Abstract
Intermittent serodetection of mouse parvovirus (MPV) infections in animal facilities occurs frequently when soiled bedding sentinel mouse monitoring systems are used. We evaluated induction of seroconversion in naïve single-caged weanling ICR mice (n = 10 per group) maintained on 5-fold serially diluted contaminated bedding obtained from SCID mice persistently shedding MPV1e. Soiled bedding from the infected SCID mice was collected, diluted, and redistributed weekly to cages housing ICR mice to represent chronic exposure to MPV at varying prevalence in a research colony. Sera was collected every other week for 12 wk and evaluated for reactivity to MPV nonstructural and capsid antigens by multiplex fluorescent immunoassay. Mice were euthanized after seroconversion, and DNA extracted from lymph node and spleen was evaluated by quantitative PCR. Cumulative incidence of MPV infection for each of the 7 soiled bedding dilution groups (range, 1:5 to 1:78125 [v/v]) was 100%, 100%, 90%, 20%, 70%, 60%, and 20%, respectively. Most seropositive mice (78%) converted within the first 2 to 3 wk of soiled bedding exposure, correlating to viral exposure when mice were 4 to 7 wk of age. Viral DNA was detected in lymphoid tissues collected from all mice that were seropositive to VP2 capsid antigen, whereas viral DNA was not detected in lymphoid tissue of seronegative mice. These data indicate seroconversion occurs consistently in young mice exposed to high doses of virus equivalent to fecal MPV loads observed in acutely infected mice, whereas seroconversion is inconsistent in mice chronically exposed to lower doses of virus.
Abbreviations: mfi, median fluorescent intensity; MFI, multiplex fluorescent immunoassay; MPV, mouse parvovirus; NS1, nonstructural protein 1; qPCR, quantitative PCR; SCID, severe combined immunodeficiency; VP2, viral capsid protein 2
Mouse parvovirus (MPV) is among the most prevalent infectious agents detected in contemporary laboratory mouse colonies2,7,10 and can have deleterious effects on research because of in vitro and in vivo immunomodulatory effects, tumor suppression, and contamination of cell cultures and tissues originating from mice.11-13 The potential for MPV transmission among mice in research facilities is enhanced by its environmental stability,6 potential to induce persistent infection in mice,8 and difficult eradication from infected laboratory mouse colonies. Despite the availability of highly sensitive and specific diagnostic assays,9,14,15 detection of MPV infections in contemporary laboratory mouse colonies remains problematic, with intermittent detection even under conditions of enzootic colony infections. The widespread use of sentinel mice exposed to soiled bedding as the primary detection system, a relatively short period of viral transmission postinfection in immunocompetent mice, and a fairly high viral dose required to induce productive infection are considered key factors that result in intermittent detection of MPV contamination in mouse colonies. As a result, MPV infections present important and costly challenges to contemporary laboratory animal research facilities.
Several studies have investigated the horizontal transmission of MPV to sentinel mice. Experimentally infected SENCAR mice transmitted MPV1a to naïve sentinels both by direct contact and soiled bedding exposure, predominantly during the first 3 wk after inoculation.17 Similarly, experimentally infected Swiss Webster mice transmitted MPV1d within 2 wk to sentinels by direct contact or through various amounts of soiled bedding.18 Interestingly, transmission to sentinel mice appeared to be enhanced in mice maintained in individually ventilated caging as compared with static microisolation caging in the cited study. Naturally infected BALB/c mice when 1 mo old, but not when 2, 3, and 6 mo old, transmitted MPV to direct contact sentinels.16 Recent studies completed in our laboratory1 indicate that C.B-17/Icr-Prkdcscid mice inoculated with MPV1e as neonates persistently shed high levels of virus in their feces over several months. Undiluted contaminated bedding collected at any time point during this period consistently transmitted MPV1e to weanling C3H sentinel mice exposed for 2 wk. Similarly inoculated neonatal BALB/c mice shed high levels of virus, with transmission to sentinels, for only 2 wk after inoculation.1 In all of these reports, the period of exposure of sentinel mice to soiled bedding was limited (2 wk or less), with no repeated exposure opportunities, as might be expected under field conditions with an infected colony. In the present study, we simulated a typical sentinel monitoring program and determined whether chronic exposure to various concentrations of MPV1-contaminated bedding, reflective of a broad range of disease prevalence scenarios within any given affected room, can induce seroconversion in sentinel mice.
