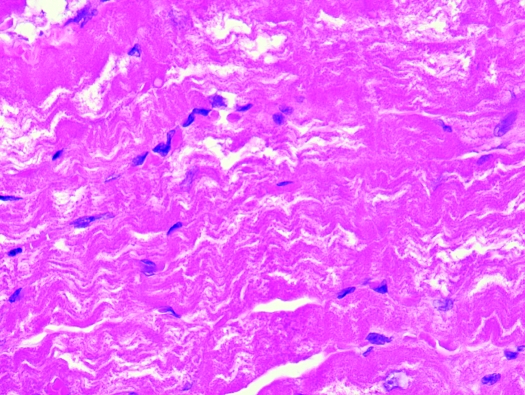
Heart, S. mystax. Myocardial wavy fibers characterized by marked angulations of cardiac myocytes. Hematoxylin and eosin stain; magnification, ×400.
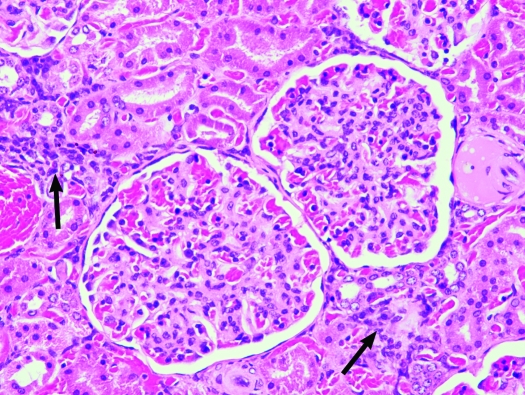
Renal lesions consisted of mesangial cell proliferation with variable thickening of the mesangial matrix, mild to moderate interstitial fibrosis, mild to marked tubular proteinosis, and multifocal lymphoplasmacytic interstitial inflammation (Figure 6). Renal lesions were present in all 4 (100%) research-naïve animals, all 13 (100%) research males, all 5 (100%) research females, and in 2 of the 4 (50%) tamarins born in captivity. Mild (grade 1+) renal lesions were characterized by small scattered foci of primarily lymphocytes and plasma cells within the interstitium; moderate to marked (grades 2+ and 3+) renal lesions were characterized by increased lymphocytes and plasma cells within the interstitium, increasing amounts of pale eosinophilic homogenous proteinaceous material deposited within the mesangial matrix, thickening of Bowman capsules, interstitial expansion by collagenous connective tissue (fibrosis), glomerular sclerosis, mineralization, proximal tubular epithelial degeneration, and tubular proteinosis. Prominent vascular changes associated with higher-grade disease included medial hypertrophy, perivascular fibrosis, and, occasionally, focal cortical infarction.
Figure 6.
Kidney, S. mystax. Membranoproliferative glomerulonephritis with mild multifocal lymphoplasmacytic interstitial foci (arrows). Hematoxylin and eosin stain; magnification, ×200.
