Abstract
The Thrifty Gene hypothesis theorizes that during evolution a set of genes has been selected to ensure survival in environments with limited food supply and marked seasonality. Contemporary environments have predictable and unlimited food availability, an attenuated seasonality due to artificial lighting, indoor heating during the winter and air conditioning during the summer, and promote sedentariness and overeating. In this setting the thrifty genes are constantly activated to enhance energy storage. Psychosocial stress and sleep deprivation are other features of modern societies. Stress-induced hypercortisolemia in the setting of unlimited food supply promotes adiposity. Modern man is becoming obese because these ancient mechanisms are efficiently promoting a positive energy balance. We propose that in today’s plentifully provisioned societies, where sedentariness and mental stress have become typical traits, chronic activation of the neuroendocrine systems may contribute to the increased prevalence of obesity. We suggest that some of the yet unidentified thrifty genes may be linked to highly conserved energy sensing mechanisms (AMP kinase, mTOR kinase). These hypotheses are testable. Rural societies that are becoming rapidly industrialized and are witnessing a dramatic increase in obesity may provide a historical opportunity to conduct epidemiological studies of the thrifty genotype. In experimental settings, the effects of various forms of psychosocial stress in increasing metabolic efficiency and gene expression can be further tested.
Keywords: adiposity, neuro-endocrine stress response, thrifty genotype, energy sensing, energy balance
Introduction
A secular trend is the long-term, continuous change of a variable over years or decades [1]. Well-known secular trends are increased stature [2] and earlier puberty [3]. Increased food intake and decreased physical activity [4] characterize the modern obesity epidemic [5]. Body mass index (BMI) has progressively risen in humans, independent of age, gender and ethnicity [6-8]. The increase in populations’ stature and weight may in part be the expression of improved sociodemographic, nutritional and health conditions allowing greater manifestation of genetic potential [9-11]. Neel suggested that in modern times excessive calories and sedentariness may have enhanced the functions of genes coping with unpredictable food availability [12]. Therefore, the obesity epidemic could be a consequence of times of “gluttony and sloth” [13]. As in industrialized countries famines or seasonality effects on food availability are rare [14], this phenomenon is currently disadvantageous.
Whether there has been a secular increase in the level of psychosocial stress is difficult to ascertain: among young people suicide attempts and antidepressants usage have been increasing along with anxiety [15]. During evolution men have been exposed to environmental challenges carrying different stressing loads. One million years ago, the main stressors were food procurement, procreation and survival against climate changes and predators’ aggression [16, 17]. In the last ten thousand years, the human-nature interaction has evolved from a passive to an active inhabitation and the environment has rapidly changed [18]. The agricultural and industrial revolutions are important events which have dramatically changed the environment, humans’ lifestyle, and social interactions, while generating substantial psychosocial stress. In the last 50 years in industrialized societies there have also been major dietary and lifestyle changes [19]. More recently, a progressive decrease in the number of hours of sleep has been reported [20, 21]. Sleep deprivation induces specific alterations in neuroendocrine systems, which include an increase in the stress hormone cortisol favoring central fat deposition, a decrease in the adipostatic signal leptin and an increase in the orexogenic signal ghrelin, inducing increased appetite and food intake [22]. This phenomenon may be contributing to the current epidemic of obesity. The “stress” genes which have been selected under pressure in ancient environments [23] may have not adapted to these rapid environmental changes.
The aim of this paper is to provide a novel synopsis of different disciplines including anthropology, urbanistic, and physiology as they offer insights into how control of energy balance and neuroendocrine responses to stress are conjuring to regulate body weight.
Evolution of Weight Gain and Gene-environment Interaction
Effects of seasonality
The human genome has been shaped into its configuration via erratic food availability and physical requirements for food procurement and reproduction [24-26]. Food dictated daily activities, influenced procreation and sustained the extra-energetic demands of mating efforts, pregnancy, and lactation [27, 28]. Seasonality had an essential impact on dietary intake and energy stores. Nowadays in developing countries such as the Gambia, seasonality has still a powerful effect on energy balance, as food is more available with the harvest during the dry season and reaches its nadir during the rainy season. Body weight fluctuations between the two seasons could be as high as 10%: during the rainy season the physical demands are increased, whereas they decline during the dry season [29, 30]. Hominins were probably exposed in ancestral times to similar variations in food availability [16, 31].
Thrifty genes and evolutionary pressures
The genes involved in the regulation of energy balance have most likely been exposed to substantial selective pressure. The thriftiness of a gene could be mediated by metabolic, adipogenic, physiological or behavioral mechanisms [32]. The thrifty genotype is most likely a multigenic trait and the “cost-effective” gene candidates meeting these criteria may be several: substrate cycling, mitochondrial proton leakage, uncoupled oxidative phosphorylation, Na+/K+ pumping, protein turnover, and immunity. Genotypes favoring a sustained positive energy balance can be classified in the following genotypes: (1) Thrifty: low metabolic rate and decreased thermogenesis; (2) Hyperphagic: poor appetite regulation and overfeeding propensity; (3) Sedentary: propensity to be physically inactive; (4) Low lipid oxidation; and (5) Adipogenetic: high lipid storage capacity [33]. We surmise that energy-sensing mechanisms, defined as “genergostat”, may exist in living organisms; these mechanisms could strategically modulate the activity of thrifty and profligate genes in conditions of positive and negative energy balance, respectively. We suggest the existence of a hypothetical “orexgenergostat” that would sense the positive energy balance and consequently activate the anabolic genes while inactivating the catabolic pathways; during negative energy balance an “anorexenergostat” would induce the opposite effects ( Fig. 1).
Fig. 1).
Fig. 1.
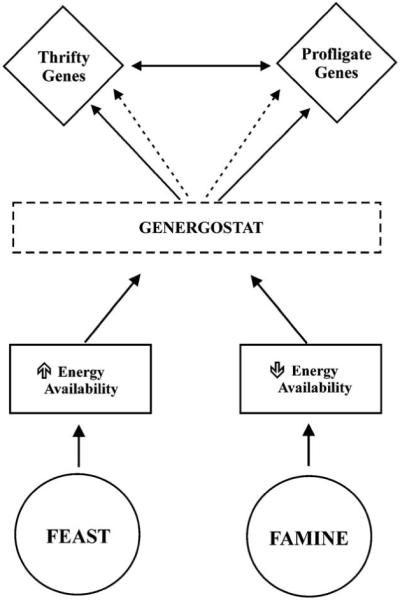
Genergostat, a single sensor model: This model proposes that thrifty and profligate genes are regulated by a single energy-sensing mechanism which responds differently to states of energy surplus and energy deficit. In a state of positive energy balance (Feast) the increase in energy availability activates (solid lines) an energy-sensing controller (genergostat) which turns on the thrifty genes and deactivates (dotted lines) the profligate ones. These two set of genes also interact with each other (double arrow lines). The system in a state of negative energy balance (Famine) would operate in the opposite direction. “Genergostat” is mediated by two independent, mechanisms activated by states of energy surplus (orex-genergostat) or depletion (anorex-energostat). For example, in a state of negative energy balance (Famine) the decrease in energy availability induces an activation (solid lines) of the anorex-energostat and an inhibition (dotted lines) of the orex-genergostat. The sensor would then activate the catabolic genes (profligate) and inhibit the anabolic (thrifty) ones to exert their catabolic action. A feedback mechanism between these genes can be envisaged to modulate their actions (double arrow lines). The system in a state of positive energy balance (Feast) would operate with a similar but opposite mechanism.
Evolution and Energy Sensing Mechanisms
Storage sensing mechanisms
Energy sensing mechanisms are continuously operating. Bacteria and yeasts have a limited capacity for energy storage and their replication critically depends on substrate availability [34]. In the presence of oxygen and cofactors, nutrients are channelled into the citric acid cycle and into the mitochondrial respiratory chain to produce energy-rich phosphate compounds such as adenosine triphosphate (ATP). Unicellular organisms can modify their energy requirements according to substrate availability and ATP levels seem to be the most important signal of energy status [34].
