SUMMARY
Toll-like receptor (TLR)-dependent pathways control the production of IFNαβ, a key cytokine in innate immune control of viruses including mouse cytomegalovirus (MCMV). The lymphotoxin (LT) αβ-LTβ receptor signaling pathway is also critical for defense against MCMV and thought to aid in the IFNβ response. We find that upon MCMV infection, mice deficient for lymphotoxin (LT)αβ signaling cannot mount the initial part of a biphasic IFNαβ response, but show normal levels of IFNαβ during the sustained phase of infection. Significantly, the LTαβ-dependent, IFNαβ response is independent of TLR signaling. B, but not T, cells expressing LTβ are essential for promoting the initial IFNαβ response. LTβR expression is required strictly in splenic stromal cells for initial IFNαβ production to MCMV and is dependent upon the NF-κB-inducing kinase (NIK). These results reveal a TLR-independent innate host defense strategy directed by B cells in communication with stromal cells via the LTαβ cytokine system.
INTRODUCTION
Type I interferons (IFNαβ) play a major role in the innate immune defense against viral pathogens through direct inhibition of viral replication in infected cells and by regulating the survival and differentiation of key innate effector cells, such as NK cells and T and B cells of the adaptive immune system. INFαβ promotes activation and maturation of antigen presenting dendritic cells (DC), facilitating the bridge between innate and adaptive immunity (Hoebe et al., 2004; Kawai and Akira, 2006; Pascual et al., 2006). INFαβ production is initiated by recognition of viral components through innate sensing receptors including the Toll-like receptor (TLR) family, as well as RNA helicase and Pyrin-dependent systems (reviewed in Benedict and Ware, 2005a, 2005b; Kato et al., 2006; Meylan and Tschopp, 2006; Werts et al., 2006). Although all nucleated cells can produce INFαβ in response to virus infection, plasmacytoid DC (pDC) produce high levels of INFα in response to infection with many different viruses, utilizing largely TLR-dependent pathways (Delale et al., 2005; Liu, 2005; Siegal et al., 1999).
Mouse cytomegalovirus (MCMV, a β-herpesvirus) has emerged as an important model revealing host defense strategies controlling pathogen persistence and latency (Mocarski, 2004; Pollock et al., 1997; Reddehase, 2002). Control of MCMV requires both innate and adaptive host defenses, with INFαβ signaling serving as a key component of innate immunity (Orange and Biron, 1996). MCMV replicates efficiently in most visceral organs during the first week of infection, with persistent replication in the salivary gland continuing for 4−8 weeks en route to establishing lifelong latency (Mocarski and Courcelle, 2001). INFαβ and NK cells largely limit early replication in the spleen of C57BL/6 (B6) mice, whereas T cells are required for eventual control of acute infection and reactivation from latency. The pDC has been identified as a major INFαβ-producing cell type in the spleen at times when INFα levels have been reported to peak in the serum of B6 mice (∼36 hr) (Dalod et al., 2003; Dalod et al., 2002; Krug et al., 2004).
Evidence that pDC-derived INFαβ is key for host defense to MCMV infection in vivo also comes from data indicating that mice deficient in TLR9 or MyD88 (the downstream adaptor molecule required for TLR9 signaling) show depressed levels of serum INFαβ at 36 hr postinfection, and these mice are more susceptible to MCMV infection (Delale et al., 2005; Krug et al., 2004). TLR3 signals through the adaptor molecule TRIF, and TRIF−/− mice show increased MCMV replication, although the TLR3-TRIF pathway appears less important than TLR9 (Hoebe et al., 2003; Tabeta et al., 2004).
The lymphotoxin (LT)αβ-LTβ receptor signaling pathway (Schneider et al., 2004; Ware, 2005) is critical for effective host defense against MCMV (Banks et al., 2005; Benedict et al., 2001). Mice deficient in genes encoding LTα, LTβ, and LTβR exhibit increased viral replication in the spleen and liver and increased morbidity, whereas mice deficient in LIGHT, a second ligand for the LTβR, show a modest increase in MCMV replication. The LTβR signaling pathway is thought to aid in promoting an effective IFNβ response, which is linked to survival of cells of the adaptive immune system. LTα−/− mice exhibited a severe defect in the induction of IFNβ mRNA in the spleen. Mice deficient in LTβR signaling also displayed a profound increase in the apoptosis of both T- and B-lymphocytes 3 to 4 days after infection with MCMV, although these cells were not directly infected. INFαβ receptor deficient mice shared this lymphocyte apoptosis phenotype in response to MCMV infection implicating an important connection between the LT and IFN pathways that allows the adaptive immune system to survive MCMV infection (Banks et al., 2005). The LTβR pathway is critically important for the formation and homeostasis of microenvironments in lymphoid organs that promote the efficiency of the adaptive immune responses (Ehlers et al., 2003; Kabashima et al., 2005). Recent findings suggest that MCMV's strategy of immune evasion involves targeting an LTβR-dependent homeostatic pathway. LTβR signaling regulates stromal cell expression of homeostatic chemokines, such as CCL21 and CXCL13, required for trafficking and positioning of T and B cells to their respective niches in the spleen (Cyster, 2005). Interestingly, MCMV infection selectively downregulates expression of CCL21, altering T cell positioning in the splenic white pulp (Benedict et al., 2006).
The role of the LTβR pathway in regulating the innate INFαβ response presented a conundrum in view of evidence indicating that TLR-dependent pathways are important in both controlling INFαβ production and for resistance to MCMV. Here, we identify a TLR-independent pathway controlling the initial INFαβ response to MCMV in the spleen. This pathway involves B lymphocytes expressing LTαβ that crosstalk with LTβR-expressing stromal cells. MCMV preferentially infects the stromal cells in the spleen, which produce the majority of the initial INFαβ. The results reveal an unorthodox strategy of the naive adaptive immune system controlling the innate host INFαβ response to a viral pathogen.
RESULTS
The Type I IFN Response to MCMV in B6 Mice Is Biphasic
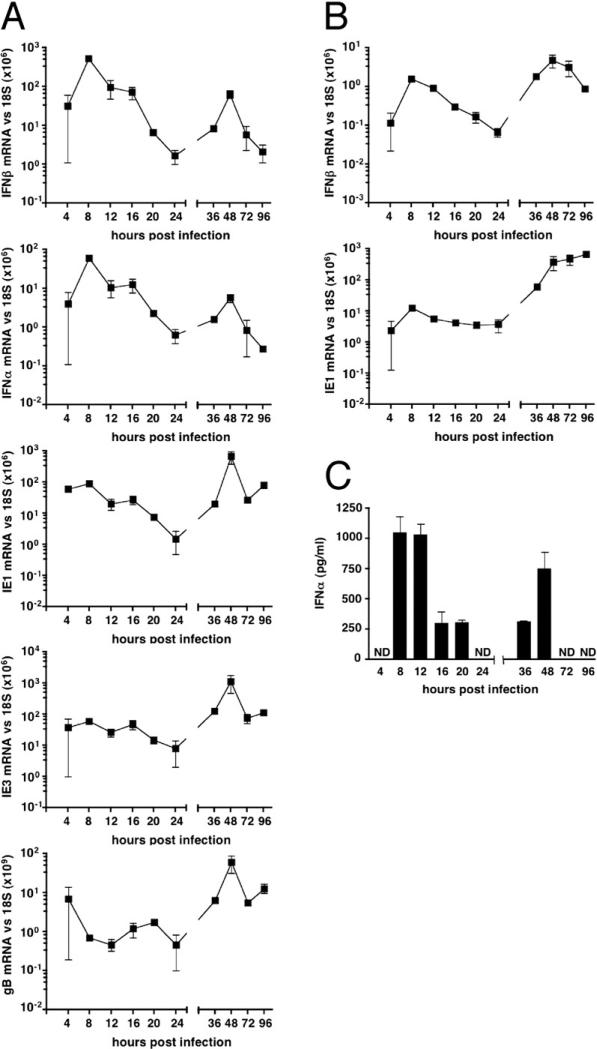
Infection of B6 mice with MCMV induced a biphasic IFNβ and INFα response in the spleen as detected by quantitative PCR (Figure 1A). The initial splenic IFNβ and INFα mRNA induced by infection reached maximal accumulation by 8 hours postinfection and then declined over the next 16 hr (Figure 1A). At the peak response, the relative induction of mRNA was 104-fold increased for IFNβ and 105-fold for INFα over baseline expression in naive mice. A second, more sustained accumulation of IFNβ and INFα mRNA occurred between 36−72 hr. The IFNβ mRNA levels that accumulated in the initial phase was substantially greater than achieved in the sustained phase (587 mRNA relative units/day versus 17 mRNA units/day) indicating the initial phase accounts for the majority of the IFNβ transcriptional activity at these infection conditions.