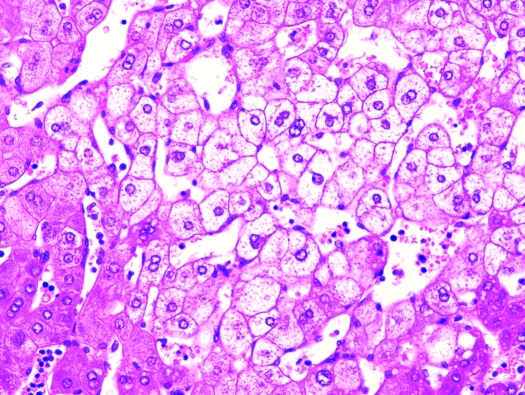
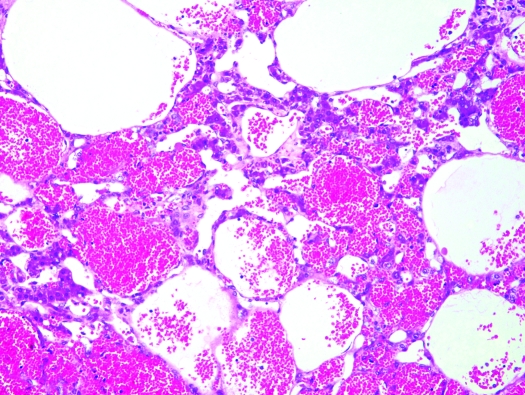
In liver, biliary hyperplasia, periportal fibrosis, hepatocellular vacuolar change, and multifocal lymphoplasmacytic infiltrates were present histologically to various degrees in the majority of animals (Figure 7). Liver lesions were observed in all 4 (100%) research-naïve tamarins, 9 of 13 (69%) research males, all 5 (100%) research females, and in 3 of 4 (75%) captive-born animals. Mild (grade 1+) hepatic lesions were characterized by few scattered aggregates of lymphocytes and plasma cells within the parenchyma and mild multifocal to locally extensive hepatocellular cytoplasmic vacuolization. Grade 2+ disease was characterized by increased lymphoid infiltrates with moderate to marked hepatocellular vacuolization, and grade 3+ hepatic lesions were characterized by diffuse hepatocellular vacuolization with multifocal to locally extensive areas of hepatocellular loss or multifocal to coalescing vacuoles (peliosis hepatis). Multifocal to coalescing vacuoles within the hepatic parenchyma consistent with peliosis hepatis, a rare disorder characterized by empty or blood-filled spaces of variable size in the liver, was present in 7 (21.2%) tamarins (Figure 8). Investigators followed serum levels of hepatic enzymes during acute viral infection; concentrations were elevated, as expected (data not shown). No viral inclusion bodies or lipidosis was noted. The cardiac, renal, and hepatic lesions were found in both experimental and research-naïve tamarins. Other lesions present to a lesser degree were pneumonia (4 cases) and renal infarcts (3 cases). Only the captive-born, 8 mo-old male, did not show any lesions at histologic examination; all of the other 32 animals in the study showed at least 2 of the described lesions.
Figure 7.
Liver, S. mystax. Diffuse hepatocellular vacuolar change and swelling. Hematoxylin and eosin stain; magnification, ×200.
Figure 8.
Liver, S. mystax. Peliosis hepatis characterized by blood-filled spaces of variable size in the liver. Hematoxylin and eosin stain; magnification, ×100.
Discussion
Several lesions were common among adult S. mystax. The lesions did not appear to be related to the experimental treatments, except perhaps for the hepatic lesions, which might have been due to the viruses injected. However, hepatic lesions were found in research-naïve animals also. In most cases, the animals had cleared the viral infection at the time of death. Shedding of hepatitis A virus in feces lasts from about week 2 to 7 after exposure, but considerable variation can occur. All animals with normal immune systems clear their infections approximately within this timeframe. Only those that are immunosuppressed or that have defective immune systems might demonstrate prolonged shedding, but this pattern would be very rare.27 Similarly, infection with hepatitis GB virus type B has an acute course in tamarins and has been proposed as a potential surrogate model for the study of hepatitis C virus infections of humans. Hepatitis GB virus type B is phylogenetically most closely related to hepatitis C and causes an acute, self-resolving hepatitis, indicated by increases in alanine aminotransferase levels and changes in liver histology, in tamarins. Although present in serum, hepatitis GB is not shed in feces. Viremia peaks around 6 to 8 wk after infection and clears by approximately 16 wk.22
The etiology of CCP is unknown, but there are 2 hypotheses. The first hypothesis considers the disease to be a postinflammatory condition, whereas the second theory considers CCP to be congenital ectopia.1,11,38 Some investigators believe that both hypotheses are correct, because 2 different types of CCP exist. Diffuse CCP is thought to be associated with a concurrent or previous inflammatory event,11 whereas the localized form appears unrelated to an inflammatory condition but to congenital ectopia.1 Other hypotheses suggested include factors such as diet, intraluminal pressure, and weakness of the muscularis mucosae.25 In animals, colitis resembling CCP has been reported to occur in dogs,20,36 pigs,37 pygmy goats,26 rhesus monkeys,29 and tamarins.15 CCP was diagnosed in an aged pygmy goat involved in a long-term progesterone study.26 In this case, the authors suggested that CCP was associated to the long-term administration of progesterone to the animal.26 In the pigmy goat, microscopic examination showed an inflammatory exudate in the colonic mucosa