Materials and Methods
Mice.
Untimed pregnant C.B-17/Icr-Prkdcscid/Crl (SCID) female mice were obtained from Charles River Laboratories (Wilmington, MA), and male and female weanling Hsd:ICR mice were obtained from Harlan Sprague Dawley (Indianapolis IN). All mice were reported to be free of murine viruses, pathogenic bacteria, and endo- and ectoparasites by the suppliers. All mice were housed in sterilized static microisolation caging on aspen chip bedding, NIH-31 diet (Harlan Teklad, Madison, WI) and hyperchlorinated water were provided ad libitum, and all animal manipulations were performed in a class IIA biological safety cabinet by using standard microisolation technique. Animals were housed in a biocontainment facility at a temperature of 22 to 24 °C, relative humidity of 40% to 60%, 12 to 15 air exchanges hourly, and a 12:12-h light:dark cycle. The University of Arizona Institutional Animal Care and Use Committee approved all animal procedures.
Animal infections.
MPV1e originally was isolated from naturally infected mice and is maintained by oral inoculation of naïve mice with filter-sterilized tissue homogenate obtained from infected mice.1 The ID50 was determined in weanling BALB/c mice by oral inoculation. Mouse pups (age, 1 d; n = 12) were inoculated oronasally with 100 ID50 MPV1e, weaned at 4 wk of age into sex-matched groups of 4 mice per cage, and maintained for the duration of the sentinel exposure study. Weekly throughout the entire 12-wk study, 10 fecal pellets were collected from the floor of each cage of these infected SCID mice and stored frozen at –80 °C until fecal viral load could be determined by quantitative polymerase chain reaction (qPCR).
Sentinel exposure.
Each of 7 large new biohazard bags labeled with the bedding dilution (1:5, 1:25, 1:125, 1:625, 1:3125, 1:15,625, and 1:78,125) received 7.2 l of clean bedding. Three cages containing 1 week's accrual of soiled bedding from weaned MPV1e-infected mice was placed into the 1:5 bedding dilution bag (that is, a total of 1.8 l soiled bedding from MPV-infected SCID mice). The biohazard bag was closed with sufficient air in the bag to allow thorough mixing and then was manually tumbled end over end for 2 min. We then removed 1.8 l of mixed bedding from the 1:5 bedding dilution and placed it into the 1:25 bedding dilution bag, and the bedding was remixed as described above. This process was repeated to generate the remaining 5-fold bedding dilutions. After mixing was complete, 600 ml of bedding from each dilution was placed into each of 10 sterilized empty shoebox cages. A single 4-wk-old Hsd:ICR mouse was placed into each cage that contained diluted bedding, with the most dilute bedding group (1:78125) completed first, followed by the next most diluted bedding group (1:15625), and so on. Cages were changed weekly in the same order (most diluted to most concentrated soiled bedding group), with each cage refilled with aliquots of freshly prepared bedding dilutions as just detailed. Blood was collected from each Hsd:ICR mouse every other week by submandibular venipuncture into hematocrit tubes. During the processing of 70 blood samples collected after the initial 2 wk exposure to soiled bedding, 24 specimens were lost in a centrifuge accident. Blood was collected from these mice 1 wk later, and the results from these samples are included along with the other available 2-wk exposure data. After centrifugation in a hematocrit centrifuge, serum was harvested and evaluated by multiplex fluorescent immunoassay (described later). Sentinels were euthanized by carbon dioxide inhalation after seroconversion was detected. Final blood samples were collected by cardiocentesis; the resulting sera were diluted 1:5 in PBS and stored at –80 °C until use. In addition, approximately 20 mg each of spleen and lymph node was harvested from each sentinel mouse after euthanasia and stored frozen at –80 °C until tissue DNA was extracted. Tissue DNA was subsequently stored at –20 °C until evaluated by qPCR. Mice that did not seroconvert by the end of the 12-wk bedding exposure period were euthanized and processed as described.