Figure 1. Analysis of the IFNαβ Response during MCMV Infection.
B6 mice were infected with MCMV (2 × 105 PFU i.p.), organs or serum were harvested at the indicated times for analysis. (A) The mRNA levels in the spleen for IFNβ, INFα, and MCMV ie1, ie3, and gB was determined by quantitative RT-PCR (qPCR) analysis of total cell RNA. All qPCR values are shown normalized to the levels of 18S rRNA. The baseline mRNA level in mock-infected B6 mice for IFNβ was 2 × 10−3/18S × 106 and INFα 3 × 10−4/18S × 106.
(B) Liver mRNA from infected B6 mice as in (A).
(C) Serum INFα levels were determined by ELISA (ND, not detectable). Each time point represents 3 to 6 mice per group, mean ± SEM.
Expression of MCMV transcripts for immediate early (ie)-1 and 3, and the envelope glycoprotein B (gB, an early/late gene product) were readily detected in the spleen 4−8 hr postinfection (Figure 1A). The levels of ie1 mRNA decreased over the next 16 hr (∼100-fold), paralleling the expression profile of INFα and IFNβ mRNA. In contrast, ie3 and gB mRNA remained relatively constant over the first 24 hr (Figure 1A). As the infection progressed, viral gene expression dramatically increased, reaching maximum abundance after two days, corresponding closely to the peak production of infectious virus, which occurs in the spleens of B6 mice 3 to 4 days postinfection (Scalzo et al., 1990). This biphasic pattern of viral ie3 and gB mRNA expression in the spleen suggests that the initial replication cycle of MCMV was probably completed by ∼30−36 hr postinoculation (consistent with the replication cycle in cultured fibroblasts), followed by spread and additional rounds of virus replication over the next 2−4 days. The initial INFαβ response in the spleen was proportional to the pfu in the inoculum of MCMV, as revealed by the identical ratio of INFαβ mRNA to ie1 mRNA at different doses of virus (Figure S1).
The liver, also a major site of MCMV replication, showed a similar biphasic INFαβ response, although ie1 mRNA levels remained relatively constant over the first 24 hr and increased dramatically from 2 to 4 days postinfection (Figure 1B). In comparison to the spleen, the accumulation of IFNβ mRNA was ∼100-fold lower at the initial times of infection (Figure 1B). Serum levels of INFα also showed a biphasic response with maximum serum levels occurring by 8−12 hr and a second peak at 48 hr concurrent with the first round of virus spread within organs. Serum INFα was not detected during peak virus production over the 72−96 hr period (Figure 1C). BALB/c mice also displayed a biphasic INFαβ mRNA response to MCMV infection (Figure S2). These data suggest that the bulk of INFαβ transcriptional activity and serum INFαβ response to MCMV infection occur during the initial phase.
The LTαβ-LTβR System Is Required for Initial IFNαβ Response to MCMV Infection in the Spleen
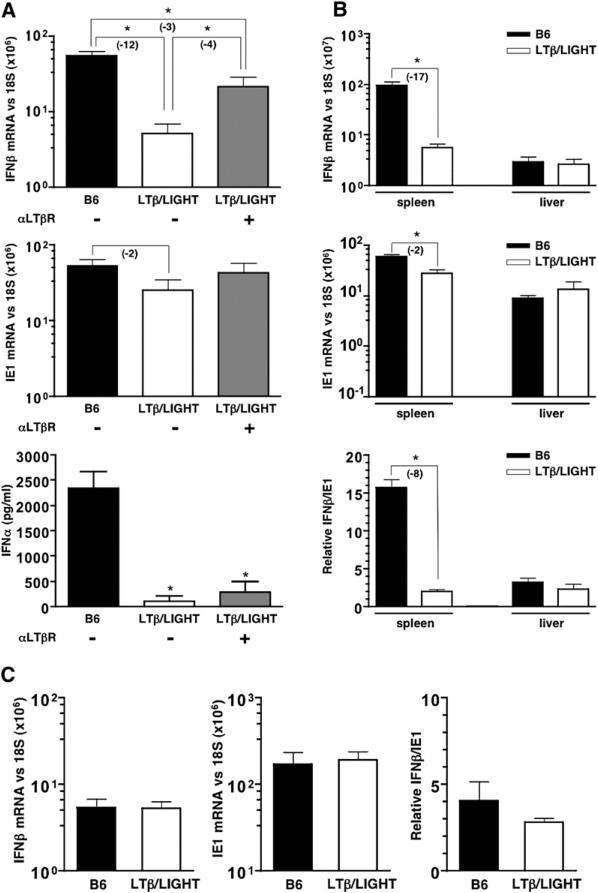
Although LTα−/− mice exhibit a defect in the induction of IFNβ mRNA in the spleen at 8 hr postinfection (Banks et al., 2005), it was not clear whether LTβR signaling was needed for both phases of the INFαβ response in B6 mice. Mice deficient in both ligands for LTβR, LTβ, and LIGHT (LTβ/LIGHT−/−) were used to attenuate physiological signaling while retaining the option to activate LTβR signaling using a specific agonist antibody. LTβ/LIGHT−/− mice exhibited reduced IFNβ mRNA levels in the spleen at 8 hr postinfection, consistent with results previously observed in LTα−/− mice (Banks et al., 2005) (Figure 2A). Notably, a minor decrease in ie1 mRNA expression at this time was also observed in LTβ/LIGHT−/− mice (∼2-fold) (Figure 2B), and an analogous decrease in ie1 mRNA levels was also seen in LTα−/− mice (Figure S3). By contrast, hepatic expression of IFNβ and ie1 mRNA in LTβ/LIGHT−/− mice was comparable to wild-type B6 mice, indicating that LTβR signaling was dispensable in the liver (Figure 2B). Moreover, splenic IFNβ and ie1 mRNA levels measured 48 hr after infection in LTβ/LIGHT−/− mice were not significantly different from B6 mice (Figure 2C). These results indicate that LTβR-dependent signaling is required for the initial INFαβ response in the spleen. Serum levels of INFα were also dramatically reduced (∼50-fold) in the LTβ/LIGHT−/−-deficient mice 8 hr postinfection (Figure 2A) even though INFαβ mRNA levels were not compromised in the liver. This result implicates the spleen as the major source of INFαβ during the initial response to MCMV infection.
Figure 2. LTβ/LIGHT−/− Mice Are Defective in Their Initial IFNαβ Response to MCMV.