composed of lymphocytes, occasional eosinophils and neutrophils, suggesting a chronic inflammatory event possibly as the precursor for the development of CCP, but diarrhea was not noted.26 In 2 dogs diagnosed with CCP,20 partly bloody diarrhea, vomitus, and painful defecation were the most common clinical signs and were managed by changes in the diet, accompanied by treatment with sulfasalazine and corticosteroids.20 In pigs,37 dysentery was present, and the gross lesions were pseudomembranous, croupous, or even diphteric inflammation of the intestine. A conspicuously small pyogenic component was observed in the inflammatory exudate in the propria mucosae. In addition, the lesions were situated exclusively at the sites of lymphatic follicles.37 This finding differs from those in humans and other species in which CCP has been diagnosed. In 4 rhesus macaques, inflammation was considered to have played a primary role in the development of CCP.29 However, the cause of the intestinal inflammation was not determined.29 In tamarins (Saguinus labiatus and S. mystax), the disease and severity of lesions were clearly associated with time in captivity and the authors speculated that the diet possibly played a role in the development of CCP.15 In the tamarin report, clinically, most animals showed chronic, intermittent, diarrhea.15 The most common finding at necropsy in tamarins with CCP was a diffusely darkened colonic and cecal mucosa that, as suggested by the authors, could be easily overlooked unless the disease is severe, in which case very small, approximately 1 to 3 mm, yellowish cysts are seen bulging in the mucosa.15 This condition is very different from the colitis commonly reported in the cotton-top tamarin (Saguinus oedipus), in which early lesions are characterized by neutrophils in the lamina propria and epithelial layers of crypts, progressing to crypt abscesses with focal necrosis and ulceration.9 In addition, in many instances, if an affected cotton-top tamarin survives long enough, colonic adenocarcinoma develops.8 This progression to neoplasia does not occur in S. mystax and S. labiatus. Despite these reports, there are no reliable CCP animal models because cases in animals are sporadic, with a very low incidence in most species, except perhaps in tamarins, where 12.5% of the animals examined postmortem in a breeding colony showed lesions compatible with CCP.15 In the present study, the intestinal lesions resembled the diffuse form of CCP, possibly related to a postinflammatory event, as suggested by some investigators.11 However, inflammatory cell infiltrate was not present in all cases, and diarrhea was not reported. The tamarins may have had soft stools but no frank diarrhea or may have shown constipation instead of diarrhea, as occasionally occurs in humans with CCP.32 Due to the high incidence of CCP in captive S. mystax and its apparent association with time in captivity, an important predisposing factor may be the diet provided to these animals in captivity.15 Various ingredients in the diet or the physical properties of the ingredients may act as chronic irritants to the intestinal mucosa, causing an increase in intestinal peristalsis. This effect, combined with a possible defect or weakness in the colonic muscularis mucosae, may predispose these animals to mucosal gland herniation, as seen in early lesions, and consequently CCP. We do not believe that hepatitis A and GB type B infections are associated with CCP because the intestinal lesions occurred in research naïve animals in this and previous studies.13,15
Of the 33 tamarins we evaluated, 12 showed either clinical signs or signs at necropsy of congestive heart failure or cardiomegaly. However, 1 animal with no gross lesions or clinical signs of cardiomyopathy showed marked myocardial fibrosis on histologic examination. This tamarin probably experienced sudden cardiac death with subtle or no previous clinical signs. To our knowledge, myocardial fibrosis in tamarins has not been reported previously. However, interstitial myocardial fibrosis was noted occasionally in a breeding colony of Saguinus labiatus and S. mystax,14 and cardiomyopathy was a common cause of death in a research colony of S. mystax.7 Myocardial fibrosis is a common finding in humans with left ventricular hypertrophy due to chronic arterial hypertension.28 In pressure overload hypertrophy, continuous structural remodeling of the fibrillar collagen matrix occurs along with an increase