Serology.
The multiplex fluorescent immunoassay (MFI) format was used to evaluate sera for the presence of virus-specific antibodies. Baculovirus-expressed recombinant nonstructural protein 1 (rNS1) from minute virus of mice prototype strain and recombinant MPV1b viral capsid protein 2 (rVP2) were prepared essentially as previously described.9,15 Purified rNS1 and rVP2 were covalently coupled to carboxylated polystyrene microspheres (Luminex, Austin, TX) at a coupling concentration of 25 μg of protein per 5 × 106 microspheres according to manufacturer's recommended protocols. Ovalbumin, A92L mouse fibroblast cell lysates, and Hi-Five insect cell lysates were similarly coated to microspheres to serve as control antigens. Lysates were prepared by three freeze-thaw cycles as previously reported.9,15 Microspheres were stored at 4 °C in the dark until use. Evaluation of mouse sera for rNS1- and rVP2-specific antibodies was performed automatically (LiquiChip Workstation, Qiagen, Valencia, CA) as described previously.1 Briefly, antigen-coated microspheres were incubated for 60 min with dilute sera at a final dilution of 1:500 in 100 μl diluent, washed twice, incubated with phycoerythrin-conjugated F(ab')2 fragment goat antimouse IgG secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA), washed twice, and resuspended in stop solution containing formalin. The microplate then was shaken for 5 min and analyzed. Baseline values (rNS1, 125; rVP2, 125) to discriminate negative and positive samples were determined previously as the mean plus 2 standard deviations of results obtained for 70 sera from mice known to be negative for murine parvovirus infection. Results are reported as the median fluorescent intensity (mfi) of 100 antigen-coated microspheres. Sera were to be excluded if they exceeded baseline values for 2 of the 3 control antigens, although in this experiment, none of the sera evaluated exceeded the mfi baseline for any of the control antigens.
qPCR.
DNA was extracted from tissues and feces and screened by an MPV-specific qPCR assay as previously described.2,14 Briefly, DNA was extracted using a genomic DNA extraction kit (MagneSil KF, Promega, Madison, WI) and a robotic extraction station (KingFisher, Thermo Labsystems, Franklin, MA) according to the manufacturer's recommendations. qPCR reactions were performed by using an automated system (Mx3000P QPCR System, Stratagene, Cedar Creek, TX), and products were analyzed with the accompanying software. Each 20-μl reaction consisted of 1× TaqMan buffer (50 mM KCl, 10 μM EDTA, 10 mM Tris-HCl [pH 8.3], and 60 nM passive reference dye); 5.5 mM MgCl2; 200 μM (each) dATP, dCTP, and dGTP; 400 μM dUTP; 300 nM primers; 125 nM probe; 0.2 U uracil-N-glycosylase (AmpErase, PE Applied Biosystems, Foster City, CA); 0.5 U Taq polymerase (AmpliTaq Gold, PE Applied Biosystems, Foster City, CA); and 2 μl template DNA (approximately 50 ng DNA). Thermal cycling conditions consisted of 50 °C for 2 min for incubation of uracil-N-glycosylase, polymerase activation at 95 °C for 10 min, and then 45 cycles of 95 °C for 15 s followed by 60 °C for 1 min. Samples were considered positive if they exhibited a mean fluorescence of greater than 0.1 and a cycle threshold of less than 35. These qPCR findings were considered the ‘gold standard’ against which MFI assays were compared for purposes of estimating their sensitivity and specificity in the context of our study. We used 10-fold serial dilutions of cloned amplicon DNA (range, 107 to 100 template copies) to generate a standard curve, with linear regression of this standard curve (R2 = 0.993), and then it was used to estimate the numbers of copies of the viral genome detected in each DNA sample by qPCR. The absolute viral copy number per fecal pellet was calculated by multiplying this estimate by the dilution factors used during DNA extraction and qPCR set-up.
