Abstract
Embryo electrofusion and tetraploid blastocyst microinjection is a modification of the traditional embryonic stem cell (ES cell)-based method to generate targeted mutant mice. Viability of tetraploid embryos is reportedly lower than with diploid embryos, with considerable interstrain variation. Here we assessed fetus and pup viability after ES cell microinjection of tetraploid blastocysts derived from outbred, hybrid, and inbred mice. Two-cell mouse embryos (C57BL/6NTac [B6], n = 788; B6D2F1/Tac [BDF1], n = 1871; Crl:CD1(ICR) [CD1], n = 1308) were electrofused; most resultant tetraploid blastocysts were injected with ES cells and surgically transferred into pseudopregnant recipient mice. Reproductive tracts were examined at midgestation for embryologic studies using B6 and BDF1 blastocysts; implantation sites and viable fetuses were counted. Pregnancies were carried to term for studies of targeted mutant mice using BDF1 and CD1 blastocysts, and pup yield was evaluated. Electrofusion rates of 2-cell embryos did not differ among B6, BDF1, and CD1 mice (overall mean, 92.8% ± 5.4%). For embryologic studies, 244 B6 blastocysts were surgically transferred and 1 fetus was viable (0.41%), compared with 644 BDF1 blastocysts surgically transferred and 88 viable fetuses (13.7%). For targeted mutant mouse studies, 259 BDF1 blastocysts were surgically transferred yielding 10 pups (3.9%); 569 CD1 blastocysts yielded 44 pups (7.7%).
Abbreviations: B6, C57BL/6NTac; BDF1, B6D2F1/Tac; CD1, Crl:CD1(ICR); E, embryonic day; ES cell, embryonic stem cell; GFP, green fluorescent protein; KSOM-AA, potassium simplex optimized medium with amino acids
The use of targeted mutant mice is widespread in the scientific community for innumerable research applications.1,5,12,14-16,19 These mice typically are generated through introduction of genetically manipulated embryonic stem cells (ES cells) into diploid blastocyst embryos, resulting in a chimeric mouse that contains cells from both the donor embryo and the ES cells. This method of manipulating the mouse genome has many powerful applications for research in genetics and in studying mouse models of human disease.3,7,8,12,13,16,21,24 In particular, the targeted nature of homologous recombination within pluripotent ES cells allows for highly specific genetic manipulations, as compared with the random insertional mutagenesis that occurs when transgenic animals are created by pronuclear injection. Confirmation of the genotype and germline transmission typically is followed by multiple breeding steps to generate a suitable research colony.
Mice derived completely from ES cells can be generated by microinjection into tetraploid rather than diploid blastocysts.18,24 However some reports have shown a small fraction of surviving donor tetraploid cells in fetuses.6,10,13 When ES cells are injected into tetraploid blastocysts, the tetraploid cells contribute only to the placenta and yolk sac and cannot contribute to the somatic cells of the developing mouse, so that the resultant pup develops only from the ES cells that have been introduced. Although tetraploid cells initially contribute to all layers of the preimplantation embryo, at gastrulation tetraploid cell differentiation is restricted to the primitive endoderm and trophectoderm, which form the extraembryonic tissues.6 Tetraploid cells may be present at embryonic day (E) 6.5 to 7.5 in the epiblast, which forms the embryo proper; persistence of tetraploid cells in the embryo appears to be strain-dependent, and these cells usually are excluded from epiblast lineages by E7.5 to 10.5.13 Consequently, tetraploid embryos that are injected with ES cells develop into pups with tissues that are completely ES-cell–derived, with the key advantage that the procedure produces mice whose gametes are all ES-cell–derived.9,18 This process can be roughly, although easily, visualized if the ES cells express green fluorescent protein.11 Mice generated by tetraploid injection are reported to be phenotypically normal when compared with mice generated in parallel by diploid injection.20
The tetraploid method is limited by a number of factors, and its success appears to be highly variable depending on blastocyst strain or ES cell strain, passage number, and quality of in vitro cell preparation. Whereas most ES cells used to date for diploid blastocyst injection are of 129 mouse background strain, F1 hybrid ES cells (C57BL/6 x 129) typically are used for tetraploid blastocyst injection. The use of either pure 129 or C57BL/6 ES cells for tetraploid blastocyst microinjection is feasible,2,23 but to date F1 ES cells have proven to be more robust.7 Consequently if a researcher wants to use a mouse with a pure 129 or C57BL/6 background for study,22 backcrossing steps will need to be done. Viability of embryos from tetraploid injections is reportedly lower than with diploid embryos, with considerable strain variation.24 In addition, in 1 study, outbred Swiss Webster blastocysts exhibit greater developmental potential with the tetraploid technique than do blastocysts from hybrid mice.6 Therefore we performed a retrospective study of work done in a core facility using 3 types of mice for embryologic and targeted mutant mouse research. Implantation sites, viable fetuses, and pups resulting from injection into outbred, hybrid, and inbred tetraploid blastocysts were evaluated whenever feasible, within the constraints of differing methods and objectives of unrelated studies.