At a macro-molecular level, a state of positive energy balance induces a preferential accretion of fat due to its high energy density (9 kcal/gram), to the lower energetic cost of adipose tissue deposition compared to protein and carbohydrates, and to a virtually unlimited storage capacity. Glycogen stores are much smaller (about 500 g) and their turn-over in muscle and liver [35] may be regulated by finer mechanisms [36]. Proteins have a primary structural and regulatory role but they may be mobilized and become a source of energy during negative energy balance [37].
The signals connecting changes in energy stores with the pathways regulating energy balance can have long-term and short-term signaling properties. Storage-sensing (fat, protein, glycogen) mechanisms have probably long or medium term effects on energy balance, whereas changes in extra- or intracellular nutrients (glucose, amino acids, free fatty acids) may represent short-term signals ( Fig. 2). Glucose sensors are present in areas of the brain involved in appetite regulation such as the arcuate nucleus, lateral hypothalamus, and nucleus of the tractus solitarius [38]. In pancreas and liver, glucose sensors have a critical role in regulating carbohydrate metabolism and they may contribute to the pathogenesis of type 2 diabetes [39]. Amino acids can centrally modulate appetite; the intra-cerebral administration of leucine has anorexigenic effects mediated by leptin [40]. Similarly, the central administration of free fatty acids induces a decrease in the hypothalamic expression of NPY [41]. The role of ketones, lactate and free fatty acids as metabolic biomarkers and regulators of energy balance is still poorly understood.
Fig. 2). Glucose sensors are present in areas of the brain involved in appetite regulation such as the arcuate nucleus, lateral hypothalamus, and nucleus of the tractus solitarius [38]. In pancreas and liver, glucose sensors have a critical role in regulating carbohydrate metabolism and they may contribute to the pathogenesis of type 2 diabetes [39]. Amino acids can centrally modulate appetite; the intra-cerebral administration of leucine has anorexigenic effects mediated by leptin [40]. Similarly, the central administration of free fatty acids induces a decrease in the hypothalamic expression of NPY [41]. The role of ketones, lactate and free fatty acids as metabolic biomarkers and regulators of energy balance is still poorly understood.
Fig. 2.
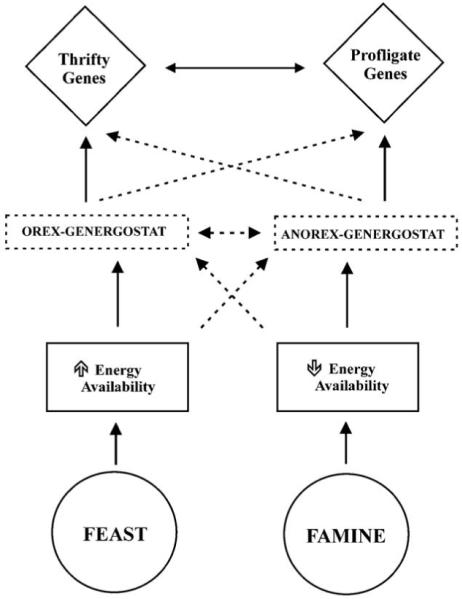
The second model proposes that thrifty and profligate genes are regulated by two independent mechanisms activated by states of energy surplus (orex-genergostat) or depletion (anorex-energostat). For example, in a state of negative energy balance (Famine) the decrease in energy availability induces an activation (solid lines) of the anorex-energostat and an inhibition (dotted lines) of the orex-genergostat. The sensor would then activate the catabolic genes (profligate) and inhibit the anabolic (thrifty) ones to exert their catabolic action. A feedback mechanism between these genes can be envisaged to modulate their actions (double arrow lines). The system in a state of positive energy balance (Feast) would operate with a similar but opposite mechanism.
Is ATP the genergostat?
In  Fig. 2 we offer a hierarchical representation of the possible energy sensing regulatory mechanisms used by mammals. Aerobic metabolism is more energy efficient as it yields more ATP than glycolysis [34]. Therefore, oxygen availability, carbon dioxide production and generation of reactive oxygen species (ROS) can signal cellular oxidative capacity [42]. ATP is short lived and continuously utilized; any changes in substrate availability or energy demands may modify the ATP/AMP ratio, which activates energy sensors such as the AMP-activated protein kinase (AMPk). This kinase is activated by increased AMP/ATP ratio and has a highly conserved function across species [43]. Once activated, AMPk turns on catabolic pathways that generate ATP, as it switches off ATP-consuming processes. The mammalian target for rapamycin (mTOR) is an evolutionarily conserved kinase sensing changes in global energy availability [44]. MTOR is activated by leptin to reduce appetite and in several cell types promotes macromolecular biosynthesis [45]. These molecules, which may fit the theoretical description of the genergostat, may have evolved to take on a novel role to respond to metabolic perturbations at the cellular level. In addition, hormones and cytokines such as insulin, leptin, and adiponectin may contribute and interacts with these ancient energy controllers to maintain energy balance at the whole body level [46].
Fig. 2 we offer a hierarchical representation of the possible energy sensing regulatory mechanisms used by mammals. Aerobic metabolism is more energy efficient as it yields more ATP than glycolysis [34]. Therefore, oxygen availability, carbon dioxide production and generation of reactive oxygen species (ROS) can signal cellular oxidative capacity [42]. ATP is short lived and continuously utilized; any changes in substrate availability or energy demands may modify the ATP/AMP ratio, which activates energy sensors such as the AMP-activated protein kinase (AMPk). This kinase is activated by increased AMP/ATP ratio and has a highly conserved function across species [43]. Once activated, AMPk turns on catabolic pathways that generate ATP, as it switches off ATP-consuming processes. The mammalian target for rapamycin (mTOR) is an evolutionarily conserved kinase sensing changes in global energy availability [44]. MTOR is activated by leptin to reduce appetite and in several cell types promotes macromolecular biosynthesis [45]. These molecules, which may fit the theoretical description of the genergostat, may have evolved to take on a novel role to respond to metabolic perturbations at the cellular level. In addition, hormones and cytokines such as insulin, leptin, and adiponectin may contribute and interacts with these ancient energy controllers to maintain energy balance at the whole body level [46].
Evolution and Energy Balance
Different thermodynamic scenarios
Low physical activity level, high-energy intake and possibly sleep deprivation, a factor previously overlooked, may have a critical role in the obesity epidemic, as they have changed the genome-environment interaction. The first law of thermodynamics, which states that energy cannot be destroyed but only transformed, is useful to describe the energy exchanges occurring in living organisms [47]. Three thermodynamic scenarios (steady state, positive and negative energy balance) are discussed. The first scenario ( Fig. 3) shows a system in equilibrium. When energy intake and physical activity are in balance, the fluxes of energy between the components of the organism (triangle) as well as the internal energy, defined as the energy available in the organism to perform work, are steady.
Fig. 3) shows a system in equilibrium. When energy intake and physical activity are in balance, the fluxes of energy between the components of the organism (triangle) as well as the internal energy, defined as the energy available in the organism to perform work, are steady.
Fig. 3.
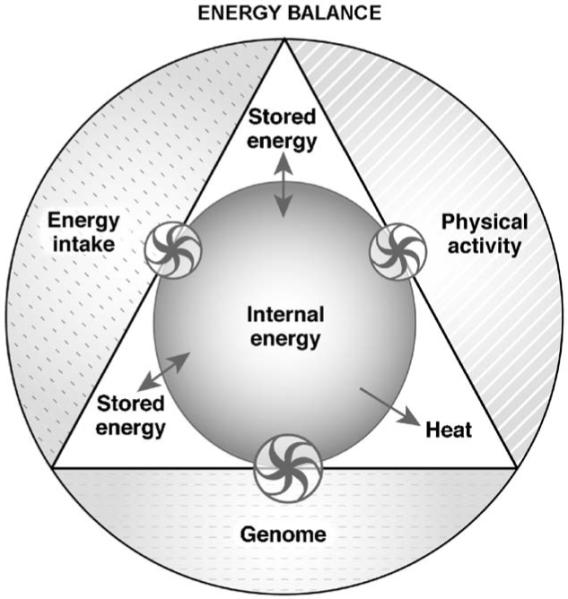
System (entire figure) in energy balance. The areas of the components (physical activity, energy intake) are equal, as no perturbation has been induced on the internal energy (inner circle) of the organism (triangle). The internal energy is table because energy fluxes between body stores (stored energy) and internal energy are constant and energy intake and physical activity match heat generation (heat). The model assumes: 1) Resting energy expenditure is constant; 2) Genome is a regulator of the energy sensing mechanisms and it does not contribute to the energetic fluxes; 3) Energy intake increases internal energy; 4) Physical activity decreases internal energy by increasing heat production. The size of the arrows of the wheels is proportional to the effect and the direction of the arrows indicates the movement of the energy fluxes. Double arrows (↔) indicate stable energy fluxes. Wheels represent the regulatory interactions between the three components (genome, physical activity, energy intake) and the mechanisms controlling the internal energy.