Statistical analysis.
Evaluation of diagnostic test performance (sensitivity, specificity, and predictive value) among MFI serologic assays from antemortem blood samples compared with qPCR results from postmortem tissue samples was done by standard methods, including calculation of their exact 95% confidence intervals, kappa statistics for test agreement, and McNemar χ2 statistic to evaluate beyond-chance concordance of findings.19 Differences with P values less than or equal to 0.05 were considered significant.
Results
MPV1-contaminated bedding.
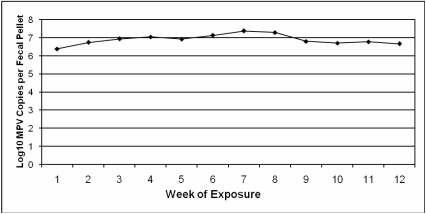
Each of 12 SCID mice (4 mice per cage) were inoculated oronasally with MPV1e (100 ID50 per mouse) within 24 h of birth. Starting at 5 wk after inoculation, an estimate of MPV fecal viral load was determined weekly for all cages used as source bedding by qPCR of DNA extracted from 10 pooled fecal pellets collected from each cage of infected SCID mice. The mean MPV fecal viral load during the exposure period was 1.3 × 107 MPV DNA copies per fecal pellet, with relatively consistent levels of fecal virus shedding throughout the course of the experiment (Figure 1). At study completion, infection of all MPV-inoculated SCID mice was confirmed by qPCR of their lymph nodes and spleens (mean lymphoid tissue viral load = 1.1 × 107 viral DNA copies per 2 μl tissue DNA).
Figure 1.
Mean MPV DNA copies per fecal pellet (expressed in log10 scale) from MPV-infected SCID mice used to produce MPV-contaminated bedding during the 12-wk sentinel mouse exposure period, as determined by qPCR.
Sentinel exposure to MPV1-contaminated bedding dilutions.
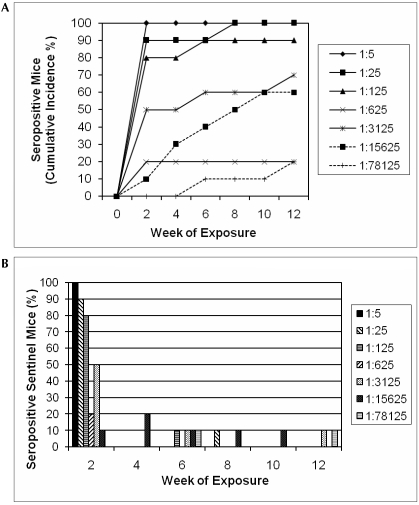
Weanling Hsd:ICR mice (n = 10 per bedding dilution group) were housed individually and exposed to 5-fold serially diluted bedding obtained from SCID mice persistently shedding MPV1e (dilution range, 1:5 to 1:78,125). Fresh bedding dilutions were prepared weekly. Every other week, mice were bled by submandibular venipuncture, and sera were evaluated by MFI for rNS1- and MPV rVP2-specific antibodies. Based on serologic findings, the cumulative incidence of MPV infection for the 7 soiled bedding dilution groups over the 12-wk study period was 100% (10 mice seropositive of 10 mice tested), 100% (10 of 10), 90% (8 of 9 seropositive; 1 additional mouse in this group found dead at 6 weeks postexposure was strongly tissue qPCR positive), 20% (2 of 10), 70% (7 of 10), 60% (6 of 10), and 20% (2 of 10), as depicted in Figure 2 A. The majority of seropositive mice (78%) converted during the first 2 to 3 wk of exposure to soiled bedding, when they were 4 to 7 wk of age (Figure 2 B).