Materials and Methods
Animals.
C57BL/6NTac (B6, inbred) and B6D2F1/Tac (BDF1, hybrid) mouse strains were chosen because they are the most commonly used strains for diploid blastocyst ES cell injection and for tetraploid blastocyst ES cell injection, respectively.1,4,7,12,21,23 The Crl:CD1(ICR) (CD1, outbred) stock was used for comparison because these mice were used for the Institute's embryo transfer recipient colony, and ICR stock has been used successfully in tetraploid blastocyst injections.10 C57BL/6NTac, B6D2F1/Tac (Taconic, Hudson, NY), and Crl:CD1(ICR) (Charles River Laboratories, Wilmington, MA) mice were housed on autoclaved bedding and provided autoclaved water and food ad libitum in an AAALAC-accredited facility. Studies were approved by the Massachusetts Institute of Technology Committee on Animal Care. Animals were housed at 20.0 to 21.1 °C and 30% to 70% humidity on a 14:10-h light:dark cycle. Mice were specific pathogen-free for the following agents: mouse hepatitis virus, minute virus of mice, Sendai virus, mouse rotavirus, pneumonia virus of mice, Theiler disease virus, lymphocytic choriomeningitis virus, reovirus 3, Ectromelia, Adenovirus, Polyomavirus, K virus, internal and external parasites, cilia-associate respiratory bacillus, Mycoplasma pulmonis, Citrobacter freundii biotype 4280, Helicobacter spp., Salmonella spp., and Campylobacter spp.
Study design.
This study was a retrospective analysis of tetraploid blastocyst injections performed in a core service facility. The goal of the research studies for which the blastocyst injections were performed was either midgestational tissue harvest for embryology studies or generation of pups for use as targeted mutant mouse models. Different ES cell lines and clones were used, depending on the particular research question of interest.
Creation of tetraploid embryos.
Female mice were superovulated at 3 to 4 wk old by intraperitoneal injection of 5 IU pregnant mare serum (Sigma-Aldrich, St Louis, MO) followed by 5 IU human chorionic gonadotropin (Sigma-Aldrich, St. Louis, MO) 47 h later, and then bred immediately to B6, BDF1, or CD1 stud males. Pseudopregnant CD1 mice were physiologically primed to receive injected blastocysts, regardless of strain, by mating to vasectomized CD1 males.
Zygotes of each strain or stock were collected by standard methods17 at 0.5 d postcoitus and cultured to 2-cell stage in microdrops of potassium simplex optimized medium with amino acids (KSOM-AA; Chemicon International, Temecula, CA) overlaid with mineral oil (Sigma-Aldrich) in an incubator at 37 °C, 5% CO2. Electrofusion and blastocyst injection were performed as described previously.17 Briefly, 2-cell embryos (B6, n = 788; BDF1, n = 1871; CD1, n = 1308) were fused (Cellfusion CF-150/B, BLS, Budapest, Hungary) in a 250-μm gap electrode chamber containing 0.3 M mannitol with 0.3% bovine serum albumin (Sigma-Aldrich). An initial electrical field of 2 V was applied to the embryos, followed by peak pulses of 50 V for 35 μs. Embryos were observed for fusion after 40 min and then were cultured to blastocyst stage in filtered KSOM-AA. The time course of embryonic development from electrofused stage to blastocyst stage varied for B6 and BDF1 embryos, with approximately 30% of embryos requiring an additional 18 h of culture prior to blastocyst stage; B6 tetraploid embryos were more prone to variation. Viability of successfully injected embryos was not affected by this variable embryo development; the ability to inject blastocysts was affected because not all blastocysts were at the appropriate stage for injection at the scheduled injection time. The variation was mitigated by using a 0.2-µm filter (Corning, NY) for preparation of KSOM culture medium. There was no evidence that the medium was contaminated; improved success from using filtered medium occurred for unexplained reasons.