Wild-type (B6) and LTβ/LIGHT−/−-deficient mice were infected with MCMV plus or minus treatment with an agonistic anti-LTβR antibody (±αLTβR) at the time of infection. Spleens were harvested 8 hr postinfection and (A) IFNβ and ie1 mRNA levels were determined by qPCR. Serum INFα levels measured by ELISA (n = 4−6 mice per group from two independent experiments, mean ± SEM) (bottom panel). (B) Spleens and livers from MCMV infected B6 and LTβ/LIGHT−/− mice were analyzed by qPCR 8 hr postinfection for IFNβ and IE1 mRNA levels (top two panels). The ratio of IFNβ to ie1 mRNA (×100) is shown (bottom panel, n = 3 mice per group, mean ± SEM). (C) Spleens from B6 and LTβ/LIGHT−/− mice were harvested 48 hr postinfection with MCMV, and IFNβ and ie1 mRNA levels were determined by qPCR (n = 3 mice per group ± SEM). Mice were injected with 2 × 105 PFU of MCMV i.p. for all experiments. Statistical significance (*p < 0.05) was determined using the Student's t test, and fold differences are shown in parentheses.
Administration of an agonistic anti-LTβR antibody to the LTβ/LIGHT−/− mice at the time of MCMV infection partially restored IFNβ mRNA and serum INFα (Figure 2A). These results indicated that the LTαβ/LIGHT-LTβR pathway regulates the initial INFαβ response to MCMV in the spleen, but not in the liver. Furthermore, the ability of an agonist anti-LTβR mAb to partially restore the splenic IFNβ response suggests that signaling through the LTβR is required during the infection to promote an optimal INFαβ response.
The Initial IFNαβ Response to MCMV Is TLR Independent
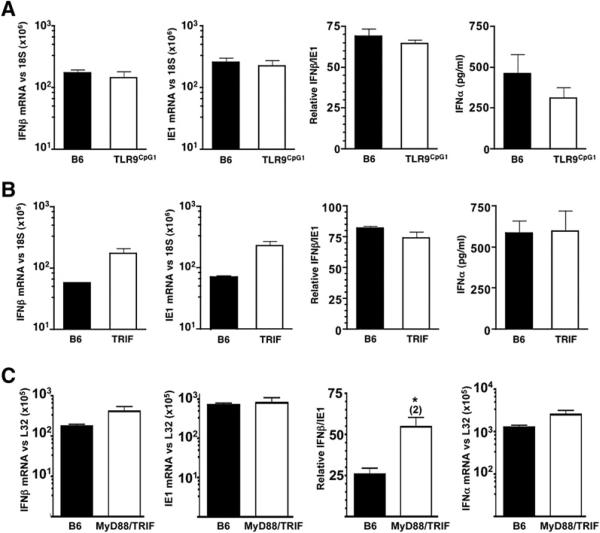
Toll-like receptor (TLR) sensing of pathogens plays a key role in innate immune defenses. Importantly, mice deficient in both TLR9 and TLR3 signaling display increased susceptibility to MCMV infection with reduced serum levels of INFαβ during the sustained phase (Krug et al., 2004; Tabeta et al., 2004). TLR9Cpg1 mice, which are unresponsive to unmethylated CpG-oligodeoxynucleotides (Tabeta et al., 2004), showed normal INFαβ mRNA accumulation in the spleen 8 hr post-MCMV infection. Additionally, TLR9Cpg1 had near normal levels of serum INFα (Figure 3A) and IFNβ (data not shown) after initial infection. MCMV infection of TRIFLps2/Lps2-deficient mice, which cannot activate the TLR3 pathway (Hoebe et al., 2003; Yamamoto et al., 2003), resulted in a slightly higher induction of IFNβ mRNA in the spleen and in serum INFα 8 hr postinfection (Figure 3B). INFα mRNA levels paralleled serum protein levels for both TLR9Cpg1- and TRIFLps2/Lps2-deficient mice (data not shown). To determine whether other TLRs might function redundantly in TLR9Cpg1 or TRIFLps2/Lps2 mice, mice deficient in both MyD88 and TRIF signaling pathways were infected (Figure 3C). These mice showed no decrease in either IFNβ or INFα mRNA levels, and ie1 levels were also normal 8 hr postinfection. Taken together, these results indicate that the LTβR-dependent, initial INFαβ response to MCMV in the spleen is independent of TLR signaling and suggests a specific LTαβ-expressing cell population promotes the initial INFαβ response.
Figure 3. The Initial Splenic IFNαβ Response to MCMV Infection is TLR Independent.
(A) TLR9CpG1, (B) TRIFLps2/Lps2, and (C) MyD88/TRIFLps2/Lps2 were infected with MCMV (2 × 105 PFU i.p.), and the INFαβ, IE1, and ratio of IFNβ/IE1 (×100) mRNA levels were determined by qPCR at 8 hr postinfection in the spleen; serum INFα levels were determined by ELISA. Statistical significance (*p < 0.05) was determined using the Student's t test, and fold differences are shown in parentheses. n = 3−6 mice per group ± SEM.
The Adaptive Immune System Promotes the Initial IFNαβ Response to MCMV
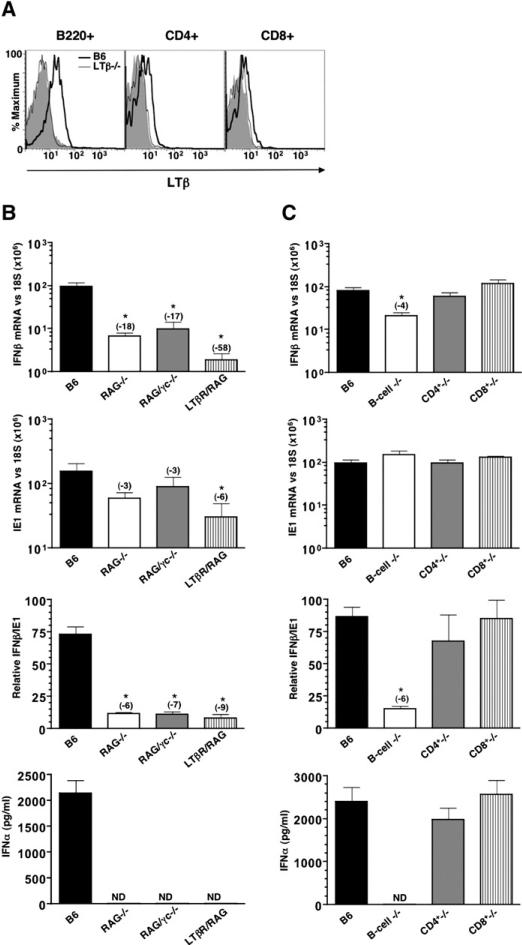
The initial INFαβ response to MCMV is rapid, suggesting that either constitutive or quickly activated LTβR signaling may be required for promoting this response. A variety of cell types including activated T cells, B-lymphocytes, and NK cells express LTβ as well as DC and lymphoid tissue inducer (LTi) cells. However, naive B cells and CD4+ T cells in the spleen constitutively express LTβ on their cell surface, as detected by flow cytometry (Figure 4A). To determine the cellular source of LTβ, we analyzed MCMV in RAG−/− (which lack T- and B cells) and RAG/γc−/− mice (which, in addition, lack NK and LTi cells, due to the absence of the common γ chain of the IL-2/4/7/9/15/21 receptors). Additionally, splenic DC subpopulations in RAG−/− mice are normal, while DC in RAG/γc−/− mice are skewed, with less CD4+ DC (C.D.T., unpublished data). However, both RAG−/− and RAG/γc−/− mice showed a substantial reduction in the accumulation of IFNβ mRNA compared to wild-type mice (Figure 4B). Splenic ie1 mRNA levels in both RAG−/− and RAG/γc−/− mice at eight hr postinfection trended lower but were not statistically different from wild-type (Figure 4B). However, normalization of IFNβ to ie1 mRNA revealed a defective initial IFNβ mRNA response (∼6-fold) in RAG−/− and RAG/γc−/− mice, very similar in magnitude to LTβ/LIGHT−/− mice. Mice deficient in both RAG and LTβR were severely compromised in their initial INFαβ response (>50-fold), which was commensurate with a substantial reduction in ie1 mRNA levels (∼6-fold). However, the IFNβ/ie1 mRNA ratio revealed a comparable, viral “dose-dependent” INFαβ defect to that seen in both RAG−/− and LTβ/LIGHT−/− mice (Figure 4B). Serum INFα levels in RAG−/−, RAG/γc−/−, and RAG/LTβR−/− mice during the initial response to MCMV were undetectable by the ELISA (Figure 4B).
