Abstract
BACKGROUND
Enhanced androgen receptor (AR) activity by increased testosterone availability may play important roles in prostate cancer progressing to castration resistant state. Comparison of expression profiles in androgen dependent and independent prostate tumors demonstrated a marked increase of the expression of UDP-glucuronosyltransferase 2B15 (UGT2B15), an androgen catabolic enzyme. We investigated mechanisms controlling the differential expression of UGT2B15 and B17 in response to androgen treatments.
METHODS
Gene expression was determined by RT-PCR. The association of AR with UGT2B15/B17 genes was determined by Chromatin immuno-precipitation (CHIP). RNA interference was used to knock-down gene expression.
RESULTS
UGT2B15 and B17 genes were not expressed in AR negative prostate cancer cell lines, PC3 and DU145, while they were expressed in AR positive cell lines, LNCaP, LNCaP-abl (an androgen independent LNCaP sub-line), and VCaP. The expression levels of UGT2B15/B17 were up-regulated in LNCaP-abl comparing to those in LNCaP. These results suggest the requirement of AR for the expression of UGT2B15/B17. Treatment with DHT down-regulated the expression of UGT2B15/B17 in LNCaP in a time and dose dependent manner and this down-regulation was competitively antagonized by flutamide and bicalutimide, suggesting a pathway mediated by AR. Further CHIP experiments demonstrated the direct interaction of AR with the promoter regions of UGT2B15/B17 genes. Knocking down AR expression in LNCaP significantly reduced the expression of UGT2B15/B17 and completely inhibited the DHT-induced down-regulation of UGT2B15/B17 genes.
CONCLUSIONS
We demonstrated that UGT2B15 and B17 are primary androgen-regulated genes and AR is required for both their basal expression and their androgen-regulated expression.
Keywords: prostate cancer, androgen, androgen receptor, UGT2B15, UGT2B17
INTRODUCTION
Prostate cancer (PCa) is the most common noncutaneous malignancy in North American men and the second leading cause of cancer death in this population. Androgen and androgen receptor (AR) play crucial roles in PCa development and progression [1]. AR is a ligand-activated transcription factor belonging to the nuclear hormone receptor super family. Upon binding to androgenic ligands, AR is thought to dissociate from chaperone proteins, dimerize, bind to androgen response elements (AREs) of target genes, recruit transcriptional coregulators, and result in the either stimulation or repression of androgen–responsive genes’ transcription [1,2]. For many decades, androgen deprivation therapies have been used in the treatment of PCa. Most patients are initially responsive to androgen deprivation but eventually relapse with castration resistant PCa (CRPC).
Studies have shown associations between enhanced AR activity and progression to CRPC [3–9]. Mechanisms that may stimulate or reactivate the AR transcriptional activity in CRPC include: (1) increased AR expression, (2) AR mutations, allowing to interact with weak androgens or other ligands, (3) increased expression of AR coactivators and activation of signal transduction pathways (i.e., growth factor, receptor tyrosine kinase, and AKT pathways), that enhance AR responses to low levels of androgens, and (4) increased testosterone uptake or synthesis. Comparison of the expression profiles in androgen dependent and independent prostate tumor cells demonstrated marked increases of AR expression level in CRPC tumor samples and the increased expression of multiple genes involved in androgen synthesis (including 3β-hydroxysteroid dehydrogenase 2, HSD3B2; aldo-keto reductase family 1 member C3, AKR1C3; type 1 5α-reducatse; SRD5A1) and catabolism (including aldo-keto reductase family 1 member C2, AKR1C2; aldo-keto reductase family 1 member C1, AKR1C1; and uridine diphosphate-glycosyltransferase 2 (UGT2) B15 (UGT2B15 [10,11])). The increased expression of HSD3B2, AKR1C3, SRD5A1 implicates enhanced intracellular conversion of adrenal androgens to testosterone (T) and dihydrotestosterone (DHT) as a mechanism by which prostate cancer adapts to androgen deprivation. The increased expression of AKR1C2, AKR1C1, and UGTB15 in CRPC presumably leads to a rapid catabolism or inactivation of the DHT produced by 5α-reductase. AKR1C1 and AKR1C2 convert DHT to the inactive 5α-androstane-3α,17β-diol, and 5α-androstane-3β,17β-diol, which are subsequently glucuronidated via UGT2B15/B17 and eliminated into circulation. UGT2B17 is also capable of directly glucuronidating both T and DHT [12]. The increased expression of androgen catabolic enzymes in CRPC seems paradoxical, given that the increase of T and DHT was implicated in cancer progression. To facilitate our understanding of the role of the regulated expression of enzymes mediating androgen metabolism, we investigated potential mechanisms underlying the differential expression of these enzymes using a series of prostate cancer cell lines. Surprisingly, we found that AR is essential for the regulated expression of UGT2B15 and B17 genes.