Figure 2.
(A) Cumulative incidence of MPV rVP2-seropositive mice in each bedding dilution group during the 12-wk exposure period. (B) Percentage of mice within each bedding dilution group that converted to seropositivity at each time point. Of the 45 mice that seroconverted, 35 (78%) did so during the first exposure period.
Correlation of MFI serology and lymphoid tissue qPCR results in sentinel mice.
Seropositive mice were euthanized by carbon dioxide inhalation after initial seroconversion was detected in antemortem blood samples, almost always within 1 wk after the positive antemortem blood was collected. All remaining mice were euthanized 12 wk after their initial exposure to MPV-contaminated bedding. Serum, lymph node, and spleen were collected from all sentinel mice and evaluated by MFI and qPCR. All mice that were seropositive to both rNS1 and MPV rVP2 in antemortem MFI evaluations (n = 43) were also seropositive to both antigens in sera harvested from them postmortem, and lymphoid tissue qPCR was strongly positive in all of these mice (mean, 8.3 × 105 viral DNA copies per 2 μl tissue DNA). All mice seronegative to both rNS1 and MPV rVP2 (n = 21) throughout the antemortem and at the postmortem evaluation were also lymphoid tissue qPCR negative.
Discrepant diagnostic test results were detected in the remaining 6 mice. Five mice were seropositive to MPV rVP2 but negative to rNS1 antigen when evaluated antemortem. One of these mice became seropositive to rNS1 at postmortem examination; and its lymphoid tissue was qPCR positive (interpreted as a true positive). Three of these 5 mice displayed similar serologic results (mean MPV rVP2 mfi, 2087; rNS1, negative) and were lymphoid tissue qPCR positive (mean = 6.1 × 104 viral DNA copies per 2 μL tissue DNA) upon postmortem examination (also interpreted as true positives). The fifth mouse displayed a low MPV rVP2 MFI result initially (mfi, 227), and was seronegative to both antigens and lymphoid tissue qPCR negative at postmortem examination (interpreted as a false positive). The remaining mouse that yielded discrepant results displayed a low seropositive result to rNS1 (mfi, 177) but was negative to MPV rVP2 antigen at the postmortem serologic evaluation and was lymphoid tissue qPCR negative (also interpreted as a false positive).
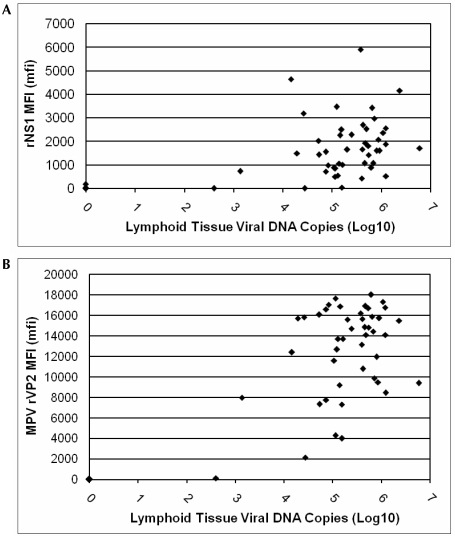
Comparing antemortem MFI assays to postmortem lymphoid tissue qPCR results revealed that the sensitivity and specificity of the rNS1 assay (with exact 95% confidence boundaries) was 95.6% (84.7%, 99.5%) and 95.8% (78.9%, 99.9%). Corresponding values of sensitivity and specificity for the rVP2 assay were 100% (92.0%, 100%) and 95.8% (78.9%, 99.9%). The kappa value for agreement between these assays was excellent at 0.81, and the McNemar test (χ2 =1.50, P = 0.22) showed they did not significantly differ in results, regardless of the bedding dilution to which mice were exposed (Figure 3).