Figure 4. The Initial Splenic IFNαβ Expression during MCMV Infection Is Dependent upon B-Lymphocytes.
(A) Flow cytometric analysis of LTβ expression by lymphocytes from B6 and LTβ−/− spleens. Anti-CD4, CD8, and B220 mAb were used to identify B and T lymphocyte subsets. Filled histograms represent staining with isotype control mAb in B6 mice (B) B6, RAG−/− (lack B and T cells), RAG/γc−/− (deficient in T, B, and NK cells), and LTβR/RAG double-deficient mice and (C) B6-, B cell (B−/−)-, CD4+ T cell (CD4+−/−)-, and CD8+ T cell (CD8+−/−)-deficient mice were infected with MCMV (2 × 105 PFU i.p.). Spleens were harvested 8 hr postinfection and IFNβ and ie1 mRNA levels were determined by qPCR and normalized to the levels of 18S rRNA (top two panels of both [B] and [C]). IFNβ/ie1 mRNA ratio (×100) was calculated and serum INFα levels were determined by ELISA; ND, not detectable) (two lower panels). Statistical significance (*p < 0.05) was determined using the Student's t test, and fold differences are shown in parentheses. n = 4−6 mice per group ± SEM.
B Cells Expressing LTβ Are Required for the Initial IFNαβ Response to MCMV
The results observed in RAG−/− mice raised the possibility that naive T and/or B cells provide the stimulus for the initial INFαβ response to MCMV infection, either directly or indirectly. As both T and B lymphocytes lack expression of LTβR, it is likely that lymphocytes deliver the ligand to LTβR-expressing cells. To delineate which of these lymphocyte populations were the responsible subset, mice genetically deficient in either B cells (Igh-6−/−), CD4+ (Cd4−/−), or CD8+ T lymphocytes (β2μ−/−) were infected with MCMV. B cell-deficient mice showed a 4-fold reduction in IFNβ mRNA at 8 hr post-MCMV infection and a drastic reduction in serum INFα (Figure 4C). In contrast, neither CD4 T cell nor CD8 T cell-deficient mice showed a reduction in splenic IFNβ mRNA or serum INFα (Figure 4C). The expression level of ie1 mRNA was similar in all groups tested.
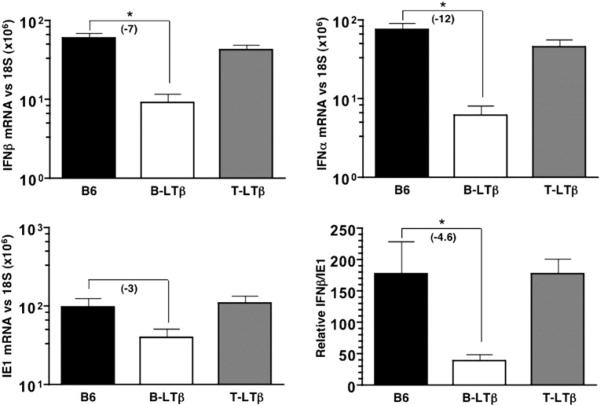
To directly test whether the lack of LTβ expression by B cells was responsible for the compromised initial INFαβ response to MCMV, mice conditionally deficient in ltβ in B- or (B-LTβ) or T cells (T-LTβ) were utilized (Tumanov et al., 2003). Strikingly, B-LTβ mice, but not T-LTβ mice, displayed a dramatic reduction in IFNβ (7-fold) and INFα mRNA (12-fold) (Figure 5). B-LTβ-deficient mice also showed ∼3-fold reduction in ie1 mRNA in the spleen that when normalized to ie1 mRNA yielded an ∼5-fold decrease in INFαβ mRNA, a result virtually identical to that seen in mice lacking the entire B lymphocyte compartment or deficient in LTβ/LIGHT. Taken together, this result highlights the critical importance of LTβ expressed in B cells to promote the initial INFαβ response to MCMV infection.
Figure 5. LTβ Expressed by B Cells Is Required for the Initial IFNαβ Response to MCMV.
B6, B-LTβ, and T-LTβ (mice lacking LTβ specifically in B and T cells, respectively) were infected with MCMV (2 × 105 PFU i.p.). Spleens were harvested 8 hr postinfection and the IFNβ, INFα, and ie1 mRNA levels were detected by qPCR and normalized to 18S rRNA. The ratio of IFNβ/ie1 mRNA (×100) is shown. Serum INFα levels were determined by ELISA. Statistical significance (*p < 0.05) was determined using the Student's t test, and fold differences are shown in parentheses. n = 4−6 mice per group; average of two independent experiments ± SEM.
LTβR-Expressing Stromal Cells Produce the Initial IFNαβ during MCMV Infection via a NIK-Dependent Pathway
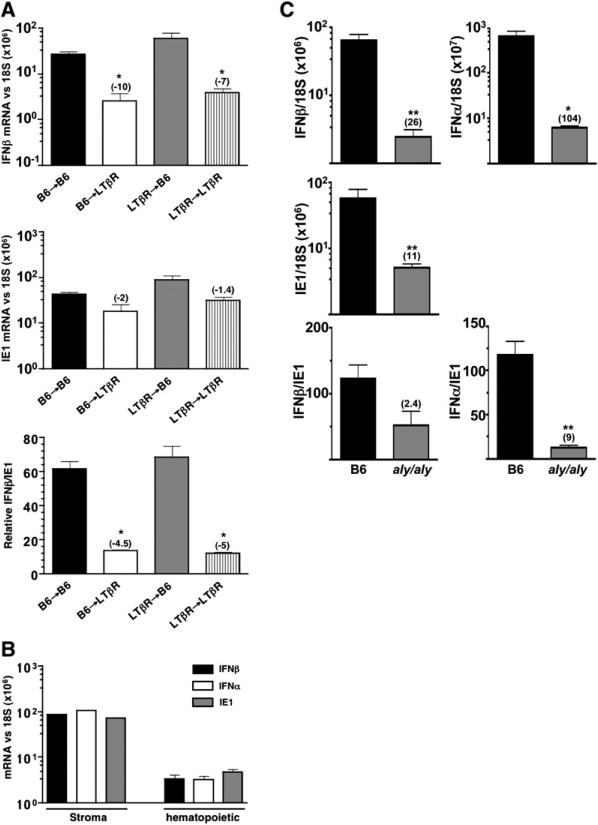
The identification of B-lymphocytes as the critical, LTβ-expressing cells promoting the initial INFαβ response to MCMV in the spleen was surprising given that naive B cells are not infected by MCMV (Banks et al., 2005) and lack expression of the LTβR (Force et al., 1996). To determine whether the hematopoietic or radio-resistant stromal compartments require expression of the LTβR, bone marrow chimeric mice were generated between LTβR−/− or wild-type mice. LTβR−/− recipients reconstituted with wild-type B6 bone marrow (B6→LTβR) exhibited reduced IFNβ mRNA accumulation (∼10-fold) when compared to wild-type controls (B6→B6) at 8 hr postinfection (Figure 6A). This IFNβ deficiency was comparable to that seen in mice defective for LTβR expression in both cellular compartments (LTβR→LTβR). In contrast, wild-type mice that received LTβR-deficient bone marrow (LTβR→B6) produced normal levels of IFNβ mRNA. Thus, stromal cells require expression of LTβR to mount the initial INFαβ response to MCMV (Figure 6A).