Male F1 hybrid ES cells were prepared by standard methods.18 Strain 129-3 ES cells were C57BL/6J × 129S4, passage 13; V6.5 ES cells were C57BL/6J × 129S4, passage 14 or 16 (Tables 1 and 2 ). These ES cells give rise to mice with agouti coat color and have proven efficient in producing high percentage chimeras with germline transmission by diploid blastocyst injection in our facility. Tetraploid blastocysts were injected with 10 to 15 ES cells each by using a Piezo microdrill (Primetech, Ibaraki, Japan) or a Leitz micromanipulator (Leica Microsystems, Bannockburn, IL) and surgically transferred into pseudopregnant recipient CD1 mice (n = 7 to 21 embryos/recipient; mean, 13 ± 3.9; median, 10; mode, 10). The number of blastocysts transferred per recipient potentially affects embryo survival; because similar numbers of blastocysts were surgically transferred per recipient dam as for typical diploid experiments in our lab, this variable was minimized for the present study.
Table 1.
Fetus yield from tetraploid blastocyst injection of ES cell clones
| Blastocyst strain |
|||||||||
| B6 |
BDF1 |
CD1 |
|||||||
| Fetuses |
Fetuses |
Fetuses |
|||||||
| Clone | No. of blastocysts injected | No. | Yield (%)a | No. of blastocysts injected | No. | Yield (%) | No. of blastocysts injected | No. | Yield (%) |
| JW | 57 | 0 | 0.0 | 111 | 6 | 5.4 | |||
| Unknownb | 187 | 1 | 0.5 | ||||||
| GFP | 157 | 19 | 12.1 | ||||||
| B6 | 26 | 4 | 15.4 | ||||||
| FLK | 60 | 0 | 0.0 | 29 | 0 | 0.0 | |||
| F2 | 70 | 25 | 35.7 | ||||||
| F3 | 50 | 18 | 36.0 | ||||||
| FBLIM2 | 60 | 4 | 6.7 | ||||||
| 13713 | 50 | 7 | 14.0 | ||||||
| B6-2GFP | 60 | 5 | 8.3 | ||||||
For all experiments, 129-3 ES cells at passage 13 were used.
Fetus yield = no. of fetuses harvested/no. of blastocysts injected.
The same unknown clone was used on multiple injection days.
Table 2.
Pup yield from tetraploid blastocyst injection of ES cell clones
| Blastocyst strain |
||||||||||||
| BDF1 |
CD1 |
|||||||||||
| ES cell line | Passage | Clone | No. of blastocysts injected | No. of pups born | Pup yield (%)a | No. of live pups born | Live pup yield (%) | No. of blastocysts injected | No. of pups born | Pup yield (%) | No. of live pups born | Live pup yield (%) |
| 129-3 | 13 | F1-1P2 | 30 | 2 | 6.7 | 1 | 3.3 | |||||
| 129-3 | 13 | p53 | 90 | 1 | 1.1 | 1 | 1.1 | |||||
| 129-3 | 13 | ES | 60 | 2 | 3.3 | 2 | 3.3 | |||||
| V6.5 | 14 | WT | 139 | 7 | 5.0 | 5 | 3.6 | 109 | 6 | 5.5 | 2 | 1.8 |
| V6.5 | 16 | R-2E1 | 71 | 9 | 12.7 | 5 | 7.0 | |||||
| V6.5 | 16 | M-1D4 | 181 | 15 | 8.3 | 11 | 6.1 | |||||
| V6.5 | 16 | R-6B1 | 68 | 4 | 5.9 | 1 | 1.5 | |||||
| V6.5 | 16 | R-3F1 | 80 | 8 | 10.0 | 6 | 7.5 | |||||
Pup yield = no. of pups born/no. of blastocysts injected.
Embryology studies.
Initially, B6 and BDF1 donor embryos were used for embryology studies; an additional experiment was performed using CD1 donor embryos. After tetraploid blastocyst injection, reproductive tracts of recipient dams were examined at midgestation (E9.5 to 10.5), and implantation sites and fetuses were counted. Implantation sites were identified as grossly visible foci on the uterus where an embryo had implanted but later was aborted or resorbed (that is, nonviable embryos). Viable fetuses were identified as being developmentally appropriate for the gestational age. Relative contributions of ES cell and tetraploid blastocysts to the embryo have been documented elswhere.11,13 F1 hybrid ES cells expressing green fluorescent protein (GFP) were used as a rough tracking guide for measuring ES cell contribution for some experiments when fetuses were harvested at E9.5 to 10.5 for analysis. We noted the overall fluorescence of fetal tissues at midgestation, which demonstrated ES cell content at a stage when coat color data was not available (data not shown). However due to the poor fetus yield from using B6 blastocysts with several different ES cell clones in initial experiments, this strain was omitted from subsequent experiments.