Increased physical activity and decreased energy intake define a state of negative energy balance ( Fig. 4), a common scenario when our ancestors were hunting. Sleep was probably curtailed and erratic at times, which in turn may have increased appetitive behavior and promoted food-seeking behavior. The paucity of food was not compensated by a reduction in physical activity, vigilance was sustained and working efforts were strenuous. The resulting reduction in internal energy levels would activate energy mobilization.
Fig. 4), a common scenario when our ancestors were hunting. Sleep was probably curtailed and erratic at times, which in turn may have increased appetitive behavior and promoted food-seeking behavior. The paucity of food was not compensated by a reduction in physical activity, vigilance was sustained and working efforts were strenuous. The resulting reduction in internal energy levels would activate energy mobilization.
Fig. 4.
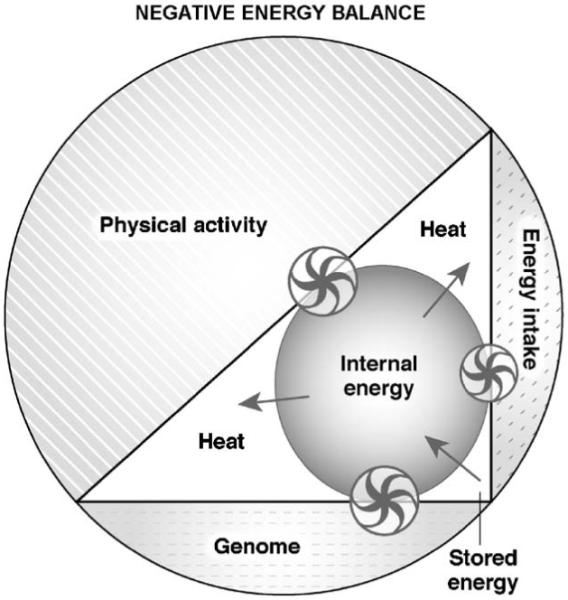
System (entire circle) in negative energy balance. The contribution of the three components to the system has changed. The genome by assumption remains stable but physical activity and energy intake have increased and decreased, respectively. The effect of these changes is a decrease in internal energy via heat generation and the energy to restore the internal energy comes from energy intake and body stores. The model is based on the same assumptions stated in legend to  Fig. 3. The size of the arrows of the wheels is proportional to the effect and the direction of the arrows indicates the movement of the energy fluxes. Wheels represent the regulatory interactions between the three components (genome, physical activity, energy intake) and the mechanisms controlling the internal energy.
Fig. 3. The size of the arrows of the wheels is proportional to the effect and the direction of the arrows indicates the movement of the energy fluxes. Wheels represent the regulatory interactions between the three components (genome, physical activity, energy intake) and the mechanisms controlling the internal energy.
Modern times are characterized by increased food availability, decreased physical activity, and curtailed sleep resulting in a state of positive energy balance ( Fig. 5). The net result is a rise in internal energy, which is predominantly stored.
Fig. 5). The net result is a rise in internal energy, which is predominantly stored.
Fig. 5.
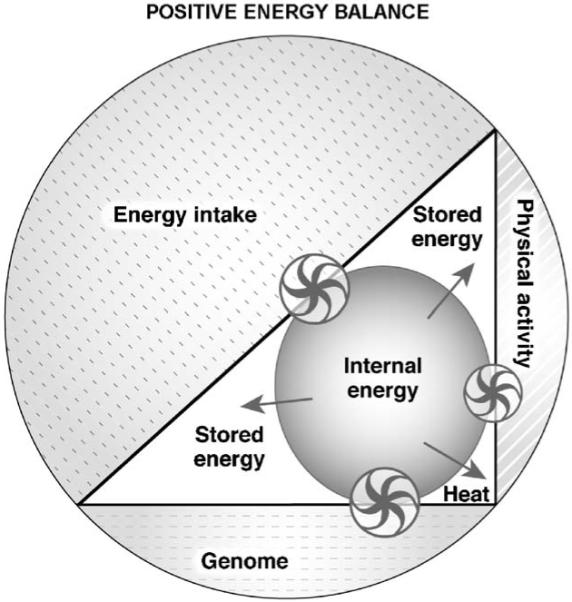
System (outer circle) in positive energy balance. The contribution of the three components to the system has changed. The genome by assumption remains stable but energy intake and physical activity have increased and decreased, respectively. The effect of these changes is an increase in internal energy and the restoration of the internal energy is achieved by storing the energy as tissue and, to some extent, by heat generation. The model is based on the same assumptions stated in legend to  Fig. 3. The size of the arrows of the wheels is proportional to the effect and the direction of the arrows indicates the movement of the energy fluxes. Wheels represent the regulatory interactions between the three components (genome, physical activity, energy intake) and the mechanisms controlling the internal energy.
Fig. 3. The size of the arrows of the wheels is proportional to the effect and the direction of the arrows indicates the movement of the energy fluxes. Wheels represent the regulatory interactions between the three components (genome, physical activity, energy intake) and the mechanisms controlling the internal energy.
Evolution of Weight Gain and Psychosocial Stress
Food availability and social structure
Baboons and chimpanzees live in relatively small and stable groups characterized by dominant-subordinate relationships, which influence the stress system, social behavior, feeding, reproduction and health status [48, 49]. These societies can be at times hierarchically stable or unstable [50]. In stable societies the subordinate animals experience greater stress as they are subjected to the dominance of the most powerful males, who control mating, reproduction and feeding. Dominant rhesus monkeys eat approximately 20% more calories than subordinates who have to steal food and consume it covertly [51]. Rats likewise live in dominant/subordinate hierarchies in which subordinate animals adapt their feeding rhythms to avoid the dominant animals [52].
In unstable societies the dominant animals experience greater stress as they continuously compete to maintain their rank and assert their control over breeding opportunities and access to food. In nonhuman primates an interaction between food intake and social status has been observed. If in socially housed cynomolgous females the dominant-subordinate roles are experimentally reversed, current subordinates display more behavioral depression and increased cortisol levels, which in turn stimulates appetite, favors fat deposition, decreases peak luteal progesterone levels and increases anovulatory cycles [48]. A similar scenario may have been apparent during hominin evolution. Early hominins and more recent ancestors (Homo ergaster, and Homo neanderthalis) probably lived in small groups (10-50 subjects) whereas feeding involved common access to a discrete resource, such as a fruiting tree or an animal carcass [24, 49, 53].
Hormonal fingerprints and group dynamics
The relative contribution of the specific neuroendocrine profiles to obesity based on social rank has started to be characterized only recently. The physiological and behavioral responses to different emotional and social stressors have been highly conserved throughout phylogenesis [54-57]. Therefore, it is likely that the neuro-endocrine responses elicited by a stressor were substantially similar in our ancestors. In primates, subordinate individuals have a different neuro-immune-endocrine fingerprint: they are relatively hypercortisolemic, have an increased NPY release, an enhanced appetite, leptin resistance [58-60], and central fat deposition [61, 62]. Similar patterns are observed in modern human societies: in a large prospective study of British civil servants, the Whitehall study, a dose-response relationship was found between exposure to work stressors, high cortisol levels, central adiposity and risk of metabolic syndrome [63-65].
Information on the social structure of our ancestors prior to the Holocene, approximately 10 000 years ago, is sparse and can be inferred studying fossils and paintings. Foraging populations led a semi-nomadic life dictated by climate, danger, and resource availability. Egalitarism in ancestral societies was achieved through sharing of resources [66]. Differences in social status nevertheless existed and influenced reproductive success and survival [48, 49, 55].