Figure 6. LTβR-Expressing Splenic Stromal Cells Promote IFNαβ Production during MCMV Infection via a NIK-Dependent Pathway.
(A) Various bone marrow chimeric mice (e.g., irradiated wild-type mice receiving LTβR−/− bone marrow = LTβR→B6) were infected with MCMV for 8 hr (1 × 105 PFU i.v.). Splenic IFNβ (top panel) and ie1 (middle panel) mRNA expression was determined by qPCR and normalized to 18S rRNA levels. The ratio of IFNβ/IE1 mRNA (×100) is shown in the bottom panel. Statistical significance (*p < 0.05) was determined using the Student's t test comparing each experimental group with the B6→B6 control mice, and the fold difference is shown in parentheses. n = 3−5 mice per group ± SEM.
(B) Stromal cells from MCMV (2 × 105 PFU i.v.) infected spleens (pooled from 5 infected mice) were separated from hematopoietic cells (see Experimental Procedures). Total cell RNA was then isolated from either the stromal or hematopoietic cell fractions, and qPCR was performed to determine IFNβ, INFα, and ie1 mRNA levels. (C) aly/aly mice or wild-type littermate controls (n = 4) were infected with MCMV, and spleens were analyzed by qPCR for INFαβ and ie1 mRNA levels at 8 hr postinfection. Infection conditions and data analysis is identical to that in (A).
Separation of the spleen into stroma and hematopoietic fractions revealed that IFNβ and INFα mRNA were 25- to 30-fold enriched in the stromal cell fraction from mice infected for 8 hr with MCMV (Figure 6B). Moreover, viral ie1 mRNA expression also preferentially localized to the stromal compartment (∼15-fold enriched) consistent with previous immunohistochemical (Benedict et al., 2006) and electron microscopy studies (Mercer et al., 1988) that stromal cells are the major site of MCMV infection in the spleen. Together, these results indicate that radioresistant stromal cells expressing the LTβR are both the target of initial infection and the major producer of INFαβ in response to MCMV.
LTβR activation of the NF-κB pathway requires the activity of the NF-κB-inducing kinase (NIK) (Dejardin et al., 2002; Basak et al., 2007), and LTβR-mediated, canonical NF-κB signaling is also dependent upon NIK (Basak et al., 2007). To test whether NIK is required for the initial INFαβ response in the splenic stroma, aly/aly mice (functional mutation in NIK) (Xiao et al., 2001) were infected with MCMV. Strikingly, aly/aly mice showed a reduction in both IFNβ and INFα mRNA at 8 hr postinfection (∼26- and 104-fold, respectively), with a commensurate reduction in ie1 mRNA (∼11-fold).
Discussion
The type I Interferon response is a key contributor to successful host defense against viral pathogens, such as MCMV (Banks et al., 2005; Dalod et al., 2002; Salazar-Mather et al., 2002). Here, we define the cellular interactions required to initiate the earliest innate INFαβ response to a β-herpesvirus. In response to MCMV, the LTαβ-LTβR pathway is required for the induction of INFαβ in the spleen, which constitutes a major source of initial INFαβ production. Our results reveal that B-lymphocytes provide the source of LTβ required to promote the initial innate INFαβ response in LTβR-expressing stromal cells, and it is cells in the stromal compartment that are both infected and producers of INFαβ during MCMV infection. Induction of initial INFαβ mRNA and protein regulated by the LTβR pathway is independent of TLR-signaling pathways, indicating that a significant difference exists in the molecular mechanisms promoting the initial and sustained phases of the INFαβ response to MCMV infection. Together, the results reveal a frontline innate host defense strategy directed by B cells of the adaptive immune system communicating with stromal cells via the LTαβ-LTβR system.
The biphasic pattern of INFαβ induction in the spleen and liver reflects the initial host response to the primary inoculum, with the subsequent sustained phase resulting from the secondary response to viral spread and continued cycles of replication at later times. The initial INFαβ mRNA response to MCMV peaked at 8 hr, paralleled by a rise in serum INFα, rapidly declined and was followed by sustained expression for the next few days. The accumulated peak of IFNβ mRNA levels indicated the bulk of the INFαβ transcriptional activity occurred during the initial phase of the infection, and the magnitude of the response in the spleen was substantially higher than in the liver. In addition, the level of INFα in the serum, reflecting accumulation from all tissue sources, was dramatically reduced in the LTβ/LIGHT−/−-deficient mice, despite an uncompromised INFαβ mRNA response in the liver. This evidence, together with the relative abundance of INFαβ mRNA in the spleen, supports the idea that the LTβR pathway regulates the major source of type I IFN during MCMV infection.
Our results and those of others indicate that TLR-dependent defense pathways control INFαβ expression in response to MCMV during the later, sustained phase. This conclusion is based on the uncompromised INFαβ response to MCMV during the initial phase in TLR9CpG, TrifLps2/Lps2, and MyD88/TRIFLps2/Lps2 mice. The complexity of the sustained phase of the INFαβ response is apparent from studies showing TLR, MyD88, and plasmacytoid DC are important at 36 hr post-MCMV infection (Andoniou et al., 2005; Delale et al., 2005; Krug et al., 2004; Tabeta et al., 2004), but additional results indicate their influence may decline at 44 hr postinfection (Andoniou et al., 2005; Delale et al., 2005; Krug et al., 2004). These data further support the idea that the initial INFαβ response regulated by the LTβR pathway is distinct from the TLR-dependent pathways functioning in plasmacytoid DC. These results indicate the LTαβ-IFN axis is an independent mechanism from conventional paradigms in which myeloid lineage cells largely promote innate defenses to pathogens. Additionally, that LTβ/LIGHT−/− mice fail to control acute MCMV replication in both the spleen and liver at 72 hr postinfection (Banks et al., 2005) indicates mice lacking LTβR-signaling are compromised in additional aspects of innate defenses to MCMV infection.
The conditional deletion of ltβ in B cells, as well as mice lacking B lymphocytes (RAG−/−, Igh-6tm1Cgn), demonstrated B cell expression of LTβ is the key factor for promoting the initial INFαβ response in the spleen. B cell-expressed LTβ is required for maturation of the normal splenic architecture (Tumanov et al., 2002, 2003) although B-LTβ mice develop a normal complement of lymph nodes and Peyer's patches, which depends on expression of LTβ in lymphoid tissue inducer cells. However, the splenic architecture (marginal zone, segregation of T and B cells) in B-LTβ mice is not as severely disrupted as in mice harboring the null genotype, but the IFNβ/ie1 mRNA ratio in the spleens of B-LTβ and LTβ/LIGHT−/− mice were similar in response to MCMV infection. Additionally, although dendritic cell subpopulations are altered in the spleen LTβR→B6 bone marrow chimeric mice (Kabashima et al., 2005; De Trez et al., 2008), the initial INFαβ response to MCMV was normal in these mice. Finally, although LTβ/LIGHT−/− mice lack both marginal zone and marginal metallophillic macrophage populations, they mount a normal INFαβ response to MCMV at 48 hr, and our studies in LTβR→B6 mice indicate LTβR expression by these macrophages is not required for the initial response. Together, these results indicate that alterations in myeloid cell subsets or the lymphatic vasculature are not likely to account for the reduced initial INFαβ production in LTβ/LIGHT−/− mice. Moreover, the partial restoration of the IFNβ mRNA response in LTβ/LIGHT−/− mice treated with an agonist anti-LTβR indicates that LTβR signaling contributes during the virus infection to induce IFNβ. The limited effect of acutely delivered anti-LTβR suggests that differentiative effects on stromal cells may be necessary (Drayton et al., 2004) to fully restore the INFαβ response. The agonist LTβR antibody may also enhance the regulation of cellular trafficking across the high endothelial venules, which is regulated by LTβR signaling, perhaps increasing the initial dissemination of MCMV to the spleen from the peritoneal cavity (Browning et al., 2005; Drayton et al., 2003). Strikingly, the bone marrow chimeric mice indicated that LTβ-expressing B cells exclusively communicate with, or regulate the differentiation of, LTβR expressing stromal cells, which produce the majority (>95%) of the INFαβ and IE1 mRNA following MCMV infection. Along these lines, a role for constitutively expressed LTβ by B cells in regulating the microarchitecture of the spleen was recently shown to play an important role in containing VSV and LCMV infection (Junt et al., 2006). LTαβ is exclusively positioned in the membrane indicating the conversation between B cells and stromal cells occurs through direct cell contact. The radioresistant stromal compartment includes many distinct cell types that could be infected, including endothelial cells, myocytes, and fibroblasts in addition to specialized parenchymal cells identified by specific chemokine expression, such as CXCL13 (Cyster, 2003). The exact lineage of stromal cells infected by MCMV in the spleen is currently unknown.