Pup studies.
For experiments in which the research goal was generation of targeted mutant mouse models, BDF1 or CD1 tetraploid-injected blastocysts were carried to full-term gestation. Non-GFP F1 hybrid ES cells carrying various mutations were used for these experiments (Table 2). Pups were assigned an ES cell score as a percentage of donor embryo coat color contribution relative to ES cell coat color contribution, based on the typical scoring system used for diploid blastocyst injection:
 |
Statistics.
Regression analysis (Microsoft Excel, Microsoft Corporation, Redmond, WA), the Fisher exact test, unpaired Student t test, and analysis of variance (GraphPad Prism, GraphPad Software, San Diego, CA) were performed for ES cell clones, ES cell lines, and mouse strains.
Results
Electrofusion.
Electrofusion was highly efficient, and electrofusion rates did not differ among inbred, hybrid, and outbred embryos (Table 3). These data are consistent with high rates of electrofusion reported previously.24 The percentages of fused tetraploid embryos developing to the blastocyst stage were: B6, 32.3% (244 of 754); BDF1, 51.5% (903 of 1754); and CD1, 70.1% (n = 820 of 1172). B6 embryos did not develop as well as BDF1 or CD1 (2-tailed P < 0.0001 for each by the Fisher exact test) and CD1 embryos developed at greater rates than did BDF1 (2-tailed P < 0.0001 by the Fisher exact test). In light of the unexpected success of CD1 embryos in their development to the blastocyst stage, not all of the CD1 blastocysts were injected, due to time and personnel limitations.
Table 3.
Embryo electrofusion rates
| Electrofusion |
||||
| Embryo strain | Strain type | No. of 2-cell embryos (2n) | No. of successful fusions (4n) | Fusion rate (%) |
| C57BL/6NTac | inbred | 788 | 754 | 95.7 ± 3.1 |
| B6D2F1/Tac | hybrid | 1871 | 1754 | 93.7 ± 4.3 |
| Crl:CD1(ICR) | outbred | 1308 | 1172 | 89.6 ± 7.3 |
Embryology studies.
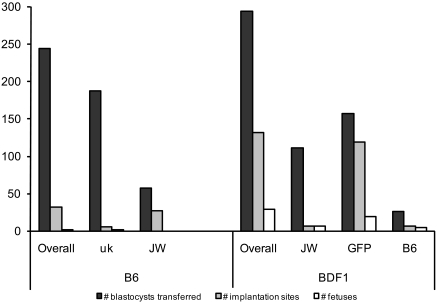
Fetal development was assessed at midgestation for 9 different clones of the ES cell line 129-3 (Table 1). After ES cell injection, fetus viability (E9.5 to 10.5) was much higher in BDF1 hybrids than in B6 inbreds (Figure 1 and Table 1). Of 244 B6 blastocysts surgically transferred, 32 implantation sites were detected (13.1%), and only 1 fetus was viable (0.49%). Of 294 BDF1 blastocysts surgically transferred, 131 implantation sites were detected (44.6%), and an additional 29 fetuses were viable (9.9%). Only 1 ES cell line, JW, was injected into both B6 and BDF1 blastocysts, allowing a controlled comparison between the blastocyst strains. Analysis of the total number of fetuses per JW-injected blastocyst showed no difference between the 2 blastocyst strains (P = 0.18 by the Fisher exact test). When data from the JW ES cell line injections were examined at the level of number of fetuses per dam, there was no difference between the B6 and BDF1 blastocysts (unpaired Student t test, 2-tailed P = 0.4129). Notably, of the 6 dams that received BDF1 blastocysts injected with JW cells, only 2 dams carried viable fetuses. Therefore 4 recipient dams carried no viable fetuses from JW cells injected into BDF1 blasts, explaining why the presence of 3 dams carrying no viable fetuses from JW cells injected into B6 blasts was not statistically unexpected. Because preliminary experiments using B6 blastocysts yielded poor results for both embryo implantation and fetus recovery, this strain was omitted from further studies. The nature of this retrospective study from a core service facility precluded the ability to obtain further B6 data. To ensure that recovered fetuses were truly ES-cell–derived rather than chimeras, all subsequent embryology experiments used GFP-expressing ES cells to track ES cell contribution. Further BDF1 tetraploid-injected blastocysts (n = 350) yielded 59 fetuses, all of which displayed extensive GFP fluorescence, for an overall BDF1 success rate of 13.7% (88 of 644).