Evolution of the stress response: Interplay between the hypothalamic-pituitary-adrenal axis and the sympathoadrenal system; metabolic consequences
The stress responses have been advantageous during the hominin evolution [67, 68]. However, with increased food availability there has been increased arousal and preparedness for a “fight-or-flight” reaction [69]. Disputes may have initiated avoidance and resignation as coping strategies in subordinate subjects rather than active responses, as the latter were likely to generate harsher penalties [48, 70, 71]. In a state of positive energy balance and low physical demands, an increased cortisol secretion may have been the predominant stress responses in emotionally stressed subordinate subjects ( Fig. 6).
Fig. 6).
Fig. 6.

System in positive energy balance and influence of the stress responses (hypothalamus-pituitary-adrenal (HPA) axis and symphatoadrenal system (SAS)) on the system (entire figure). The increase in energy intake produces a rise in the internal energy (inner circle) which is primarily stored and, to some extent, dissipated as heat to restore the equilibrium of the organism (triangle). The induction of a stress response via the activation of the HPA and SAS produces different effects. The HPA interacts with the genome to modulate energy fluxes but exerts also a stimulating effect (solid line) on energy intake and fat deposition and an inhibiting effect (dotted line) on heat generation via a modulation of the immune system. The SAS has the opposite effects as induce a decrease in appetite and increase in heat generation and fat mobilization. The size of the arrows and wheels is proportional to the effect and the direction of the arrows indicates the movement of the energy fluxes. The model is based on the same assumptions stated in legend to  Fig. 3. Double arrows (↔) indicate reciprocal influences. Wheels are non-energetic, regulatory interactions between the three components (genome, physical activity, energy intake) and the mechanisms controlling the internal energy.
Fig. 3. Double arrows (↔) indicate reciprocal influences. Wheels are non-energetic, regulatory interactions between the three components (genome, physical activity, energy intake) and the mechanisms controlling the internal energy.
The two main components of the stress system, the hypothalamic-pituitary-adrenal and the sympathetic system have integrated metabolic effects which are complex and depend significantly on insulin secretion and insulin sensitivity [72]. Changes in energy balance modulate the interaction between these systems to control fuel partitioning, disposal, accretion and distribution. Studies in rats and humans have demonstrated the tissue specific actions of the three systems after manipulation of energy intake [73-76]. One of the main findings was the observation of a threshold effect of corticosteroid levels on insulin action and energy metabolism [77]. At low levels, corticosteroids amplify the effects of insulin on the liver and increase gluconeogenesis and lipogenesis. At this level of activation of the stress system muscle metabolism seems relatively unaffected. In the adipose tissue, insulin and corticosteroids promote adipocyte differentiation and lipid deposition by increasing lipoprotein lipase activity in a depot-specific manner [78], that is, visceral fat is more responsive to the action of corticosteroids due to a higher number of corticosteroid receptors [79].
If the stressor becomes more severe and sustained resulting in higher levels of corticosteroids, the scenario becomes more complex. In a state of positive energy balance fat accumulates due to the synergistic action of corticosteroids and insulin; protein turnover in the muscle is increased to an extent depending on the corticosteroid/insulin ratio. In a state of negative energy balance, insulin and glucose levels are low, the corticosteroid/insulin ratio is increased resulting in a more pronounced protein turnover. Stress-induced increased sympathetic activity favours fat mobilization (lipolysis), glucose production (gluconeogenesis), and mobilization (glycogenolysis). These mechanisms are highly conserved across species and the different modus operandi of the metabolic pathways becomes more elaborate as we ascend the phylogenetic scale and metabolic control starts to be more closely intertwined with cognition.
In summary, modern lifestyle stress occurs in a sleep-deprived, sedentary, energy abundant environment, which induces cortisol release and enhances visceral fat accumulation, a risk factor for metabolic syndrome and type 2 diabetes.
The preagriculture era
Prior to the emergence of agriculture, hunting, scavenging and gathering maintained physical demands [16, 17]. The activities were performed in open spaces and, most likely, done in small groups of individuals. It is likely that the neuro-endocrine response was primarily directed at improving muscular efficiency to capitalize gathering, hunting and scavenging and raise the level of attention, readiness and awareness [16, 25, 26, 80, 81]. Thus, the involvement of the sympato-adrenal system may have been more pronounced than the HPA axis, as catecholamines (adrenalin, noradrenalin) enhance awareness and alertness (vision, hearing, attention), readiness (increased blood pressure and redistribution of blood to brain and muscles from gastrointestinal system, increased respiratory rate) and metabolic support (increased lipolysis, glycogenolysis, gluconeogenesis, while lessening internal cues (decreased hunger and pain) [23, 82, 83].
Evolution and encephalization
Table 1 outlines the anthropometric and energetic characteristics of our progenitors [84]. Indirect estimate of body size through fossils has been possible in few of the species that have populated the Earth in the last 4-5 millions years [85, 86]. Hunting, scavenging and gathering were the principal feeding practices of early hominins in the Lower and Middle Palaeolithic eras (2500000-30000 years ago, approx). Hominin became able to produce rudimentary hand shaped tools [53, 85]. The morphometric evolution of hominins proceeded with a progressive increase in height, weight, and brain volume (Table 1). Increased body size was the expression of a better nutritional status [87-90]. The encephalization allowed greater structural, cognitive, and problem-solving ability in the setting of an improved diet (essential fatty acids) [87, 89-91]. The positive correlation between resting energy expenditure and brain weight both in men (r =0.72, p<0.05) and women (r=0.78, p<0.05) indicate the metabolic impact of a bigger, and supposedly more active, brain.
Table 1.
Early hominid species, existence dates, encephalization and proxy estimates of body mass index (BMI), body surface area (BSA), and resting energy expenditure (REE)
| Taxon | Dates (MY) | Height (m) | Weight (kg) | BMI (kg/m2) | BSA (m2) | REE (kcal/d) | Brain weight (g) | Brain weight/Body weight | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | |||
| Australopithecus afarensis | 3.9-3.0 | 1.51 | 1.05 | 45 | 29 | 19.74 | 26.30 | 1.38 | 0.88 | 1216 | 703 | 434 | 9.6 | 15.0 |
| Australopithecus africanus | 3.0-2.4 | 1.38 | 1.15 | 41 | 30 | 21.53 | 22.68 | 1.24 | 0.95 | 1095 | 762 | 448 | 10.9 | 14.9 |
| Paranthropus boisei | 2.3-1.4 | 1.37 | 1.24 | 49 | 34 | 26.11 | 22.11 | 1.33 | 1.06 | 1175 | 849 | 514 | 10.5 | 15.1 |
| Paranthropus robustus | 1.9-1.4 | 1.32 | 1.10 | 40 | 32 | 22.96 | 26.45 | 1.19 | 0.95 | 1049 | 759 | 523 | 13.1 | 16.3 |
| Homo habilis | 1.9-1.6 | 1.31 | 1.00 | 37 | 32 | 21.56 | 32.00 | 1.14 | 0.88 | 1009 | 708 | 601 | 16.2 | 18.8 |
| Homo rudolfensis | 2.4-1.6 | 1.60 | 1.50 | 60 | 51 | 23.44 | 22.67 | 1.62 | 1.44 | 1433 | 1158 | 736 | 12.3 | 14.4 |
| Homo ergaster | 1.9-1.7 | 1.80 | 1.60 | 66 | 56 | 20.37 | 21.88 | 1.84 | 1.58 | 1625 | 1263 | 849 | 12.9 | 15.2 |
| Homo sapiens | existing | 1.75 | 1.61 | 58 | 49 | 18.94 | 18.90 | 1.71 | 1.50 | 1507 | 1198 | 1350 | 23.3 | 27.6 |
BMI calculated as weight (kg) divided by squared height in meters
BSA is calculated using the formula of DuBois el al.[125] [BSA=weight (kg)0.425 × height (cm)0.725 × 0.007184]
REE calculated using the formula of Roberston and Reid [126] based on BSA and attribution of specific age-energy coefficient. All hominids were assumed to have an age of 25 years. Age Coefficients (AC) were: Male (M) = 36.8; Female (F) = 33.4. The formula is: AC × BSA × 24. Table modified from McHenry et al. (2000) [84] and Aiello et al. (2002) [80]
The increased energy requirements of the brain of early hominids were compensated by a decrease in gut dimensions related to a more digestible and assimilable diet [92, 93]. The progressive encephalization allowed an evolving, species-typical computational and neural architecture of the human mind. More effective neural circuitries allowed solving the ecological problems facedby hunter-gatherers [68, 87, 94].