The importance of this B cell-stromal cell interaction is evident in LT deficient mice, which are highly susceptible to infection with MCMV (for review see Ware, 2005). Increased morbidity occurs in LTα-, LTβ-, or LTβR-deficient mice or wild-type mice treated with LTβR-Fc decoy in response to MCMV (Banks et al., 2005; Benedict et al., 2001). Mice deficient in INFαβ receptor phenocopy LT-deficient mice, which undergo massive apoptosis of T and B lymphocytes during infection that are not directly infected by the virus (Banks et al., 2005). LTβR expression was required in both the hematopoietic and stromal compartments of the spleen to completely protect against this apoptosis induced by MCMV, implicating crosstalk occurs between the myeloid and stromal compartments to induce INFαβ and promote survival of lymphocytes (Banks et al., 2005). Our results in aly/aly mice strongly indicate this LT-dependent crosstalk requires NIK, likely involving activation of NF-κB, a known key player in the regulation of IFNβ transcription (Thanos and Maniatis, 1995). Together, these results indicate that the initial B cell directed INFαβ response contributes to viral control, and ultimately helps to promote the survival of the adaptive immune response.
B lymphocytes play a critical role in the post natal maturation and homeostasis of the splenic microarchitecture, a structural feature designed to promote highly efficient immune responses (Cupedo et al., 2004). LTαβ-LTβR signaling pathway is required to form the marginal zone, wherein antigen-capturing macrophages, B cells and dendritic cells reside (Kraal, 1992) and regulate expression of the lymphoid tissue chemokines (CCL21, CXCL13) in specialized stromal cells creating discrete micro-niches that compartmentalize T cells to the periarteriole region surrounded by the B cell follicles (Cyster, 2003). MCMV targets the splenic microarchitecture by specifically suppressing expression of CCL21 mRNA, evident two to three days after infection (Benedict et al., 2006). The specific loss of CCL21 leaves T cells without sufficient cues to localize around the arteriole. However, MCMV did not significantly alter the B cell follicle chemokine CXCL13, unlike infection with lymphocytic choriomeningitis virus, which reduces expression of both these splenic chemokines (Mueller et al., 2007) suggesting the LT-IFN pathway might protect the B cell micro-niche during MCMV infection.
The commitment of genomic information encoding resistance to MCMV (estimated at 1%–3% of the host genome) suggests herpesviruses provide powerful selective pressures for diversification of host resistance mechanisms (Beutler et al., 2005). Likewise, the accumulation of numerous immune modulating mechanisms in the viral genome, many derived from “captured” cellular genes, indicates a long coevolutionary history. That two distinct human herpesviruses, HSV-1 and HCMV, target receptors (HVEM and BTLA) involved in LT-dependent signaling, underscores this coevolutionary relationship (Cheung et al., 2005). Indeed, resistance to other pathogens is provided to the host as a consequence of infection with either γ- and β-herpesviruses, suggesting a strong symbiotic relationship has emerged from the host-virus interplay (Barton et al., 2007). In the context of evolution, the LTαβ-LTβR pathway, functioning as a developmental and homeostatic regulator, may have evolved as an adaptive countermeasure to promote an early IFN response in the cells initially infected by the virus, perhaps in response to the functional paralysis of macrophages and DC by MCMV (Andrews et al., 2001; Popkin et al., 2003). The discovery of this B cell-dependent pathway also suggests an unrecognized route to INFαβ production that may operate in autoimmune diseases, such as lupus erythematosus, where both infection and genetic factors contribute to pathogenesis (Baccala et al., 2007).
EXPERIMENTAL PROCEDURES
Mice
Wild-type C57BL/6 (B6), B6 CD45.1, BALB/c, 129, C57BL/10, B6.129S2-Igh-6tm1Cgn/J (B−/−), B6.129S2-Cd4tm1Mak/J (CD4−/−), and B6.129P2-B2mtm1Unc/J (CD8−/−) were purchased from the Jackson Laboratory, and RAG2−/− RAG2/(γc)−/−(C57BL/6J × C57BL/10SgSnAi)-[KO]γc-[KO]RAG2) double-deficient mice were from Taconic. LTβ/LIGHT (Scheu et al., 2002) and LTβR−/−(Futterer et al., 1998) were backcrossed five generations (n = 5) to B6 mice. LTα−/− mice were generated as described (De Togni et al., 1994) and were obtained from Jackson Labs. LTβR−/− mice were crossed onto RAG1−/− (C57BL/6) mice at LIAI. The conditional LTβ-deficient mice were derived by breeding LTβ “floxed” mice with CD19-Cre knockin mice for the B cell-specific deletion, and CD4-Cre transgenic mice were used for T cell-specific deletion (Tumanov et al., 2003). TLR9CpG1 mice and TrifLps2/Lps2 mice generated on the C57BL/6 background by ENU mutagenesis were obtained from Dr. Bruce Beutler (Hoebe et al., 2003; Tabeta et al., 2004). MyD88/TRIFLps2/Lps2 doubly deficient mice were obtained from Dr. Sujan Shresta, (constructed by Dr. S. Akira and Dr. B. Beutler). All mice were used at 6−12 weeks and were age and sex matched. Mice were treated with the agonist rat anti-mouse LTβR at 100 μg injected i.p. at the time of infection.
For bone marrow transplantation, recipient mice were lethally irradiated (1000 rad) and reconstituted with total donor bone marrow cells (107 cells/recipient mouse) by injection into the retro-orbital sinus of the recipients; recipient mice were given antibiotics in the drinking water for 3 weeks. The chimeric mice were analyzed 7 weeks post transfer; recipient mice were analyzed with CD45 allelic markers (LTβR−/− CD45.2 or B6 CD45.1) to verify reconstitution.
Virus
Mouse CMV (Smith strain) was prepared from salivary glands from infected BALB/c mice as described previously (Benedict et al., 2001). Preparations were derived from a virus stock acquired from the ATCC (VR-1399) that was propagated twice through mouse salivary glands for amplification/generation of a “seed stock.” This seed stock was then used to infect mice to generate viral stocks for use in all experiments. In addition, all stocks were centrifuged through a 20% sorbitol cushion and resuspended in PBS. The infectious virus in the preparation was quantified by plaque assay on NIH 3T3 cells (Reddehase et al., 1985). All breeding and experimental protocols were performed under the approval by LIAI Animal Care Committee.