Figure 1.
Implantation sites and fetal viability at E9.5–10.5 (B6 and BDF1 fetuses). For B6, 2 ES cell clones were used (uk and JW). For BDF1, 3 ES cell clones were used (JW, GFP, and B6). Of 244 B6 tetraploid blastocysts surgically transferred, 32 implantation sites were detected, and 1 fetus was viable. Of 294 BDF1 tetraploid blastocysts surgically transferred, 131 implantation sites were detected, and 29 fetuses were viable.
Regression analysis showed no statistical difference among ES cell clones in BDF1 (P = 0.79), therefore a clone effect could be ruled out. One experiment from 29 CD1 blastocysts that yielded no fetuses was not included in the analysis because of insufficient sample size. Ideally the experiments would be performed prospectively in both B6 and BDF1 blastocysts in sufficient numbers to achieve higher statistical power.
Pup studies.
BDF1 (n = 259) and CD1 (n = 569) tetraploid-injected blastocysts were carried to term (implantation sites were not counted for fetuses carried to term; Table 4). Eight clones from 2 ES cell lines were used for experiments that were carried to term (3 clones from 129-3 and 5 clones from V6.5; Table 2). Ten BDF1 pups were born by natural birth to 7 dams (1 to 2 pups/litter; mean, 1.4 pups/litter). Seven pups were alive at birth; 4 of these pups were harvested for analysis postpartum, and the remaining 3 pups survived to adulthood. These pups were scored as ES-cell–derived in light of 100% agouti coat and were fertile. Of the 44 CD1 pups born by natural birth to 23 dams (1 to 4 pups/litter; mean, 1.9 pups/litter), 27 pups were alive and showed 100% agouti coat; 16 of these pups survived to adulthood and were fertile, resulting in an overall CD1 success rate of 2.8% (16 of 569).
Table 4.
4n blastocyst injection: fetus and pup yields
| 4n blastocyst injection |
||||||
| Yield (%)a |
||||||
| Embryo strain | Strain type | No. of blastocysts injected and transferred | No. of fetuses | No. of pups born | Fetuses | Pups |
| C57BL/6NTac | inbred | 204 | 1 | na | 0.5 | na |
| B6D2F1/Tac | hybrid | 634 | 88 | na | 13.9 | na |
| 249 | na | 10 | na | 4.0% | ||
| Crl:CD1(ICR) | outbred | 560 | na | 44 | na | 7.9% |
na, not applicable
Fetus yield = no. of fetuses/no. of blastocysts transferred. Pup yield = no. of pups /no. of blastocysts transferred.
Regression analysis showed no statistical difference among ES cell clones (P = 0.87) or ES cell lines (P = 0.92). Because the laboratory is a core facility, most experiments were performed by using genetically altered ES cells. Of the experiments described in the present study, 3 tetraploid experiments (139 BDF1 blastocysts, 109 CD1 blastocysts) were performed by using unmanipulated wild-type ES cells (Table 1; WT, V6.5 passage 14). Pup yield from these wild-type ES cells was similar for both BDF1 (7 of 139) and CD1 (6 of 109). Although no other clones were used for BDF1 and CD1 simultaneously, the overall pup yield for BDF1 was 10 of 259 (3.9%) and for CD1 was 44 of 569 (7.7%).
Investigators chose natural birth over caesarean section due to the decreased personnel and animal resources required. Dams were observed throughout gestation for abdominal enlargement as a sign of pregnancy, and intervention was available during labor if dams showed signs of dystocia. Overall, dams that appeared to be pregnant at 20 d postcoitus gave birth naturally with no intervention. Most of the time, lack of viable pups was associated with dams that did not appear pregnant at 20 d postcoitus, so those fetuses had most likely resorbed earlier during gestation, rather than dying during the periparturient period.