The Upper Palaeolithic era (40000-11000 years ago, approx.) saw a rapid advancement in social, cognitive and technological skills. Human language developed in this period with fundamental implications for brain development. Agriculture, farming and animal domestication changed food availability, as food was stocked for longer periods. In agricultural societies a dominant role was associated with control of food supplies and easier access to food. This power was particularly evident during famines, which affected mainly individuals lacking control over their food supply. Seasonality and periodic shortages of food were still an integral part of humans’ life [16, 24, 85].
The agriculture era and the industrial revolution
Genetic adaptations may have occurred in the post-agricultural age through assortative mating [95] and periodic exposure to food insecurity, famine, migrations, colonization and slavery, which were likely to select genes conferring an advantage on survival in different environments [31, 96].
With the appearance of the first civilisations, progress became an increasingly localized phenomenon, with Western populations changing more rapidly [97]. The Industrial Revolution dramatically modified the genome-environment interaction. Mechanization, food storage, transport and mobility, social and urbanistic structure, working demand, changing social relationships, scientific discoveries and increased life expectancy are all dynamics still evolving in modern societies and altogether are imposing a burden on the relatively unchanged human genome [19, 98-100].
The Energetic System of Modern Man and his Stressful Surroundings
The most recent changes in food processing and eating habits have converted the seasonal experience into a state of incessant, positive energy balance. The human genome responds by running the energy-sparing programs coded by the thrifty genes. The causative factors leading to weight gain are numerous and all contribute, to a different extent, to the global burden of obesity in developing and developed countries [101, 102].
Stress and evolution
Emotions and cognitive functions depend on brain development and encephalization [67, 79, 103]. Compared to modern humans, early hominins presumably had simpler mental circuits and a stressful or joyful situation may have evoked simpler reactions [90, 104, 105]. Encephalization, the development of tools and the use of language with the consequent effects of communication on human relations probably coincided with the rapid growth of brain volume and expansion of cortical areas and neurocircuitry [68, 103].
Animals perceive food shortage as stressful, which increases vigilance, post-pones sleep, and enhances food-seeking behavior [106]. Similarly, humans facing unpredictability in food availability were presumably stressed during periods of negative energy balance and increased food seeking behaviors and physical exertion while curtailing sleep. The stress system response would have been an activation of the catecholaminergic system over the HPA system to enhance fuel availability, level of awareness and physical performance and the activation of lipolysis, glycogenolysis and gluconeogenesis. Insulin orchestrated the energy fluxes towards fat deposition in states of chronic stress [73].
The modern thermodynamic scenario in industrialized countries
The environment has dramatically changed in the last century and especially in the last 20 years. The advent of the computer has further reduced physical activity and intensified mental efforts [107]. The modern, computer-dependent, sleep-deprived, physically-inactive humans live chronically stressed in a society of food abundance. Desk jobs, computers, and high-energy food are commonly associated. Mental task jobs can lead to isolation, as workers are assembled in a rigid hierarchical structure where a few individuals have high power, job latitude and reward, and the majority are left in unidirectional, narrow roles with small reward/effort ratios [108-111].
The sprawling urban environment is not conducive to walking and physical activity and may be playing a major role that only now is starting to emerge. Families are smaller and co-dependent relationships, characteristic of more primitive societies, are declining. Global mobility has changed and migratory fluxes from developing countries to industrialized nations are larger, creating more prominent social disparities and inter-ethnic tensions [64, 112-115].
Between 1980 and 2005 the prevalence of obesity, as indicated by BMI, has tripled from 8 to 22 %. Over the same period of time energy expenditure due to physical activity has not declined [116] and caloric intake has not increased. Thus, the search for other obesogenic factors. In a seminal review article [117], it has been hypothesized that a reduction in variability in ambient temperature, along with other factors, may be one of the contributory reasons to the obesity epidemic. In the last 25 years, both house heating during the winter and air conditioning in the summer have indeed become much more prevalent. As during cold exposure the metabolic rate increases and in a hot environment the propensity to feeding is diminished, these two factors may theoretically have contributed via different mechanisms to increasing body weight. The Thrifty Gene hypothesis attempts to explain the high prevalence of obesity in Pimas mostly in terms of adaptation to an environment with limited and unpredictable food resources. Little attention has however been paid so far to the fact that essential adaptive abilities in an ambient such as the desert would also include the ability to endure hot days as well as cold nights, and the capability to limit water losses in a hot environment. As high body mass per se has been indicated as a key factor both in the adaptation to a cold or a hot environment, high BMI may have indeed protected the Pima Indians towards extreme daily excursion [118]. No functional studies of adaptation to cold have been conducted in Pima Indians therefore this testable hypothesis has not been directly verified yet, however genetic data may provide circumstantial evidence that Pima Indians under selective pressure may have evolved mechanisms to better cope with cold. Specifically, uncoupling protein 5 long form, one of isoform which uncouples fuel oxidation from ATP synthesis, is more represented in this population (approximately 2/3 of the subjects in a small series of 36 Pimas) than the other two isoforms, the uncoupling protein 5 short form and the uncoupling protein 5 short form insert [119]. This more abundant form correlated positively with resting metabolic rate and lipid oxidation during an insulin clamp.
The brown adipose tissue (BAT) is the only tissue whose main function is heat production; this tissue is largely represented in rodents and in hibernating animals like bears [120]. In recent evolutionary history such as during the Ice Age humans had to cope with major changes in environmental temperature. It has been hypothesized that the presence of BAT allowed the Neanderthal man to survive during the Ice Age in Europe. Higher on in the phylogenetic scale the role of this tissue has greatly diminished, and BAT until recently was deemed to be mostly reductional in Homo sapiens. Recently, the widespread notion that BAT disappears in humans after the first few years of life has been challenged by empirical evidence. This is not a trivial issue, as a small amount of BAT, such as 40-50g can increase energy expenditure by 10% preventing in the long-term obesity, while ensuring in the short-term cold adaptation. Back in the 80’s, BAT had been found in a small autoptic series of outdoor workers in Finland [121].
Novel imaging techniques such as FDG PET have suggested, based on studies mostly conducted in patients with cancer to detect metastases [122], that: A) BAT may be present in adults mostly in the supraclavicular areas up to 80% of the cases; B) BAT seems to be more easily detected by imaging if the subject is in a cold environment; C) in condition of thermoneutrality BAT becomes more evident in the feeding than in the fasting state; D) it seems more common in younger patients and in women; and E) in a large convenience clinical series, BAT seemed to be associated with lower BMI and better insulin sensitivity. It is not clear however whether the BAT detected by nuclear imaging methods is metabolically active. Studies specifically designed to assess BAT in different physiological conditions (feeding/fasting, cold/warm environment, sleep/wake) are needed. More recently, in a large convenience clinical series BAT was correlated by imaging with improved insulin sensitivity, although it is not clear how much of the BAT tissue, as revealed by imaging, may be functionally active. Finally, BAT has been shown to derive from a common precursor that may later differentiate into either a skeletal myocite or a BAT cell, challenging the doctrine that all adypocites derive from a common precursor [123]. Interestingly 30% of Pima Indians carry a point mutation in the beta-3 adrenergic receptor that innervates the BAT. This mutation has been associated with inability to lose weight and insulin resistance [124].
Conclusions and Future Directions
The effects of psychosocial stress on metabolism and neuroendocrine systems could be investigated by conducting studies in countries still subjected to seasonality. Epidemiological investigations in rural populations living in Sub-Saharan African countries such as Gambia or Senegal would be informative of the changes in the gene-environment interaction in fieri. In experimental settings, the relationship between stress response, adiposity, and appetite control could be investigated by the exposure to stressors including sleep deprivation and cold exposure, while assessing the ability to metabolize a standardized high fat meal. In conclusion, we have revisited from an anthropological angle the thrifty genotype hypothesis and have expanded it to include the effects of emotional stress and sleep deprivation.
Acknowledgment
This research was supported in part by the Intramural Research Programs of the National Institute of Diabetes, Digestive and Kidney Diseases.