Quantitative PCR
Gene expression was assayed by quantitative RT-PCR (Stratagene Mx4000), as described (Benedict et al., 2001). Total cellular RNA was isolated from snap frozen tissues using the TRIzol reagent and purified with RNeasy Mini RNA purification kit (QIAGEN, Chatsworth, CA). RNA was treated with DNaseI and 2 μg were used for the RT reaction. The primers for detection of murine IFNβ, INFα and the MCMV immediate early genes IE1, IE3 and late gene gB were: IFNβ, 5′-CTGGAGCAGCTGAATGGAAAG-3′ and 5′-CTCCGTCATCTCCATAGGGATCT-3′; INFα, 5′-TGCAATGACCTCCATCAGCA-3′ and 5′-TTCCTGGGTCAGAGGAGGTTC-3′; IE1, 5′-AGCTGTTGGTGGTGTCACTCAA-3′ and 5′-GGCTGGGACTCATCTTCTTCAG-3′ IE3 5′-AGCTGTTGGTGGTGTCACTCAA-3′ and GGCTGGGACTCATCTTCTTCAG-3′, gB, 5′-GGGCGAGAACAACGAGATCA-3′ and 5′-TCTTCCTGCTGTTCGTGTCG-3′. All mRNA levels were normalized to 18S rRNA in individual samples, except in one case where L32 was used. INFα primers were designed to hybridize to a region of the INFα mRNA that is 100% conserved in 13/14 murine INFα subtypes. Real-time PCR data was analyzed using the Relative Expression Software Tool (REST) for groupwise comparison and statistical analysis of relative gene expression (Pfaffl et al., 2002) and with Prism Software (Student's pairwise t test).
Interferon ELISA
INFα and IFNβ serum levels were quantified using ELISA that detects mouse IFN-αA, α1, α4, α5, α6, and α9. Briefly, plates coated with a rat anti-mouse INFα mAb (RMMA-1, PBL Biomedical Laboratories) were incubated with serum samples, followed by rabbit anti-mouse INFα polyclonal Ab (PBL Biomedical Laboratories), and horseradish peroxidase-conjugated goat anti-rabbit IgG for detection (Jackson ImmunoReaserch Laboratories) with the substrate TMB (eBioscience). Recombinant INFα (PBL Biomedical Laboratories) was used to generate a standard curve (12.5−500pg/ml). Colorimetric changes of enzyme substrates were detected at 450 nm (SpectraMax, Molecular Devices) and data analyzed with the SOFTmax Pro software.
Separation of Splenic Stroma and Hematopoietic Cells
Spleens from mice infected for 8 hours with 2 × 105 PFU of MCMV were separated into a stroma and hematopoietic fractions, RNA extracted for Real Time PCR analysis as previously described (Benedict et al., 2006). Briefly, spleens were perfused with collagenase (100 U/ml) in Hanks’ Balanced Salt Solution containing Ca2+ and Mg2 for 30 min at 37°C. Treated spleens from five mice were then pooled and extruded through a 70 μm nylon filter strainer in order to obtain sufficient amounts of stromal cell RNA. Cells extruded through the filter (hematopoietic fraction) and cells retained on filter (stromal fraction) were washed with PBS followed by lysis with Trizol. The filter-bound stromal fraction from uninfected mice contained a maximum of 15%–30% contaminating hematopoietic cells, and 4%–7% contaminating stromal cells in the hematopoietic fraction based on marker genes expressed exclusively in the hematopoietic (CD80, IL-7Rα, LTα, and LTβ) and stromal (gp38 and IL-7) compartments.
Flow Cytometry
Single cell suspensions of splenic leukocytes were preincubated with anti-FcγRII/III blocking reagent (2.4G2; BD PharMingen). Cell surface LTβ was detected with monoclonal antibody BB.F6.F6.BF2 (BD PharMingen) followed by biotinylated goat anti-hamster IgG and Streptavidin APC. For the lymphocyte subset detection the following antibodies from BD were used: PE 145−2C11 (anti-CD3), FITC RM4-4 (anti-CD4), FITC 53−6.7 (anti-CD8), and PE RA3−6B2 (anti-B220). Stained cells were analyzed on a FACSCalibur flow cytometer using CellQuest research software (BD Biosciences) and FlowJo Software.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to very much thank Sujan Shresta, Bruce Beutler, Shizuo Akira and Kasper Hoebe for mutant mice, Portia Zamos, Hideki Sanjo, Michelle Her and Lisa Hohmann for technical assistance and Jurg Tschopp, Alex Hoffmann, Claire Jacquin, and Susan Sweeny for helpful discussions. This investigation was supported in part by grants from the Public Health Service, National Institutes of Health AI48073, AI33068, CA69381 and AI057840 (C.F.W.) and AI061549 (C.A.B.). K.S. and A.L. were in part funded by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft (DFG). S.A.N. is International Research Scholar of the Howard Hughes Medical Institute. This is manuscript no. 888 from LIAI.
Footnotes
The Supplemental Data include three supplemental figures and can be found with this article online at http://www.cellhostandmicrobe.com/cgi/content/full/3/2/67/DC1/.
REFERENCES
- Andoniou CE, van Dommelen SL, Voigt V, Andrews DM, Brizard G, Asselin-Paturel C, Delale T, Stacey KJ, Trinchieri G, Degli-Esposti MA. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat. Immunol. 2005;6:1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- Andrews DM, Andoniou CE, Granucci F, Ricciardi-Castagnoli P, Degli-Esposti MA. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2001;2:1077–1084. doi: 10.1038/ni724. [DOI] [PubMed] [Google Scholar]
- Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat. Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- Banks TA, Rickert S, Benedict CA, Ma L, Ko M, Meier J, Ha W, Schneider K, Granger SW, Turovskaya O, et al. A lymphotoxin-IFN-beta axis essential for lymphocyte survival revealed during cytomegalovirus infection. J. Immunol. 2005;174:7217–7225. doi: 10.4049/jimmunol.174.11.7217. [DOI] [PubMed] [Google Scholar]
- Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW., IV Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- Basak S, Kim H, Kearns JD, Tergaonkar V, O'Dea E, Werner SL, Benedict CA, Ware CF, Ghosh G, Verma IM, et al. A fourth IkappaB protein within the NF-kappaB signaling module. Cell. 2007;128:369–381. doi: 10.1016/j.cell.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict CA, Banks TA, Senderowicz L, Ko M, Britt WJ, Angulo A, Ghazal P, Ware CF. Lymphotoxins and cytomegalovirus cooperatively induce interferon-beta, establishing host-virus detente. Immunity. 2001;15:617–626. doi: 10.1016/s1074-7613(01)00222-9. [DOI] [PubMed] [Google Scholar]
- Benedict CA, De Trez C, Schneider K, Ha S, Patterson G, Ware CF. Specific Remodeling of Splenic Architecture by Cytomegalovirus. PLoS Pathog. 2006;2:e16. doi: 10.1371/journal.ppat.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict CA, Ware CF. Poxviruses aren't stuPYD. Immunity. 2005a;23:553–555. doi: 10.1016/j.immuni.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Benedict CA, Ware CF. RIGing a virus trap. Nat. Med. 2005b;11:929–930. doi: 10.1038/nm0905-929. [DOI] [PubMed] [Google Scholar]
- Beutler B, Georgel P, Rutschmann S, Jiang Z, Croker B, Crozat K. Genetic analysis of innate resistance to mouse cytomegalovirus (MCMV). Brief. Funct. Genomics Proteomics. 2005;4:203–213. doi: 10.1093/bfgp/4.3.203. [DOI] [PubMed] [Google Scholar]
- Browning JL, Allaire N, Ngam-Ek A, Notidis E, Hunt J, Perrin S, Fava RA. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity. 2005;23:539–550. doi: 10.1016/j.immuni.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Cheung TC, Humphreys IR, Potter KG, Norris PS, Shumway HM, Tran BR, Patterson G, Jean-Jacques R, Yoon M, Spear PG, et al. Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway. Proc. Natl. Acad. Sci. USA. 2005;102:13218–13223. doi: 10.1073/pnas.0506172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupedo T, Lund FE, Ngo VN, Randall TD, Jansen W, Greuter MJ, de Waal-Malefyt R, Kraal G, Cyster JG, Mebius RE. Initiation of cellular organization in lymph nodes is regulated by non-B cell-derived signals and is not dependent on CXC chemokine ligand 13. J. Immunol. 2004;173:4889–4896. doi: 10.4049/jimmunol.173.8.4889. [DOI] [PubMed] [Google Scholar]