UDP-glucuronosyltransferases (UGTs) encompass a family of enzymes that catalyze the transfer of glucuronic acid from uridine 5′-diphosphoglucuronic acid to molecules with functional groups of oxygen, nitrogen, sulfur or carbon and subsequently render the substrates more water soluble, less toxic, and more easily excreted from the body [13]. These enzymes play a key role in the clearance of many biologically active substrates. Members of the UGT2B subfamily have a preference for glucuronidation of steroid hormones, bile acids, phenolic drugs and carcinogens and they are highly expressed in the human liver, as well as extra hepatic tissues, including kidney, prostate, mammary gland, and ovary. Two of them, UGT2B15 and B17 were found as enzymes with particularly high activity for glucuronidation of androgenic steroids including DHT, androstane-3α, 17β-diol (3α-Diol), and androsterone (ADT). UGT2B15 is expressed in luminal cells of the prostate, where DHT is formed, whereas, UGT2B17 is expressed in basal cells of the prostate, where dehydroepiandrosterone is converted into ADT and 3α-Diol [14]. In addition to facilitating the excretion, glucuronidation of androgens mediated by UGT2B15 and UGT2B17 in the prostate results in irreversible steric hindrance of androgens and abolishes the affinity of androgens to AR [15,16]. UGT2B15 and B17 are critical enzymes for the local inactivation of androgens. Knocking down UGT2B15 and B17 expression efficiently reduced the glucuronidation of DHT, 3α-DIOL and ADT, and subsequently altered the expression of androgen dependent genes [17].
Expression of UGT2B15 and/or B17 can be modulated upon the treatment with androgens or estrogens, though the involved regulatory mechanisms have not yet been previously reported. UGT2B15 and B17 transcripts were down regulated in human PCa cells upon the treatment with androgens or growth factors, and up-regulated in CRPC tumor cells derived from human bone biopsies [18–20]. The UGT2B15 transcript is up-regulated by estrogen in estrogen receptor-positive human breast cancer cell and in the CRPC LuCaP 35V xerographic cells [21,22]. Our finding that AR controls the transcription of UGT2B15 and B17 explains the differential expression of UGT2B15/B17 in response to changes in the level of androgen and/or the status of AR expression. This control mechanism may represent a key function in the homeostasis of androgens and androgen signaling processes in prostate cells.
MATERIALS AND METHODS
Cell Lines and Chemicals
LNCaP and LNCaP-abl cells were maintained in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) for LNCaP cells, 10% charcoal-stripped FBS for LNCaP-abl cells. PC3, DU145, and VCaP cell lines were maintained in DMEM medium supplemented with 10% FCS. DHT and flutamide were purchased from Sigma–Aldrich. Anti-AR (N20) antibody was from Santa Cruz Biotechnology.
Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR) and RT-PCR Analysis
All total RNAs were isolated using the RNA isolation kit from Qiagen (RNeasy Mini). Real-time RT-PCR analysis was performed as described previously [23,24]. Sense and antisense primer sets for: AR, GTTGTGTAAGGCAGTGTC and CTCAGCAGTATC-TTCAGTG; PSA, TGTGTGCTGGACGCTGGA and CACTGCCCCATGACGTGAT; UGT2B15, TGGGAA-TATTATGACTACAG and AGGGGTTTGGCTGGTT-TAC; UGT2B17, TGTGTTGGGAATATTCTGACTA-TAA and AGGGGTTTGGCTGGTTTAC; Actin, GT-GGGGCGCCCCAGGCACCA and TGGGTCATCTT-CTCGCGGTT; GAPDH, TCCACCCATGGCAAATTC and TCGCCCCACTTGATTTTGG; for both UGT2B15 and B17, GTTTTCTCTGGGGTCGATGA and TTGGC-TTCTTGCCATCAAAT.
Chromatin Immuno-Precipitation (ChIP) Assay
ChIP was performed following a previously described protocol [24,25]. Anti-AR antibody (N20, from Santa Cruz Biotechnology)was used to pull-down AR-DNA complexes. qPCR amplifications were performed to determine the amount of input DNA and AR bound DNAs. Primers, sense 5′-GTGGGCCACACTA-GCACCTT-3′ and anti-sense 5′-ATGGACGTCAGTCT-TTCTGCTG-3′ were used to detect UGT2B15/B17 promoter region derived sequences and primers, sense-5′-TGGGACAACTTGCAAACCTG-3′ and anti-sense-5′-CCAGAGTAGGTCTGTTTTCAATCCA-3′ were used to detect the PSA enhancer derived sequence.
RNA Interference (RNAi)
The assay was performed as described previously [24]. Briefly, SMARTpool RNAi targeting AR (Dharmacon, Lafayette, CO) was transfected into LNCaP cells using Lipofectamine 2000 (Invitrogen). Forty-eight hours after transfection, cells were treated ethanol vehicle or 10 nM DHT for another 24 hr. Total RNAs were then harvested using the RNA isolation kits from Qiagen and analyzed by real-time RT-PCR for AR, PSA, UGT2B15, and UGT2B17.
RESULTS
Differential Expression of UGT2B15 and B17 in Androgen-Dependent and Independent Human Prostate Cancer Cell Lines
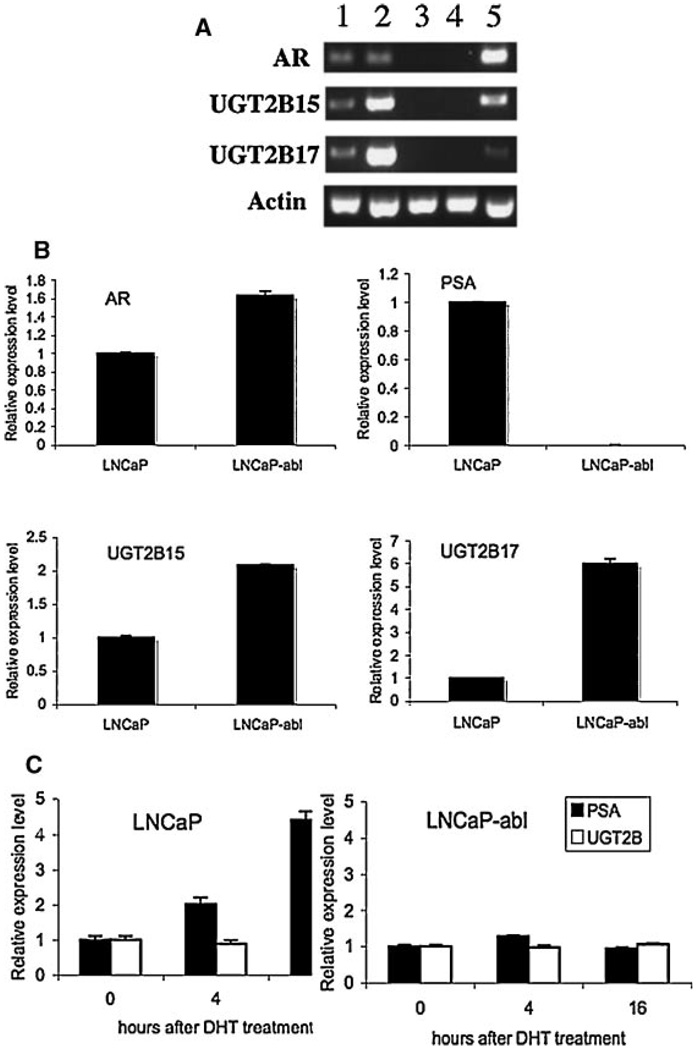
To identify potential mechanisms involved in the differential expression of UGT2B15/B17 in androgen dependent and independent prostate cancer cells, we first compared the UGT2B15/B17 expression in several AR positive prostate cancer cell lines including the androgen dependent LNCaP cell line, the LNCaP derived sub line-LNCaP-abl, which is androgen independent, and the VCaP cell line, which is androgen dependent and expresses a relatively high level of AR and two AR negative and androgen independent cell lines—PC3 and DU145. Interestingly, we found that UGT2B15 and B17 were only expressed in the AR positive cell lines as determined by RT-PCR (Fig. 1A). PC3 and DU145, which do not have the AR message, did not express UGT2B15 and B17. This observation is consistent with the hypothesis that expression of UGT2B 15 and B17 is AR dependent. In addition, UGT2B15 and B17 are expressed at a relatively high level in the androgen independent LNCaP-abl cell line compared to that of the androgen dependent LNCaP cell line, a feature comparable to the finding of the up-regulated expression of UGT2B in the androgen-independent prostate cancer tissues [10,11]. Subsequently, we used LNCaP and LNCaP-abl cell lines as models for the identification of potential mechanisms involved in the differential expression of UGT2B15 and B17 genes.