in heart weight. These changes are believed to be a compensatory mechanism that enhances tensile strength.28,39,40 However, the increased stiffness of the myocardium has a detrimental effect on cardiac elasticity and contractibility and predisposes to both atrial and ventricular arrhythmias and, possibly, atrial fibrillation.35 In addition, the greatly thickened myocardial wall, due to myocyte hypertrophy and increased interstitial fibrous tissue, impairs oxygen perfusion, leading to hypoxia and consequently myocyte necrosis and reparative fibrosis with further detrimental effect on heart function. Another mechanism for myocyte necrosis is the entrapment of myocardial cells by connective tissue which prevents oxygen perfusion. Eventually these animals develop clinical signs of heart failure and die. Interestingly, due to the high incidence of wavy cardiac myofibers in dogs with dilated cardiomyopathy, histologic examination for attenuated wavy fibers was suggested as a useful postmortem test for dilated cardiomyopathy in dogs.34 Wavy cardiac myofibers also occur frequently in owl monkeys with dilated cardiomyopathy.12 In the present study, discrete wavy fibers were noted in a few cases, with only 1 animal showing marked lesions; this animal also was diagnosed with dilated cardiomyopathy during necropsy. All animal models of cardiac fibrosis involve experimental manipulation of the animal to create the condition; no spontaneous animal model exists.31 Although the cardiac lesions in our tamarins suggest arterial hypertension, this hypothesis could not be confirmed because blood pressure was not monitored in these animals. In addition, myocardial fibrosis is unlikely to be an age-related condition because it was not a common finding in a breeding colony where animals were held for prolonged periods of time.13
Chronic glomerulonephritis is a common finding in callitrichids.4 Membranoproliferative glomerulonephritis has been noted in tamarins infected with hepatitis A virus as well as in research-naïve animals.4-6,23 Several studies suggest that glomerular disease in tamarins is immunologically related, possibly due to IgM deposits.4,6 IgM in conjunction with IgA deposits recently were reported to be the cause of glomerulopathy in tamarins.5 Mesangial IgM deposits have been noted in young tamarins, with no morphologic lesions in glomeruli.4 In the same study,4 older animals with glomerulosclerosis did not exhibit IgM deposits in glomeruli; the authors therefore reported the glomerulosclerosis as ‘old-age nephropathy.’ These studies suggest that the tamarin nephropathy is an immune-related condition and that the lesions possibly were exacerbated by hepatitis A viral antigen immune complex deposition in glomeruli in some of the animals in our study.19 No attempts were made on the present study to determine the presence of IgM or IgA deposits in glomeruli. The renal vascular changes noted in tamarins with severe kidney disease in the present study were suggestive of arterial hypertension.12 However, hypertension was not suspected in these animals antemortem, so blood pressure was not measured.
Mild diffuse hepatocellular vacuolation and periportal mononuclear cell infiltration occurs occasionally in research-naïve tamarins during routine histologic examination, particularly in wild-caught animals.14 Hepatocellular vacuolation might be pronounced in tamarins that experienced prolonged anorexia. In addition, pronounced cellular vacuolation and swelling, accompanied by portal tracts densely infiltrated with mononuclear cells, is reported to occur in tamarins experimentally infected with hepatitis A virus and is believed to be an immunologically mediated lesion following viral infection, because peak antibody titer, but not viral presence, coincides with histologic signs of liver damage.19 The experimental viral infection associated with the colonic, renal, or cardiac lesions may have contributed to death and postmortem hepatic lesions. One tamarin died almost 2 y after infection and still showed hepatocellular vacuolation (as well as cardiomegaly) at necropsy. A research-naïve tamarin showed hepatocellular vacuolation and peliosis hepatis; this animal was diagnosed grossly with cardiohepatopathy, but no myocardial fibrosis was noted on histologic examination. Histologic examination of this same tamarin did reveal marked colitis and moderate glomerulonephritis, both of which may have contributed to its poor condition and death.