Discussion
The present study analyzed fetus viability and pup births using the tetraploid blastocyst injection technique; however fetus viability may not correlate with live pup viability. Because low pup yield is common with the tetraploid method,4,7,12,20 it may be most useful initially for embryology studies. In our lab, tetraploid fetus viability, like diploid fetus viability, tended to be dependent on using filtered media with intact unhatched blastocysts. Whereas experiments using B6 blastocysts yielded only 1 viable fetus, studies using blastocysts from BDF1 and CD1 stock yielded live pups and animals that survived to adulthood and proved to be fertile, suggesting that variable survivability is dependent on strain and demonstrates the outbred vigor of the CD1 stock. However, discontinuing analysis of B6 donor embryos resulted in insufficient data to achieve statistical power. BDF1 and CD1 embryos performed equally well. Use of the CD1 stock as donors for tetraploid blastocysts was efficient in our lab and had the additional benefits of being both cost-effective due to inhouse breeding and convenient because their white coat color is easily distinguished in the progeny as donor- or ES-cell–derived from the day of birth. If an unfused CD1 embryo is injected inadvertently along with tetraploid blastocysts, the resultant pup would be a chimera instead of a completely ES-cell–derived mouse and therefore could contain some white coat color contribution from the donor CD1 blastocyst; if donor embryos from brown or black mice were used, this coat color anomaly would likely go undetected until much later. Such a scenario has occurred in our lab on rare occasion—the CD1 contribution was obvious immediately at birth so that needless time and resource expenditure were averted. Furthermore the high yield of fused CD1 embryos developing to the blastocyst stage reduced the number of animals required to provide the necessary number of donor embryos.
In addition to being a tool for genetic manipulations, embryo transfer is widely used for rederivation to eradicate mouse pathogens. Embryo transfer of mice that are not genetically modified is a straightforward procedure with a greater than 90% efficiency in our lab. The rate is slightly lower with genetically modified strains, perhaps attributable to factors such as decreased fertility or lethal or morbid phenotype. The introduction of genetic manipulations directly into embryos, as with aggregation chimera modeling, reduces embryo transfer efficiency. This decrease may be due to factors such as direct embryo cell lysis from the manipulation, cell death during in vitro culture, decreased in utero survivability after manipulation, and phenotype alterations resulting from the genetic modification. In our lab, the chimeric pup yield from diploid embryo injections is greater than 30%, (however, not all of these chimeras possess ES-cell–derived germlines); the pup yield from tetraploid injections is 6.5% as reported in the present study, and the germlines of all the mice we tested appear to be fully ES-cell–derived.
Tetraploid blastocyst injection is a valuable technology for the generation of mice with targeted mutations and for embryology research. The benefits of tetraploid blastocyst injection in targeted transgenesis are easily discernable. The timeline for generating targeted mutant mice by using diploid blastocyst injection is quite long; the current production strategy involves injecting ES cells into a diploid blastocyst, acquiring pups which range from 0% to 100% chimerism, test mating the high-percentage chimeras for germline transmission, and then intercrossing before the line can be used for research purposes. This process can take months of effort without an accurate assessment of the mouse's genotype,7,21 especially while trying to determine which (if any) chimeras carry the mutation in the germline. In contrast, tetraploid blastocyst injection produces mice that are fully ES-cell–derived and can be used immediately for studies or for generating a research colony.
Some drawbacks of the tetraploid method, such as the decreased efficiency of pup production and higher initial cost and time investments, are considerable. Although the tetraploid blastocyst microinjection itself takes the same amount of time as diploid blastocyst microinjection, the tetraploid method involves additional embryo culture time (3 d compared with 1/2 d). In addition, the electrofusion step takes an entire day, occupying time that could otherwise have been spent performing an entire diploid blastocyst microinjection procedure. The pup yield appears to be lower with the tetraploid method; however, with the diploid method, litters usually contain a combination of high-percentage chimera pups, low-percentage chimera pups, and some pups with no chimerism. Considering that germline transmission is more likely to occur in high-percentage chimeras, the yield of chimeras potentially carrying the mutation germline from the diploid method is not much greater than the yield of pups from the tetraploid method. A researcher can be reasonably confident that pups produced by the tetraploid method are useful animals because the mutation is unquestionably present in the germline. Therefore the lower pup yield from the tetraploid technique need not be a cause for alarm in the long term.