Fig. 1.
Differential expression of UGT2B15 and 17 in different PCa celllines. A: Comparison of UGT2B15 and 17 expression in AR positive and AR negative cell lines. Total RNAs were isolated from LNCaP (1), LNCaP-abl (2), DU145 (3),PC-3 (4), and VCaP (5) cell lines. Regular RT-PCR reactions were performed for ARUGT2B15, UGT2B17 and actin and the amplified cDNAs were separated on a 2.5% agarosegel. The expression level of actin was used as a control for estimating the relative amount of total RNAs applied in each reaction. B: Quantitative analysis of the expression level of UGT2B15 and B17 in LNCaP and LNCaP-abl cell lines. Real-time RT-PCR analysis was performed for measuring the relative mRNA level of AR, PSA, UGT2B15, and UGT2B17 in each cell line. Values represent the fold differences in gene expression relative to those in LNCaP cells, which was arbitrarily set as 1.0. C: Comparison of the response to DHT treatment in LNCaP and LNCaP-abl cell lines. Total RNAs were isolated from cells prior to the DHT treatment (0), and cells that were treated with 100 nMDHT for 4 hr (4) and 16 hr (16).The expression levels of PSA and UGT2B15/B17 were determined by real-time RT-PCR reactions. The level of expression in cells without DHT treatment (0) was arbitrarily set as 1.
The mRNA levels of AR, PSA, UGT2B15, and UGT2B17 were compared by qPCR in LNCaP and LNCaP-abl cells as shown in Figure 1B. The mRNA level of AR, UGT2B15, and UGT2B17 in LNCaP-abl cells is ∼1.5-fold, 2.0-fold and 6-fold, respectively, higher than those in LNCaP cell line, whereas the AR induced PSA expression in LNCaP-abl is absent. All measurements were normalized with the expression level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which is not an AR responsive gene and expressed at a similar level in both LNCaP and LNCaP-abl cell lines.
We further compared the impact of androgen treatment on gene expression in LNCaP and LNCaP-abl cell lines (Fig. 1C). As anticipated, the PSA expression can be induced in LNCaP cell line by DHT in a time dependent manner. Unlike PSA, the expression of UGT2B15 and B17 was reduced to ∼85% and ∼50% in LNCaP cells after 4 and 16 hr treatment with DHT, respectively. In contrast, expression of PSA and UGT2B15/B17 in LNCaP-abl cell line was not significantly affected upon the treatment with DHT. Thus, although both PSA and UGTB15/B17 are androgen-regulated genes, the effects of androgen on regulation of these genes are distinct.