References
- 1.Last J. A Dictionary of Epidemiology. 4th ed. Oxford University Press; Oxford: 2001. [Google Scholar]
- 2.Kac G. Secular height trend: a literature review. Cad Saude Publica. 1999;15:451–461. doi: 10.1590/s0102-311x1999000300002. [DOI] [PubMed] [Google Scholar]
- 3.Wattigney WA, Srinivasan SR, Chen W, Greenlund KJ, Berenson GS. Secular trend of earlier onset of menarche with increasing obesity in black and white girls: the Bogalusa Heart Study. Ethn Dis. 1999;9:181–189. [PubMed] [Google Scholar]
- 4.Lynn Matton ND, Wijndaele K, Philippaerts R, Duquet W, Beunen G, Claessens AL, Thomis M, Lefevre J. Secular trends in anthropometric characteristics, physical fitness, physical activity, and biological maturation in Flemish adolescents between 1969 and 2005. Am J Hum Biol. 2007;19:345–357. doi: 10.1002/ajhb.20592. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KMCM, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 6.Al-Lawati JA, Jousilahti PJ. Prevalence and 10-year secular trend of obesity in Oman. Saudi Med J. 2004;25:346–351. [PubMed] [Google Scholar]
- 7.Schober E, Rami B, Kirchengast S, Waldhor T, Sefranek R. Recent trend in overweight and obesity in male adolescents in Austria: a population-based study. Eur J Pediatr. 2007;166:709–714. doi: 10.1007/s00431-006-0312-z. [DOI] [PubMed] [Google Scholar]
- 8.O’Loughlin J, Paradis G, Meshefedjian G, Gray-Donald K. A five-year trend of increasing obesity among elementary schoolchildren in multiethnic, low-income, inner-city neighborhoods in Montreal, Canada. Int J Obes Relat Metab Disord. 2000;24:1176–1182. doi: 10.1038/sj.ijo.0801362. [DOI] [PubMed] [Google Scholar]
- 9.Rona R. Genetic and environmental factors in the control of growth in childhood. Br Med Bull. 1981;37:265–272. doi: 10.1093/oxfordjournals.bmb.a071713. [DOI] [PubMed] [Google Scholar]
- 10.Silventoinen K. Determinants of variation in adult body height. J Biol Sci. 2003;35:263–285. doi: 10.1017/s0021932003002633. [DOI] [PubMed] [Google Scholar]
- 11.Tanner J. Growth as a measure of the nutritional and hygienic status of a population. Horm Res. 1992;38:106–115. doi: 10.1159/000182580. [DOI] [PubMed] [Google Scholar]
- 12.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 13.Prentice A, Jebb S. Obesity in Britain: gluttony or sloth? BMJ. 1995;311:437–439. doi: 10.1136/bmj.311.7002.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prentice AM. Obesity - the inevitable penalty of civilisation? Br Med Bull. 1997;53:229–237. doi: 10.1093/oxfordjournals.bmb.a011610. [DOI] [PubMed] [Google Scholar]
- 15.Twenge JM. The age of anxiety? Birth cohort change in anxiety and neuroticism, 1952-1993. J Pers Soc Psychol. 2000;79:1007–1021. doi: 10.1037//0022-3514.79.6.1007. [DOI] [PubMed] [Google Scholar]
- 16.Foley R. The influence of seasonality on hominid evolution. In: Strickland SUS, editor. Seasonality and Human Ecology. Cambridge University Press; Cambridge: 1993. pp. 17–37. [Google Scholar]
- 17.Ulijaszek SJ. Human eating behaviour in an evolutionary ecological context. Proc Nutr Soc. 2002;61:517–526. doi: 10.1079/pns2002180. [DOI] [PubMed] [Google Scholar]
- 18.Wells JCK, Stock JT. The biology of the colonizing ape. Am J Phys Anthropol. 2007;134(S45):191–222. doi: 10.1002/ajpa.20735. [DOI] [PubMed] [Google Scholar]
- 19.Hill JO, Peters JC. Environmental Contributions to the Obesity Epidemic. Science. 1998;280(5368):1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 20.Webb WB, Agnew HW. Are we chronically sleep deprived? Bull Psych Soc. 1975;6:47–48. [Google Scholar]
- 21.Cizza G, Skarulis M, Mignot E. A link between short sleep andobesity: building the evidence for causation. Sleep. 2005;28:1217–1220. doi: 10.1093/sleep/28.10.1217. [DOI] [PubMed] [Google Scholar]
- 22.MacEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism. 2006;55(10 Suppl 2):S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 23.MacEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 24.Bogin B, Smith BH. Evolution of the human life cycle. Am J Hum Biol. 1996;8:703–716. doi: 10.1002/(SICI)1520-6300(1996)8:6<703::AID-AJHB2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 25.Eaton SB. What did our late paleolithic (preagricultural) ancestors eat? Nutr Rev. 1990;48:227–230. doi: 10.1111/j.1753-4887.1990.tb02943.x. [DOI] [PubMed] [Google Scholar]
- 26.Eaton SB, Eaton SB. An evolutionary perspective on human physical activity: implications for health. Comparative Biochemistry and Physiology - Part A: Mol Integr Physiol. 2003;136:153–159. doi: 10.1016/s1095-6433(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 27.Wells JCK. The evolution of human fatness and susceptibility to obesity: an ethological approach. Biol Rev Camb Philos Soc. 2006;81:183–205. doi: 10.1017/S1464793105006974. [DOI] [PubMed] [Google Scholar]
- 28.Prentice AM, Goldberg GR, Prentice A. Body mass index and lactation performance. Eur J Clin Nutr. 1994;48(Suppl 3):S78–S86. discussion S86-S89. [PubMed] [Google Scholar]
- 29.Minghelli G, Schutz Y, Whitehead R, Jequier E. Seasonal changes in 24-h and basal energy expenditures in rural Gambian men as measured in a respiration chamber. Am J Clin Nutr. 1991;53:14–20. doi: 10.1093/ajcn/53.1.14. [DOI] [PubMed] [Google Scholar]
- 30.Prentice AM, Jebb SA, Goldberg GR, Coward WA, Murgatroyd PR, Poppitt SD, Cole TJ. Effects of weight cycling on body composition. Am J Clin Nutr. 1992;56(1 Suppl):209S–216S. doi: 10.1093/ajcn/56.1.209S. [DOI] [PubMed] [Google Scholar]
- 31.Prentice AM. Starvation in humans: evolutionary background and contemporary implications. Mech Ageing Dev. 2005;126:976–981. doi: 10.1016/j.mad.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Prentice AM, Rayco-Solon P, Moore SE. Insights from the developing world: thrifty genotypes and thrifty phenotypes. Proc Nutr Soc. 2005;64:153–161. doi: 10.1079/pns2005421. [DOI] [PubMed] [Google Scholar]
- 33.Bouchard C. The biological predisposition to obesity: beyond the thrifty genotype scenario. Int J Obes (Lond) 2007;31:1337–1339. doi: 10.1038/sj.ijo.0803610. [DOI] [PubMed] [Google Scholar]
- 34.Voet D, Voet J, Pratt CW. Fundamentals of Biochemistry. Wiley; New York: 1999. [Google Scholar]
- 35.Heymsfield SB, Wang Z, Baumgartner RN, Ross R. Human body composition: advances in models and methods. Annu Rev Nutr. 1997;17:527–558. doi: 10.1146/annurev.nutr.17.1.527. [DOI] [PubMed] [Google Scholar]
- 36.Flatt J. Glycogen levels and obesity. Int J Obes. 1996;20:S1–S11. [PubMed] [Google Scholar]
- 37.Waterlow JC. Whole-Body Protein Turnover in Humans-Past, Present, and Future. Annu Rev Nutr. 1995;15:57–92. doi: 10.1146/annurev.nu.15.070195.000421. [DOI] [PubMed] [Google Scholar]
- 38.Levin BE. Metabolic sensing neurons and the control of energy homeostasis. Physiol Behav. 2006;89:486–489. doi: 10.1016/j.physbeh.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Weir GC. A defective beta-cell glucose sensor as a cause of diabetes. N Engl J Med. 1993;328:729–731. doi: 10.1056/NEJM199303113281012. [DOI] [PubMed] [Google Scholar]
- 40.Cota D, Proulx K, Smith KAB, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312(5775):927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 41.Migrenne S, Magnan C, Cruciani-Guglielmacci C. Fatty acid sensing and nervous control of energy homeostasis. Diab Metab. 2007;33:177–182. doi: 10.1016/j.diabet.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Benani A, Troy S, Carmona MC, Fioramont X, Lorsignol A, Leloup C, Casteilla L, Pénicaud L. Role for mitochondrial reactive oxygen species in brain lipid sensing: redox regulation of food in take. Diabetes. 2007;56:152–160. doi: 10.2337/db06-0440. [DOI] [PubMed] [Google Scholar]