A key obstacle to obtaining pups that survive to adulthood is the small litter sizes obtained after tetraploid ES cell injection; in our lab thus far, only 1 to 2 embryos per recipient have survived to parturition. Small litters are particularly problematic when dystocia and cannibalization occur. Dystocia was not noted in the present study, but cannibalization and still-born pups did occur. Use of carrier embryos (unmanipulated embryos that help support the pregnancy), performing caesarean sections, and providing foster dams may help to alleviate some of these complications. Surgical transfer of more tetraploid ES-cell–injected embryos per recipient may help achieve larger litter sizes. ES cell quality is another important factor with tetraploid complementation, and cannibalized pups could be offspring from lower-quality ES cells.12
Induction of tetraploidy is an invasive procedure that appears to have a high percentage of deleterious embryonic effects, as evidenced by the lower rates of midgestational fetus recovery and pup production observed in the present study compared with traditional diploid ES cell injection (in our lab, 30% of injected diploid embryos develop into pups, compared with 8.7% of tetraploid embryos). Several factors may account for these differences. Inbreeding depression (a reduction of the health and fitness of offspring produced by inbred mouse strains) would be expected to contribute to higher morbidity and mortality of manipulated inbred embryos compared with manipulated hybrid and outbred embryos. When diploid blastocyst injection embryos are collected, injected once, and surgically transferred into recipient mice on the same day, the process lasts an average of 6 h. In comparison, embryos used in the tetraploid procedure must not only survive in vitro for 3 to 4 d but also withstand the additional electrofusion manipulation. Diminished ability of embryos to tolerate the additional manipulations would be expected with inbreeding depression. Other scientists have reported a greater developmental potential of embryos in outbred Swiss Webster compared with hybrid mice using the tetraploid technique,6 and we observed increased viability in outbred CD1 mice in the present study. After electrofusion we noted increased fragility of tetraploid embryos during blastocyst injection; therefore additional lethality due to the blastocyst injection procedure would be expected in tetraploid embryos compared with diploid embryos. These factors, combined with the variables introduced by the type and quality of ES cells used, contribute to the success of fetus and live pup production efficiency by tetraploid blastocyst injection.
In this study we observed more consistent generation of ES-cell–derived mouse fetuses and pups by tetraploid blastocyst injection using hybrid BDF1 and outbred CD1 embryos compared with inbred B6 embryos. These results must be interpreted taking into consideration the difficulty achieving high statistical power associated with the limitations of a retrospective study from a core service facility. Not only does the embryo strain affect survivability of tetraploid embryos, but the origin of ES cells also affects fetus viability after tetraploid blastocyst injection. Previous studies have shown that hybrid ES cells (typically C57BL/6 × 129 strains) produce viable offspring more efficiently than do inbred ES cells (typically 129 strains).7 Furthermore, tetraploid blastocyst aggregation experiments reveal that the genetic background of ES cells as well as the embryos offer key contributions the survival of these mice.12 Therefore, both embryo choice and ES cell choice are important factors for investigators to consider when creating knockout mice by using the tetraploid blastocyst injection method. The data presented here should further increase the overall efficiency of mouse generation though tetraploid injection by highlighting the outbred CD1 stock as a robust source of blastocysts for this procedure.
Acknowledgments
This work was supported by grants T32RR07036, PO1CA26731, and P30ES02109 from the NIH. AAB and JMM are supported in part by National Cancer Institute Cancer Center Support Grant 5 P30 CA14051. We thank James Fox, Mark Whary, and Joel Lawitts for critical reading of this manuscript, and Mark Whary for assistance with statistical analyses.
References
- 1.Aiba A, Inokuchi K, Ishida Y, Itohara S, Kobayashi K, Masu M, Mishina M, Miyakawa T, Mori H, Nakao K, Obata Y, Sakimura K, Shiroishi T, Wada K, Yagi T. 2007. Mouse liaison for integrative brain research. Neurosci Res 58:103–104 [DOI] [PubMed] [Google Scholar]
- 2.Baharvand H, Matthaei KI. 2004. Culture condition difference for establishment of new embryonic stem cell lines from the C57Bl/6 and BALB/c mouse strains. In Vitro Cell Dev Biol Anim 40:76–81 [DOI] [PubMed] [Google Scholar]