Figure 3.
Scatter plots of mean number of MPV DNA copies per 2 μl DNA extract (expressed in log10 scale) detected in lymph node and spleen, correlated to (A) rNS1 and (B) MPV rVP2 MFI serology results. For both antigens, median fluorescent intensity (mfi) values greater than 125 are considered positive.
Stability of infectious MPV in soiled bedding.
Four groups of 2 weanling C3H mice each were housed for 2 weeks on MPV-contaminated bedding from infected SCID mice, which was either undiluted or diluted 1:125 and then stored at room temperature for either 4 or 8 wk. Three weeks after being housed on this bedding, sera and lymphoid tissues were harvested from the mice and evaluated by MPV MFI and qPCR, respectively. Only the 2 mice exposed to undiluted contaminated bedding stored for 4 wk seroconverted (mean MPV rVP2 mfi, 16900) and displayed high lymphoid tissue viral DNA loads (mean = 520 viral DNA copies per 2 μl tissue DNA). Mice in the remaining groups remained seronegative and displayed negative lymphoid tissue viral loads by qPCR.
Discussion
There was a high level of agreement among the 3 MPV diagnostic assays used, independent of the bedding dilution group to which mice were exposed. With estimated relative test sensitivity and specificity of 100% and 95.7%, respectively, the rVP2 MFI slightly outperformed the rNS1 MFI as compared with our gold standard assay of qPCR results in tissues harvested postmortem. High lymphoid tissue viral loads were detected in all mice that seroconverted to MPV rVP2, and all but 3 mice that were MPV rVP2 seropositive were also seropositive to rNS1. The latter results suggest exposure without productive infection, consistent with findings reported previously in mice experimentally inoculated with MPV1 at 8 and 12 wk of age.3 False-positive serology results, 1 each to rNS1 and MPV rVP2 antigen, were detected in 2 mice, as suggested by low seroreactivity and failure to detect MPV DNA by qPCR. Viral DNA was not detected by qPCR in any of the mice that were seronegative to both rNS1 and MPV rVP2 antigens.
Most sentinel mice (78%) were infected during the first 2- to 3-wk exposure period, when the mice were 4 to 7 wk of age. This finding is consistent with the relatively higher susceptibility of young mice to productive MPV1 infection as compared with that in older mice.3 However, we cannot exclude a dose-dependent phenomenon because the vast majority (83%) of mice that seroconverted during this timeframe were from the groups exposed to the 3 highest concentrations of contaminated bedding. The amount of virus to which sentinel mice were exposed was extremely high: approximately 1 × 107 MPV copies per fecal pellet on average. We estimated fecal output from our MPV-infected SCID mice to be approximately 2000 fecal pellets per cage of 4 mice per week (data not shown), which extrapolates to 2 × 1010 MPV copies in the bedding of each source cage used to prepare the dilution series each week. Therefore, the 5-fold serial dilutions that were used for sentinels in this study emulated scenarios of chronic MPV exposures ranging from 4 × 109 MPV copies at the 1:5 dilution (400 fecal pellets) to 2.5 × 105 MPV copies at the 1:78,125 dilution (less than 1 fecal pellet). That infection occurred in even 2 of the mice within the highest bedding dilution group (1:78,125) was surprising, although fragmentation of fecal pellets likely occurred during preparation of the soiled bedding dilutions, and clean bedding was used as diluent such that an inquisitive mouse might seek and possibly ingest the small amounts of foreign fecal material present. To our knowledge, the foraging behavior of mice when housed on soiled bedding originating from unrelated mice has not been studied.