Peliosis hepatis is a rare condition in humans and animals and is characterized by dilatation of hepatic sinusoids and the presence of blood-filled spaces within the liver. The disease occurs mainly in subjects exposed to toxic substances or estrogens and is often asymptomatic.3 In the current study, peliosis hepatis was present in both male and female animals and in both experimental and research-naïve tamarins. Peliosis hepatis was accompanied in all cases by moderate to marked renal and liver disease. However, the cause for the peliosis hepatis in our tamarins could not be identified. Recently, attention has been paid to the phytoestrogen content of laboratory animal diets as a possible confounding research variable.18,30 Most commonly used natural-ingredient laboratory animal diets, including the diet fed to the tamarins in the present study, use soy as a source of protein. Soy contains isoflavones, which are phytoestrogens with a chemical structure and function similar to 17-β-estradiol, a potent natural estrogen.18 According to a recent study,30 monkeys consuming a typical commercial chow diet enriched with fruits and vegetables actually have an isoflavone exposure equivalent to that used in experiments designed to demonstrate the benefits and potential adverse effects of soy. The same study30 concluded that the serum levels of biologically active isoflavones were high in animals fed a nonhuman primate commercially available diet. Prolonged exposure to isoflavones with estrogen-like activity might have induced hepatic peliosis in the tamarins in the present report. Interestingly, peliosis hepatis has recently been suggested as an early indication of idiopathic portal hypertension.3 Idiopathic noncirrhotic intrahepatic portal hypertension has an anatomic basis in increased fibrous tissue in the portal vessel wall and periportal and perisinusoidal space, with obliteration of small portal veins and areas of nodular regenerative hyperplasia.3 Coincidently, some of these lesions were present in the moustached tamarins in the current study.
The results from this study suggest that moustached tamarins have a high incidence of spontaneous diffuse CCP, myocardial fibrosis, and membranoproliferative glomerulonephritis in captivity. These diseases greatly impair the use of this species in viral hepatitis research and should be investigated further. In addition, S. mystax may be the first spontaneous animal model for the study of CCP etiopathogenesis and experimental therapy and may provide a new spontaneous nonhuman primate model for the study of myocardial fibrosis. Although experimentally manipulated animal models exist, the study of myocardial fibrosis in a spontaneous animal model likely will contribute greatly to the understanding and treatment of this disease in humans.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases, Comparative Medicine Branch, the Office of Research Support, and a NIAID contract to SoBran and BIOQUAL. We thank Drs Robert Purcell and Sue Emerson for kindly letting us use tamarin tissue samples from their previous studies, and Ms Charlene Shaver, Dr Richard Montali, Mr Brad Fisher, Ms Melissa Williams, and Mr John DeLeonardis for their comments and support.
References
- 1.Allen MS. 1966. Hamartomatous inverted polyps of the rectum. Cancer 19:257–265 [DOI] [PubMed] [Google Scholar]
- 2.Barnard D, Knapka J, Renquist D. 1988. The apparent reversal of a wasting syndrome by nutritional intervention in Saguinus mystax. Lab Anim Sci 38:282–288 [PubMed] [Google Scholar]
- 3.Berzigotti A, Magalotti D, Zappoli P, Rossi C, Callea F, Zoli M. 2006. Peliosis hepatis as an early histological finding in idiopathic portal hypertension: a case report. World J Gastroenterol 12:3612–3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brack M. 1995. Callitrichid IgM-nephropathy—an old age-related disease? Lab Anim 29:54–58 [DOI] [PubMed] [Google Scholar]
- 5.Brack M, Schroeder C, Fooke M, Schlumberger W. 1999. IgM/IgA nephropathy in callitrichids: antigen studies. Nephron 82:221–231 [DOI] [PubMed] [Google Scholar]
- 6.Brack M, Weber M. 1995. Ultrastructural and histochemical mesangial alterations in callitrichid IgM nephropathy (Primates: Platyrrhina). Nephron 69:286–292 [DOI] [PubMed] [Google Scholar]
- 7.Brasky K.2006. Personal communication.