- 3.Bronson SK, Smithies O. 1994. Altering mice by homologous recombination using embryonic stem cells. J Biol Chem 269:27155–27159 [PubMed] [Google Scholar]
- 4.Cho M, Jang M, Lee EJ, Han JY, Lim JM. 2006. An alternative method of deriving embryonic stem cell-like clones by aggregation of diploid cells with tetraploid embryos. Fertil Steril 85Suppl 1:1103–1110 [DOI] [PubMed] [Google Scholar]
- 5.Downing GJ, Battey JF., Jr 2004. Technical assessment of the first 20 years of research using mouse embryonic stem cell lines. Stem Cells 22:1168–1180 [DOI] [PubMed] [Google Scholar]
- 6.Eakin GS, Hadjantonakis A, Papaioannou VE, Behringer RR. 2005. Developmental potential and behavior of tetraploid cells in the mouse embryo. Dev Biol 288:150–159 [DOI] [PubMed] [Google Scholar]
- 7.Eggan K, Akutsu H, Loring J, Jackson-Grusby L, Klemm M, Rideout WM, 3rd, Yanagimachi R, Jaenisch R. 2001. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc Natl Acad Sci USA 98:6209–6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggan K, Rode A, Jentsch I, Samuel C, Hennek T, Tintrup H, Zevnik B, Erwin J, Loring J, Jackson-Grusby L, Speicher MR, Kuehn R, Jaenisch R. 2002. Male and female mice derived from the same embryonic stem cell clone by tetraploid embryo complementation. Nat Biotechnol 20:455–459 [DOI] [PubMed] [Google Scholar]
- 9.Fox JG, Anderson LC, Loew FM, Quimby FW. 2002. Laboratory animal medicine, 2nd ed San Diego: Academic Press; p 37 [Google Scholar]
- 10.George SHL, Gertsenstein M, Vintersten K, Korets-Smith E, Murphy J, Stevens M, Haigh JJ, Nagy A. 2007. Developmental and adult phenotyping directly from mutant embryonic stem cells. Proc Natl Acad Sci USA 104:4455–4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishiguro N, Kano K, Yamamoto Y, Taniguchi K. 2005. Tetraploid cells of enhanced green fluorescent protein transgenic mice in tetraploid/diploid-chimeric embryos. J Reprod Dev 51:567–572 [DOI] [PubMed] [Google Scholar]
- 12.Li X, Wei W, Yong J, Jia Q, Yu Y, Di K. 2005. The genetic heterozygosity and fitness of tetraploid embryos and embryonic stem cells are crucial parameters influencing survival of mice derived from embryonic stem cells by tetraploid embryo aggregation. Reproduction 130:53–59 [DOI] [PubMed] [Google Scholar]
- 13.MacKay GE, West JD. 2005. Fate of tetraploid cells in 4n-2n chimeric mouse blastocysts. Mech Dev 122:1266–1281 [DOI] [PubMed] [Google Scholar]
- 14.McKerlie C. 2006. Cause and effect considerations in diagnostic pathology and pathology phenotyping of genetically engineered mice (GEM). ILAR J 47:156–162 [DOI] [PubMed] [Google Scholar]
- 15.Melo EO, Canavessi AM, Franco MM, Rumpf R. 2007. Animal transgenesis: state of the art and applications. J Appl Genet 48:47–61 [DOI] [PubMed] [Google Scholar]
- 16.Misra RP, Bronson SK, Xiao Q, Garrison W, Li J, Zhao R, Duncan SA. 2001. Generation of single-copy transgenic mouse embryos directly from ES cells by tetraploid embryo complementation. BMC Biotechnol 1:12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagy A, Gertsenstein M, Vintersten K, Behringer R. 2003. Manipulating the mouse embryo, a laboratory manual, 3rd ed Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press [Google Scholar]
- 18.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. 1993. Derivation of completely cell culture-derived mice from early passage embryonic stem cells. Proc Natl Acad Sci USA 90:8424–8428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raman V, Pathak AP, Glunde K, Artemov D, Bhujwalla ZM. 2007. Magnetic resonance imaging and spectroscopy of transgenic models of cancer. NMR Biomed 20:186–199 [DOI] [PubMed] [Google Scholar]
- 20.Schwenk F, Zevnik B, Bruning J, Rohl M, Willuweit A, Rode A, Hennek T, Kauselmann G, Jaenisch R, Kuhn R. 2003. Hybrid embryonic stem cell-derived tetraploid mice show apparently normal morphological physiological, and neurological characteristics. Mol Cell Biol 23:3982–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seibler J, Zevnik B, Kuter-Luks B, Andreas S, Kern H, Hennek T, Rode A, Heimann C, Faust N, Kauselmann G, Schoor M, Jaenisch R, Rajewsky K, Kuhn R, Schwenk F. 2003. Rapid generation of inducible mouse mutants. Nucleic Acids Res 31:e12–e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seong E, Saunders TL, Stewart CL, Burmeister M. 2004. To knockout in 129 or in C57Bl/6: that is the question. Trends Genet 20:59–62 [DOI] [PubMed] [Google Scholar]
- 23.Shimizukawa R, Sakata A, Hirose M, Takahashi A, Iseki H, Liu Y, Kunita S, Sugiyama F, Yagami K. 2005. Establishment of a new embryonic stem cell line derived from C57BL/6 mouse expressing EGFP ubiquitously. Genesis 42:47–52 [DOI] [PubMed] [Google Scholar]
- 24.Wang ZQ, Kiefer F, Urbanek P, Wagner EF. 1997. Generation of completely embryonic stem cell-derived mutant mice using tetraploid blastocyst injection. Mech Dev 62:137–145 [DOI] [PubMed] [Google Scholar]