- 43.Carling D. The AMP-activated protein kinase cascade - a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Tokunaga C, Yoshino K-i, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun. 2004;313:443–446. doi: 10.1016/j.bbrc.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Kahn BB, Myers MG. mTOR tells the brain that the body is hungry. Nat Med. 2006;12:615–617. doi: 10.1038/nm0606-615. [DOI] [PubMed] [Google Scholar]
- 46.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabol. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Kleiber M. The fire of life: an introduction to animal energetics. Wiley; New York: 1961. [Google Scholar]
- 48.Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 49.Charlton BG. The inequity of inequality: egalitarian instincts and evolutionary psychology. J Health Psychol. 1997;2:413–425. doi: 10.1177/135910539700200309. [DOI] [PubMed] [Google Scholar]
- 50.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 51.Brennan J, Anderson JR. Varying responses to feeding competitionin a group of rhesus monkeys (Macaca mulatta) Primates. 1988;29:353–360. [Google Scholar]
- 52.Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, Ma LY, Woods SC, Sakai RR. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav. 2004;80:683–693. doi: 10.1016/j.physbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J. Origins and evolution of the Western diet:health implications for the 21st century. Am J Clin Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 54.Sternberg EM, Glowa JR, Smith MA, Calogero AE, Listwak SJ, Aksentijevich S, Chrousos GP, Wilder RL, Gold PW. Corticotropin releasing hormone related behavioral and neuroendocrine responses to stress in Lewis and Fischer rats. Brain Res. 1992;570:54–60. doi: 10.1016/0006-8993(92)90563-o. [DOI] [PubMed] [Google Scholar]
- 55.Otten W, Puppe B, Kanitz E, Schon PC, Stabenow B. Effects of dominance and familiarity on behaviour and plasma stress hormones in growing pigs during social confrontation. Zentralbl Veterinarmed A. 1999;46:277–292. doi: 10.1046/j.1439-0442.1999.00216.x. [DOI] [PubMed] [Google Scholar]
- 56.Jessop S. Modulation of the adrenocortical stress response in marine turtles (Cheloniidae): evidence for a hormonal tactic maximizing maternal reproductive investment. J Zool. 2001;254:57–65. [Google Scholar]
- 57.Perry S, Bernier NJ. The acute humoral adrenergic stress response in fish: facts and fiction. Aquaculture. 1999;177:285–295. [Google Scholar]
- 58.Gluck ME, Geliebter A, Hung J, Yahav E. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosom Med. 2004;66:876–881. doi: 10.1097/01.psy.0000143637.63508.47. [DOI] [PubMed] [Google Scholar]
- 59.Epel E, Lapidus R, MacEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 60.Bjorntorp P, Rossner S, Udden J. “Consolatory eating” is not a myth. Stress-induced increased cortisol levels result in leptin-resistant obesity. Lakartidningen. 2001;98:5458–5461. [PubMed] [Google Scholar]
- 61.Samra JS, Clark ML, Humphreys SM, MacDonald IA, Bannister PA, Frayn KN. Effects of physiological hypercortisolemia on the regulation of lipolysis in subcutaneous adipose tissue. J Clin Endocrinol Metab. 1998;83:626–631. doi: 10.1210/jcem.83.2.4547. [DOI] [PubMed] [Google Scholar]
- 62.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 63.Brunner EJ, Chandola T, Marmot MG. Prospective effect of job strain on general and central obesity in the Whitehall II study. Am J Epidemiol. 2007;165:828–837. doi: 10.1093/aje/kwk058. [DOI] [PubMed] [Google Scholar]
- 64.Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332(7540):521–525. doi: 10.1136/bmj.38693.435301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steptoe A, Kunz-Ebrecht SR, Brydon L, Wardle J. Central adiposity and cortisol responses to waking in middle-aged men and women. Int J Obes. 2004;28:1168–1173. doi: 10.1038/sj.ijo.0802715. [DOI] [PubMed] [Google Scholar]
- 66.Woodburn J. Egalitarian societies. Man. 1982;17:431–451. [Google Scholar]
- 67.Hamilton WD. The genetical evolution of human behaviour. J Theor Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 68.Cosmides L, Tooby J. In: Handbook of Emotions. 2nd Edition ed. Jones MLMH, editor. Guilford; New York: 2000. [Google Scholar]
- 69.Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann N Y Acad Sci. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- 70.Solomon MB, Foster MT, Bartness TJ, Huhman KL. Social defeat and footshock increase body mass and adiposity in male Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2007;292:R283–R290. doi: 10.1152/ajpregu.00330.2006. [DOI] [PubMed] [Google Scholar]
- 71.Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1284–R1293. doi: 10.1152/ajpregu.00437.2005. [DOI] [PubMed] [Google Scholar]
- 72.Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 73.la Fleur SE. The effects of glucocorticoids on feeding behavior in rats. Physiol Behav. 2006;89:110–114. doi: 10.1016/j.physbeh.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 74.Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271(2 Pt 1):E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- 75.Singh A, Petrides JS, Gold PW, Chrousos GP, Deuster PA. Differential hypothalamic-pituitary-adrenal axis reactivity to psychological and physical stress. J Clin Endocrinol Metab. 1999;84:1944–1948. doi: 10.1210/jcem.84.6.5746. [DOI] [PubMed] [Google Scholar]
- 76.la Fleur SE, Akana SF, Manalo SL, Dallman MF. Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology. 2004;145:2174–2185. doi: 10.1210/en.2003-1359. [DOI] [PubMed] [Google Scholar]
- 77.Dallman MF, Warne JP, Foster MT, Pecoraro NC. Glucocorticoids and insulin both modulate caloric intake through actions on the brain. J Physiol. 2007;583:431–436. doi: 10.1113/jphysiol.2007.136051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fried S, Russell CD, Grauso NL, Brolin RE. Lipoprotein lipase regulation by insulin and glucocorticoid in subcutaneous and omental adipose tissues of obese women and men. J Clin Invest. 1993;92:2191–2198. doi: 10.1172/JCI116821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–1143. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- 80.Aiello LC, Wells JCK. Energetics and evolution of the genus Homo. Ann Rev Anthropol. 2002;31:323–338. [Google Scholar]
- 81.Chakravarthy MV, Booth FW. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol. 2004;96:3–10. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- 82.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 83.Tentolouris N, Liatis S, Katsilambros N. Sympathetic system activity inobesity and metabolic syndrome. Ann NY Acad Sci. 2006;1083:129–152. doi: 10.1196/annals.1367.010. [DOI] [PubMed] [Google Scholar]
- 84.MacHenry HM, Coffing K. Australopithecus to Homo: Transformation in body and mind. Ann Rev Anthropol. 2000;29:125–146. [Google Scholar]
- 85.Wood B. Origin and evolution of the genus Homo. Nature. 1992;355(6363):783–790. doi: 10.1038/355783a0. [DOI] [PubMed] [Google Scholar]
- 86.Wood B. Human evolution. Ecce homo-behold mankind. Nature. 1997;390(6656):120–121. doi: 10.1038/36450. [DOI] [PubMed] [Google Scholar]
- 87.Duchaine B, Cosmides L, Tooby J. Evolutionary psychology and the brain. Curr Opin Neurobiol. 2001;11:225–230. doi: 10.1016/s0959-4388(00)00201-4. [DOI] [PubMed] [Google Scholar]
- 88.Elton S, Bishop LC, Wood B. Comparative context of Plio-Pleistocene hominin brain evolution. J Hum Evol. 2001;41:1–27. doi: 10.1006/jhev.2001.0475. [DOI] [PubMed] [Google Scholar]