Ten mice seroconverted between 8 and 16 wk of age when exposed weekly to various concentrations of MPV, indicating that older mice can indeed become productively infected if exposed to a sufficient dose of virus. Of the mice that remained seronegative on 1 or both assays throughout the 12 wk study period, all were from groups exposed to soiled bedding diluted to at least 1:125, indicating that the exposure dose among sentinels is critical to inducing an infection. However, the cumulative incidence among sentinel mice in each group did not correlate directly with the amount of virus in the bedding—that is, only 2 mice in the 1:625 dilution group seroconverted, whereas 7 and 6 mice seroconverted in the 1:3125 and 1:15625 dilution groups, respectively. This finding suggests interindividual variation among the mice, possibly due to either behavioral variables (for example, ingestion related to coprophagic or burrowing behavior) or to other yet-undefined stochastic processes. Alternatively, at these levels of bedding dilution, the amount of fecal fragments present may have varied and could have resulted in the variable transmission to the sentinel mice. Regardless, this situation increases the complexities associated with consistent sentinel detection of MPV.
The fecal viral loads detected in our infected SCID mice throughout the 12 wk study are similar to those we reported previously for acutely infected immunocompetent mice (> 106 copies per fecal pellet).1 As a result, each bedding dilution in our study approximates various ratios of cages containing acutely infected mice to those containing uninfected mice in the population being monitored by the sentinel. In contrast, the fecal viral load in immunocompetent mice after induction of an immune response was at least 10,000-fold lower in concentration. Therefore, our highest dilution groups in which only poor seroconversion was seen mimic viral levels shed from chronically infected immunocompetent mice. This finding suggests that sentinel-based detection of MPV occurs primarily when there are sufficient numbers of acutely infected colony mice present, particularly when acute shedding coincides with exposure of postweanling-age sentinel mice.
We also evaluated the viability of infectious MPV within soiled bedding stored at room temperature for 4 and 8 wk because parvoviruses are relatively resistant to physicochemical degradation4-6 and because MPV1 remains infective under normal environmental conditions for at least 7 d.18 Undiluted bedding collected from SCID mice persistently infected with MPV1 and stored for 4 wk (but not 8 wk) induced productive infection in exposed mice, whereas bedding diluted 1:125 did not induce infections in naïve mice after storage for 4 wk or longer. These findings suggest that the amount of infectious virus within the milieu of soiled bedding diminishes over time to levels below the critical threshold required to induce productive infection in mice. Future studies of this issue will be required to help further define any dose-dependent thresholds, which could become important components of infection control strategies within MPV-contaminated colonies.
Collectively, our findings indicate that seroconversion occurs consistently in young mice exposed to high doses of MPV1 virus equivalent to those shed by acutely infected mice, whereas seroconversion is inconsistent in mice chronically exposed to lower doses of virus that mimic those shed by MPV-infected mice after an adaptive immune response.
Acknowledgments
We thank Charles River Laboratories for providing the MPV1e isolate and SCID mice used in this study, and Marissa Steinberg, Emily Marcus, Christine Vogt, and Jessie Loganbill for technical assistance. This work was made possible by grants from the ACLAM Foundation and the National Center for Research Resources (1 R01 RR 18488-01), a component of the National Institutes of Health.