- Cyster JG. Lymphoid organ development and cell migration. Immunol. Rev. 2003;195:5–14. doi: 10.1034/j.1600-065x.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- Dalod M, Hamilton T, Salomon R, Salazar-Mather TP, Henry SC, Hamilton JD, Biron CA. Dendritic cell responses to early murine cytomegalovirus infection: Subset functional specialization and differential regulation by interferon alpha/beta. J. Exp. Med. 2003;197:885–898. doi: 10.1084/jem.20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA. Interferon alpha/beta and interleukin 12 responses to viral infections: Pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–706. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- De Trez C, Schneider K, Potter K, Droin N, Fulton J, Norris PS, Ha SW, Fu YX, Murphy T, et al. The inhibitory HVEM-BTLA pathway counter regulates LTβ-receptor signaling to achieve homeostasis of dendritic cells. J. Immunol. 2008;180:238–248. doi: 10.4049/jimmunol.180.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The Lymphotoxin-beta Receptor Induces Different Patterns of Gene Expression via Two NF-kappaB Pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- Delale T, Paquin A, Asselin-Paturel C, Dalod M, Brizard G, Bates EE, Kastner P, Chan S, Akira S, Vicari A, et al. MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo. J. Immunol. 2005;175:6723–6732. doi: 10.4049/jimmunol.175.10.6723. [DOI] [PubMed] [Google Scholar]
- Drayton DL, Bonizzi G, Ying X, Liao S, Karin M, Ruddle NH. I kappa B kinase complex alpha kinase activity controls chemokine and high endothelial venule gene expression in lymph nodes and nasal-associated lymphoid tissue. J. Immunol. 2004;173:6161–6168. doi: 10.4049/jimmunol.173.10.6161. [DOI] [PubMed] [Google Scholar]
- Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J. Exp. Med. 2003;197:1153–1163. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S, Holscher C, Scheu S, Tertilt C, Hehlgans T, Suwinski J, Endres R, Pfeffer K. The lymphotoxin beta receptor is critically involved in controlling infections with the intracellular pathogens Mycobacterium tuberculosis and Listeria monocytogenes. J. Immunol. 2003;170:5210–5218. doi: 10.4049/jimmunol.170.10.5210. [DOI] [PubMed] [Google Scholar]
- Force WR, Walter BN, Hession C, Tizard R, Kozak CA, Browning JL, Ware CF. Mouse lymphotoxin-beta receptor. Molecular genetics, ligand binding, and expression. J. Immunol. 1996;155:5280–5288. [PubMed] [Google Scholar]
- Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat. Immunol. 2004;5:971–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- Junt T, Tumanov AV, Harris N, Heikenwalder M, Zeller N, Kuprash DV, Aguzzi A, Ludewig B, Nedospasov SA, Zinkernagel RM. Expression of lymphotoxin beta governs immunity at two distinct levels. Eur. J. Immunol. 2006;36:2061–2075. doi: 10.1002/eji.200626255. [DOI] [PubMed] [Google Scholar]
- Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG. Intrinsic Lymphotoxin-beta Receptor Requirement for Homeostasis of Lymphoid Tissue Dendritic Cells. Immunity. 2005;22:439–450. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Kraal G. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Liu YJ. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Mercer JA, Wiley CA, Spector DH. Pathogenesis of murine cytomegalovirus infection: Identification of infected cells in the spleen during acute and latent infections. J. Virol. 1988;62:987–997. doi: 10.1128/jvi.62.3.987-997.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Tschopp J. Toll-like receptors and RNA helicases: Two parallel ways to trigger antiviral responses. Mol. Cell. 2006;22:561–569. doi: 10.1016/j.molcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Mocarski ES, Courcelle CT. Cytomegaloviruses and their replication. Lippincott Williams and Wilkins; Philadelphia, PA: 2001. pp. 2629–2673. [Google Scholar]
- Mocarski ES., Jr. Immune escape and exploitation strategies of cytomegaloviruses: Impact on and imitation of the major histocompatibility system. Cell. Microbiol. 2004;6:707–717. doi: 10.1111/j.1462-5822.2004.00425.x. [DOI] [PubMed] [Google Scholar]
- Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, Ahmed R, Matloubian M. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317:670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- Orange JS, Biron CA. Characterization of early IL-12, IFN-alpha beta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J. Immunol. 1996;156:4746–4756. [PubMed] [Google Scholar]
- Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: All roads lead to type I interferons. Curr. Opin. Immunol. 2006;18:676–682. doi: 10.1016/j.coi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock JL, Presti RM, Paetzold S, Virgin HW. Latent murine cytomegalovirus infection in macrophages. Virology. 1997;227:168–179. doi: 10.1006/viro.1996.8303. [DOI] [PubMed] [Google Scholar]
- Popkin DL, Watson MA, Karaskov E, Dunn GP, Bremner R, Virgin HW., IV Murine cytomegalovirus paralyzes macrophages by blocking IFN gamma-induced promoter assembly. Proc. Natl. Acad. Sci. USA. 2003;100:14309–14314. doi: 10.1073/pnas.1835673100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase MJ. Antigens and immunoevasins: Opponents in cytomegalovirus immune surveillance. Nat. Rev. Immunol. 2002;2:831–844. doi: 10.1038/nri932. [DOI] [PubMed] [Google Scholar]
- Reddehase MJ, Weiland F, Munch K, Jonjic S, Luske A, Koszinowski UH. Interstitial murine cytomegalovirus pneumonia after irradiation: Characterization of cells that limit viral replication during established infection of the lungs. J. Virol. 1985;55:264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Mather TP, Lewis CA, Biron CA. Type I interferons regulate inflammatory cell trafficking and macrophage inflammatory protein 1alpha delivery to the liver. J. Clin. Invest. 2002;110:321–330. doi: 10.1172/JCI15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo AA, Fitzgerald NA, Simmons A, La Vista AB, Shellam GR. Cmv-1, a genetic locus that controls murine cytomegalovirus replication in the spleen. J. Exp. Med. 1990;171:1469–1483. doi: 10.1084/jem.171.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K. Targeted Disruption of LIGHT Causes Defects in Costimulatory T Cell Activation and Reveals Cooperation with Lymphotoxin beta in Mesenteric Lymph Node Genesis. J. Exp. Med. 2002;195:1613–1624. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K, Potter KG, Ware CF. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol. Rev. 2004;202:49–66. doi: 10.1111/j.0105-2896.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos D, Maniatis T. Virus induction of human IFN beta gene expression requires the assembly of an enhancesome. Cell. 1995;83:1091–1100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- Tumanov A, Kuprash D, Lagarkova M, Grivennikov S, Abe K, Shakhov A, Drutskaya L, Stewart C, Chervonsky A, Nedospasov S. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 2002;17:239–250. doi: 10.1016/s1074-7613(02)00397-7. [DOI] [PubMed] [Google Scholar]
- Tumanov AV, Grivennikov SI, Shakhov AN, Rybtsov SA, Koroleva EP, Takeda J, Nedospasov SA, Kuprash DV. Dissecting the role of lymphotoxin in lymphoid organs by conditional targeting. Immunol. Rev. 2003;195:106–116. doi: 10.1034/j.1600-065x.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- Ware CF. NETWORK COMMUNICATIONS: Lymphotoxins, LIGHT, and TNF. Annu. Rev. Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- Werts C, Girardin SE, Philpott DJ. TIR, CARD and PYRIN: Three domains for an antimicrobial triad. Cell Death Differ. 2006;13:798–815. doi: 10.1038/sj.cdd.4401890. [DOI] [PubMed] [Google Scholar]
- Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.