- 8.Chalifoux LV, Bronson RT. 1981. Colonic adenocarcinoma associated with chronic colitis in cotton-top marmosets, Saguinus oedipus. Gastroenterology 80:942–946 [PubMed] [Google Scholar]
- 9.Chalifoux LV, Bronson RT, Escajadillo A, McKenna S. 1982. An analysis of the association of gastroenteric lesions with chronic wasting syndrome of marmosets. Vet Pathol Suppl 7:141–162 [PubMed] [Google Scholar]
- 10.Emerson SU, Huang YK, Nguyen H, Brockington A, Govindarajan S, St Claire M, Shapiro M, Purcell RH. 2002. Identification of VP1/2A and 2C as virulence genes of hepatitis A virus and demonstration of genetic instability of 2C. J Virol 76:8551–8559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodall HB, Sinclair ISR. 1957. Colitis cystica profunda. J Pathol Bacteriol 73:33–42 [Google Scholar]
- 12.Gozalo A, Dagle G, Montoya E, Weller R, Málaga C. 1992. Spontaneous cardiomyopathy and nephropathy in the owl monkey (Aotus spp.) in captivity. J Med Primatol 21:279–284 [PubMed] [Google Scholar]
- 13.Gozalo A, Montoya E. 1992. Mortality causes of the moustached tamarin (Saguinus mystax) in captivity. J Med Primatol 21:35–38 [PubMed] [Google Scholar]
- 14.Gozalo AS.2007. Personal communication.
- 15.Gozalo AS, Dagle GE, Montoya EJ, Weller RE. 1994. Spontaneous colitis cystica profunda in captive tamarins. J Med Primatol 23:309–312 [DOI] [PubMed] [Google Scholar]
- 16.Hansen JF, Alford PL, Keeling ME. 1984. Diffuse myocardial fibrosis and congestive heart failure in an adult male chimpanzee. Vet Pathol 21:529–531 [DOI] [PubMed] [Google Scholar]
- 17.Hubbard GB, Lee DR, Eichberg JW. 1991. Diseases and pathology of chimpanzees at the southwest foundation for biomedical research. Am J Primatol 24:273–282 [Google Scholar]
- 18.Jensen MN, Ritskes-Hoitinga M. 2007. How isoflavone levels in common rodent diets can interfere with the value of animal models and with experimental results. Lab Anim 41:1–18 [DOI] [PubMed] [Google Scholar]
- 19.Karayiannis P, Jowett T, Enticott M, Moore D, Pignatelli M, Brenes F, Scheuer PJ, Thomas HC. 1986. Hepatitis A virus replication in tamarins and host immune response in relation to pathogenesis of liver cell damage. J Med Virol 18:261–276 [DOI] [PubMed] [Google Scholar]
- 20.Kraft W, Ghermai AK, von Bomhard D. 1989. Two cases of colitis cystica profunda in dogs. Tierarztl Prax 17:299–302 [PubMed] [Google Scholar]
- 21.Lammey ML, Lee DR, Ely JJ, Sleeper MM. 2008. Sudden cardiac death in 13 captive chimpanzees (Pan troglodytes). J Med Primatol 37 Suppl 1:39–43. [DOI] [PubMed] [Google Scholar]
- 22.Lanford RE, Chavez D, Notvall L, Brasky KM. 2003. Comparison of tamarins and marmosets as hosts for GBV-B infections and the effect of immunosuppression on duration of viremia. Virology 311:72–80 [DOI] [PubMed] [Google Scholar]
- 23.Morita M, Kitajima K, Yoshizawa H, Itoh Y, Iwakiri S, Shibata C, Mayumi M. 1981. Glomerulonephritis associated with arteritis in marmosets infected with hepatitis A virus. Br J Exp Pathol 62:103–113 [PMC free article] [PubMed] [Google Scholar]
- 24.National Research Council 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 25.O'Donnell N. 1987. Enteritis cystica profunda revisited. Hum Pathol 18:1300–1301 [DOI] [PubMed] [Google Scholar]
- 26.Patton NM, Blankevoort M. 1976. Colitis cystica profunda in pygmy goats. J Comp Pathol 86:371–375 [DOI] [PubMed] [Google Scholar]
- 27.Purcell R.2007. Personal communication.