- 89.Kappelman J. The evolution of body mass and relative brain size in fossil hominids. J Human Evol. 1996;30:243–276. [Google Scholar]
- 90.Ruff CB, Trinkaus E, Holliday TW. Body mass and encephalization in Pleistocene Homo. Nature. 1997;387(6629):173–176. doi: 10.1038/387173a0. [DOI] [PubMed] [Google Scholar]
- 91.Tooby J, Cosmides L, Barrett HC. The second law of thermodynamics is the first law of psychology: evolutionary developmental psychology and the theory of tandem, coordinated inheritances: comment on Lick-liter and Honeycutt (2003) Psychol Bull. 2003;129:858–865. doi: 10.1037/0033-2909.129.6.858. [DOI] [PubMed] [Google Scholar]
- 92.Peters A, Schweiger U, Pellerin L, Hubold C, Oltmanns KM, Conrad M, Schultes B, Born J, Fehm HL. The selfish brain: competition for energy resources. Neurosci Biobehav Rev. 2004;28:143–180. doi: 10.1016/j.neubiorev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 93.Foley RA, Lee PC. Ecology and energetics of enchephalization in hominid evolution. Philos Trans R Soc London Ser B. 1991;334:223–232. doi: 10.1098/rstb.1991.0111. [DOI] [PubMed] [Google Scholar]
- 94.Cosmides L, Tooby J. Beyond intuition and instinct blindness: toward an evolutionarily rigorous cognitive science. Cognition. 1994;50:41–77. doi: 10.1016/0010-0277(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 95.Heath AC, Eaves LJ, Nance WE, Corey LA. Social inequality and assortative mating: cause or consequence? Behav Genet. 1987;17:9–17. doi: 10.1007/BF01066007. [DOI] [PubMed] [Google Scholar]
- 96.Prentice AM. Fires of life: the struggles of an ancient metabolism in a modern world. Br Nutr Found Nutr Bull. 2001;26:13–27. [Google Scholar]
- 97.Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S2–S9. doi: 10.1038/sj.ijo.0802804. [DOI] [PubMed] [Google Scholar]
- 98.Komlos J. Nutrition, Population Growth, and the Industrial Revolution in England. Social Sci Hist. 1990;14:69–91. [Google Scholar]
- 99.Cowan RS. The “IndustrialRevolution” in the Home: Household Technology and Social Change in the 20th Century. Technol Cult. 1976;17:1–23. [PubMed] [Google Scholar]
- 100.Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev. 2006;27:750–761. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- 101.Jequier E, Tappy L. Regulation of body weight in humans. Physiol Rev. 1999;79:451–480. doi: 10.1152/physrev.1999.79.2.451. [DOI] [PubMed] [Google Scholar]
- 102.James WP, Rigby N, Leach R. Obesity and the metabolic syndrome: the stress on society. Ann N Y Acad Sci. 2006;1083:1–10. doi: 10.1196/annals.1367.002. [DOI] [PubMed] [Google Scholar]
- 103.Cosmides L. The logic of social exchange: has natural selection shaped how humans reason? Studies with the Wason selection task. Cognition. 1989;31:187–276. doi: 10.1016/0010-0277(89)90023-1. [DOI] [PubMed] [Google Scholar]
- 104.Akers KG, Nakazawa M, Romeo RD, Connor JA, MacEwen BS, Tang AC. Early life modulators and predictors of adult synaptic plasticity. Eur J Neurosci. 2006;24:547–554. doi: 10.1111/j.1460-9568.2006.04921.x. [DOI] [PubMed] [Google Scholar]
- 105.Klein SB, Cosmides L, Tooby J, Chance S. Decisions and the evolution of memory: multiple systems, multiple functions. PsycholRev. 2002;109:306–329. doi: 10.1037/0033-295x.109.2.306. [DOI] [PubMed] [Google Scholar]
- 106.Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids. Brain Res. 1999;830:56–71. doi: 10.1016/s0006-8993(99)01380-3. [DOI] [PubMed] [Google Scholar]
- 107.Lopez RP, Hynes HP. Obesity, physicalactivity, and the urban environment: public health research needs. Environ Health. 2006;5:25. doi: 10.1186/1476-069X-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kivimaki M, Head J, Ferrie JE, Shipley MJ, Brunner E, Vahtera J, Marmot MG. Work stress, weight gain and weight loss: evidence for bidirectional effects of job strain on body mass index in the Whitehall II study. Int J Obes. 2006;30:982–987. doi: 10.1038/sj.ijo.0803229. [DOI] [PubMed] [Google Scholar]
- 109.Kouvonen A, Kivimaki M, Cox SJ, Cox T, Vahtera J. Relationship between work stress and body mass index among 45 810 female and male employees. Psychosom Med. 2005;67:577–583. doi: 10.1097/01.psy.0000170330.08704.62. [DOI] [PubMed] [Google Scholar]
- 110.Ostry AS, Radi S, Louie AM, LaMontagne AD. Psychosocial and other working conditions in relation to body mass index in a representative sample of Australian workers. BMC Public Health. 2006;6:53. doi: 10.1186/1471-2458-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Overgaard D, Gamborg M, Gyntelberg F, Heitmann BL. Psychological workload and weight gain among women with and without familial obesity. Obesity (Silver Spring) 2006;14:458–463. doi: 10.1038/oby.2006.60. [DOI] [PubMed] [Google Scholar]
- 112.Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn R, Syme SL. Socioeconomic status and health. The challenge of the gradient. Am Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 113.Marmot MG. Stress, social and cultural variations in heart disease. J Psychosom Res. 1983;27:377–384. doi: 10.1016/0022-3999(83)90069-7. [DOI] [PubMed] [Google Scholar]
- 114.Robert SA, Reither EN. A multilevel analysis of race, community disadvantage, and body mass index among adults in the US. Soc Sci Med. 2004;59:2421–2434. doi: 10.1016/j.socscimed.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 115.Walsh A, Walsh PA. Social support, assimilation and biological effective blood pressure levels. Int Migr Rev. 1987;21:577–591. [Google Scholar]
- 116.Westerterp KR, Speakman JR. Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. Int J Obes. 2008;8:1256–1263. doi: 10.1038/ijo.2008.74. [DOI] [PubMed] [Google Scholar]
- 117.Keith SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM, Ruden D, Pietrobelli A, Barger JL, Fontaine KR, Wang C, Aronne LJ, Wright SM, Baskin M, Dhurandhar NV, Lijoi MC, Grilo CM, DeLuca M, Westfall AO, Allison DB. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes. 2006;11:1585–1594. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- 118.Steegmann AT., Jr. Human cold adaptation: an unfinished agenda. Am J Hum Biol. 2007;19:218–227. doi: 10.1002/ajhb.20614. [DOI] [PubMed] [Google Scholar]
- 119.Yang X, Pratley RE, Tokraks S, Tataranni PA, Permana PA. UCP5/BMCP1 transcript isoforms in human skeletal muscle: relationship of the short-insert isoform with lipid oxidation and resting metabolic rates. Mol Genet Metab. 2002;75:369–373. doi: 10.1016/S1096-7192(02)00008-2. [DOI] [PubMed] [Google Scholar]
- 120.Cinti S. The role of brown adipose tissue in human obesity. Nutr Metab Cardiovasc Dis. 2006;16:569–574. doi: 10.1016/j.numecd.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 121.Huttunen P, Hirvonen J, Kinnula V. The occurrence of brown adipose tissue in outdoor workers. Eur J Appl Physiol Occup Physiol. 1981;46:339–345. doi: 10.1007/BF00422121. [DOI] [PubMed] [Google Scholar]
- 122.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 123.Farmer SR. Brown fat and skeletal muscle: unlikely cousins? Cell. 2008;134:726–727. doi: 10.1016/j.cell.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 124.Yoshida T, Umekawa T. Beta 3 adrenergic receptor polymorphism and obesity. Nippon Rinsho. 1998;56:1871–1875. [PubMed] [Google Scholar]
- 125.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Nutrition. 1989;5:303–311. 1916. [PubMed] [Google Scholar]
- 126.Robertson JD, Reid D. Standards for the basal metabolism of normal people in Britain. Lancet. 1952;3:940–943. doi: 10.1016/s0140-6736(52)90543-6. [DOI] [PubMed] [Google Scholar]