References
- 1.Besselsen DG, Becker MD, Henderson KS, Wagner AM, Banu LA, Shek WR. 2007. Temporal transmission studies of mouse parvovirus 1 in BALB/c and C.B-17/Icr-Prkdc(scid) mice. Comp Med 57:66–73 [PubMed] [Google Scholar]
- 2.Besselsen DG, Romero MJ, Wagner AM, Henderson KS, Livingston RS. 2006. Identification of novel murine parvovirus strains by epidemiological analysis of naturally infected mice. J Gen Virol 87:1543–1556 [DOI] [PubMed] [Google Scholar]
- 3.Besselsen DG, Wagner AM, Loganbill JK. 2000. Effect of mouse strain and age on detection of mouse parvovirus 1 by use of serologic testing and polymerase chain reaction analysis. Comp Med 50:498–502 [PubMed] [Google Scholar]
- 4.Boschetti N, Niederhauser I, Kempf C, Stuhler A, Lower J, Blumel J. 2004. Different susceptibility of B19 virus and mice minute virus to low pH treatment. Transfusion 44:1079–1086 [DOI] [PubMed] [Google Scholar]
- 5.Boschetti N, Wyss K, Mischler A, Hostettler T, Kempf C. 2003. Stability of minute virus of mice against temperature and sodium hydroxide. Biologicals 31:181–185 [DOI] [PubMed] [Google Scholar]
- 6.Harris RE, Coleman PH, Morahan PS. 1974. Stability of minute virus of mice to chemical and physical agents. Appl Microbiol 28:351–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacoby RO, Ball-Goodrich LJ, Besselsen DG, McKisic MD, Riley LK, Smith AL. 1996. Rodent parvovirus infections. Lab Anim Sci 46:370–380 [PubMed] [Google Scholar]
- 8.Jacoby RO, Johnson EA, Ball-Goodrich L, Smith AL, McKisic MD. 1995. Characterization of mouse parvovirus infection by in situ hybridization. J Virol 69:3915–3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livingston RS, Besselsen DG, Steffen EK, Besch-Williford CL, Franklin CL, Riley LK. 2002. Serodiagnosis of mice minute virus and mouse parvovirus infections in mice by enzyme-linked immunosorbent assay with baculovirus-expressed recombinant VP2 proteins. Clin Diagn Lab Immunol 9:1025–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingston RS, Riley LK. 2003. Diagnostic testing of mouse and rat colonies for infectious agents. Lab Animal 32:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKisic MD, Lancki DW, Otto G, Padrid P, Snook S, Cronin DC, Lohmar PD, Wong T, Fitch FW. 1993. Identification and propagation of a putative immunosuppressive orphan parvovirus in cloned T cells. J Immunol 150:419–428 [PubMed] [Google Scholar]
- 12.McKisic MD, Macy JD, Jr, Delano ML, Jacoby RO, Paturzo FX, Smith AL. 1998. Mouse parvovirus infection potentiates allogeneic skin graft rejection and induces syngeneic graft rejection. Transplantation 65:1436–1446 [DOI] [PubMed] [Google Scholar]
- 13.McKisic MD, Paturzo FX, Smith AL. 1996. Mouse parvovirus infection potentiates rejection of tumor allografts and modulates T cell effector functions. Transplantation 61:292–299 [DOI] [PubMed] [Google Scholar]
- 14.Redig AJ, Besselsen DG. 2001. Detection of rodent parvoviruses by fluorogenic nuclease polymerase chain reaction. Comp Med 51:326–331 [PubMed] [Google Scholar]
- 15.Riley LK, Knowles R, Purdy G, Salome N, Pintel D, Hook RR, Jr, Franklin CL, Besch-Williford CL. 1996. Expression of recombinant parvovirus NS1 protein by a baculovirus and application to serologic testing of rodents. J Clin Microbiol 34:440–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shek WR, Paturzo FX, Johnson EA, Hansen GM, Smith AL. 1998. Characterization of mouse parvovirus infection among BALB/c mice from an enzootically infected colony. Lab Anim Sci 48:294–297 [PubMed] [Google Scholar]
- 17.Smith AL, Jacoby RO, Johnson EA, Paturzo F, Bhatt PN. 1993. In vivo studies with an “orphan” parvovirus of mice. Lab Anim Sci 43:175–182 [PubMed] [Google Scholar]
- 18.Smith PC, Nucifora M, Reuter JD, Compton SR. 2007. Reliability of soiled bedding transfer for detection of mouse parvovirus and mouse hepatitis virus. Comp Med 57:90–96 [PubMed] [Google Scholar]
- 19.Thrusfield M. 2005. The nature of data. Thrusfield M. Veterinary epidemiology, 3rd ed. Ames (IA): Blackwell; p 152–167 [Google Scholar]