- 28.Rossi MA. 1998. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens 16:1031–1041 [DOI] [PubMed] [Google Scholar]
- 29.Scotti TM. 1975. Colitis cystica profunda in rhesus monkeys. Lab Anim Sci 25:55–61 [PubMed] [Google Scholar]
- 30.Stroud FC, Appt SE, Wilson ME, Franke AA, Adams MR, Kaplan JR. 2006. Concentrations of isoflavones in macaques consuming standard laboratory monkey diet. J Am Assoc Lab Anim Sci 45:20–23 [PubMed] [Google Scholar]
- 31.Sun Y, Weber KT. 2005. Animal models of cardiac fibrosis. Methods Mol Med 117:273–290 [DOI] [PubMed] [Google Scholar]
- 32.Sztarkier I, Benharroch D, Walfisch S, Delgado J. 2006. Colitis cystica profunda and solitary rectal ulcer syndrome, polypoid variant: two confusing clinical conditions. Eur J Intern Med 17:578–579 [DOI] [PubMed] [Google Scholar]
- 33.Tajika M, Nakamura T, Kawai H, Sawaki A, Mizuno N, Takahashi K, Yokoi T, Yatabe Y, Hirai T, Yamao K, Kato T. 2007. A case of colonic morule with colitis cystica profunda. Gastrointest Endosc 65:162–163 [DOI] [PubMed] [Google Scholar]
- 34.Tidholm A, Haggstrom J, Jonsson L. 1998. Prevalence of attenuated wavy fibers in myocardium of dogs with dilated cardiomyopathy. J Am Vet Med Assoc 212:1732–1734 [PubMed] [Google Scholar]
- 35.Timonen P, Magga J, Risteli J, Punnonen K, Vanninen E, Turpeinen A, Tuomainen P, Kuusisto J, Vuolteenaho O, Peuhkurinen K. 2007. Cytokines, interstitial collagen, and ventricular remodeling in dilated cardiomyopathy. Int J Cardiol Apr 16 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 36.Van Kruiningen HJ. 1972. Canine colitis comparable to regional enteritis and mucosal colitis of man. Gastroenterology 62:1128– 1142 [PubMed] [Google Scholar]
- 37.Vitovec J, Vladik P. 1976. Colitis cystica profunda in inflammatory psedomembraneous dysentery in pigs. Vet Med (Praha) 21:475–481 [PubMed] [Google Scholar]
- 38.Wayte DM, Helwig EB. 1967. Colitis cystica profunda. Am J Clin Pathol 48:159–169 [Google Scholar]
- 39.Weber KT, Jalil JE, Janicki JS, Pick R. 1989. Myocardial collagen remodeling in pressure overload hypertrophy. A case for interstitial heart disease. Am J Hypertens 2(12 Pt 1):931–940 [DOI] [PubMed] [Google Scholar]
- 40.Weber KT, Pick R, Jalil JE, Janicki JS, Carroll EP. 1989. Patterns of myocardial fibrosis. J Mol Cell Cardiol 21Suppl 5:121–131 [DOI] [PubMed] [Google Scholar]