Down-Regulation of UGT2B15 and B17 Expression by DHT
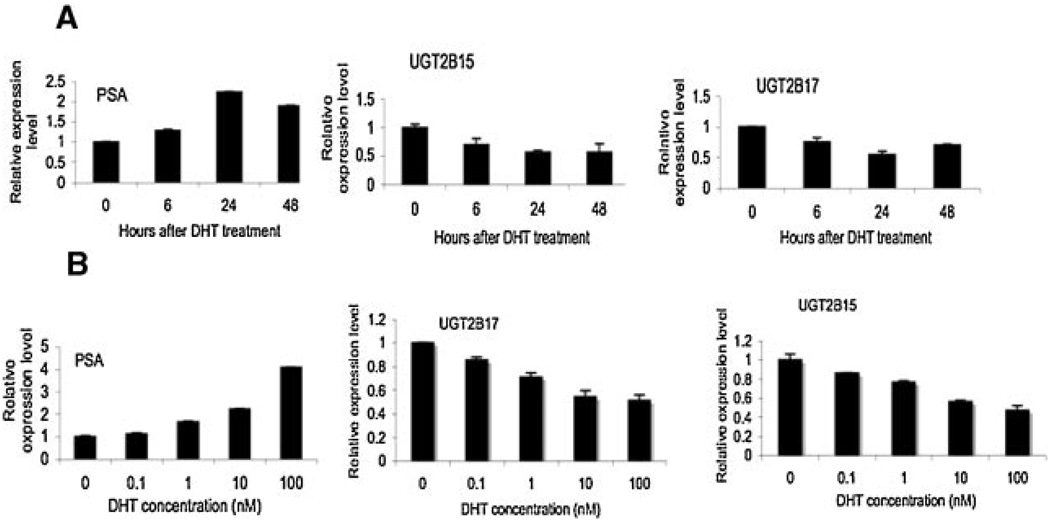
To ascertain the AR-mediated control of UGT2B15 and B17 expression, we further performed the kinetic analysis of the down-regulation of UGT2B15 and B17 by DHT in LNCaP and LNCaP-abl cell lines (Fig. 2). The expression level of PSA and GAPDH were simultaneously measured to serve as positive and negative controls in response to the treatment of DHT. The relative expression levels of all genes were normalized with the expression level of GAPDH (serving as a control for the amount of total RNA used) in each sample. As anticipated, the PSA expression can be induced in LNCaP cell line by DHT in a time and dose dependent manner. In contrast to PSA, the expression level of UGT2B15 and B17 was significantly reduced upon treatment with DHT. Increasing the incubation time of DHT treatment increased the extent of DHT induced down-regulation of UGT2B15 and B17 mRNA and at 24 hr, the down-regulation reached to the maximum level (Fig. 2). In addition, the down-regulation of UGT2B15 and B17 expression by DHT is also dose-dependent. Increasing the concentration of DHT increased the level of reduction of the UGT2B15 and B17 expression (Fig. 2). Expression of PSA and UGT2B15 and B17 in the androgen-independent LNCaP-abl cell line was not significantly affected with treatment with DHT. In sum, these results suggested the possibility that AR is involved in the control of UGT2B15 and B17 expression.
Fig. 2.
Characterization of the impact of DHT treatment on UGT2B15 and B17 expression in LNCaP cells. A: Responses to different amounts of DHT. LNCaP cells were treated with different amounts of DHT, as indicated, for 24hr.B: Responses to the period of incubation time for DHT treatment. LNCaP cells were treated with 10 nMDHT for different periods of time, as indicated. Total RNAs were isolated and the expression levels of PSA, UGT2B15 and UGT2B17 were examined by real-time RT-PCR. Values represent the fold differences in gene expression relative to the expression level at 0 time point or without DHT treatment, which was arbitrarily set as 1.0.
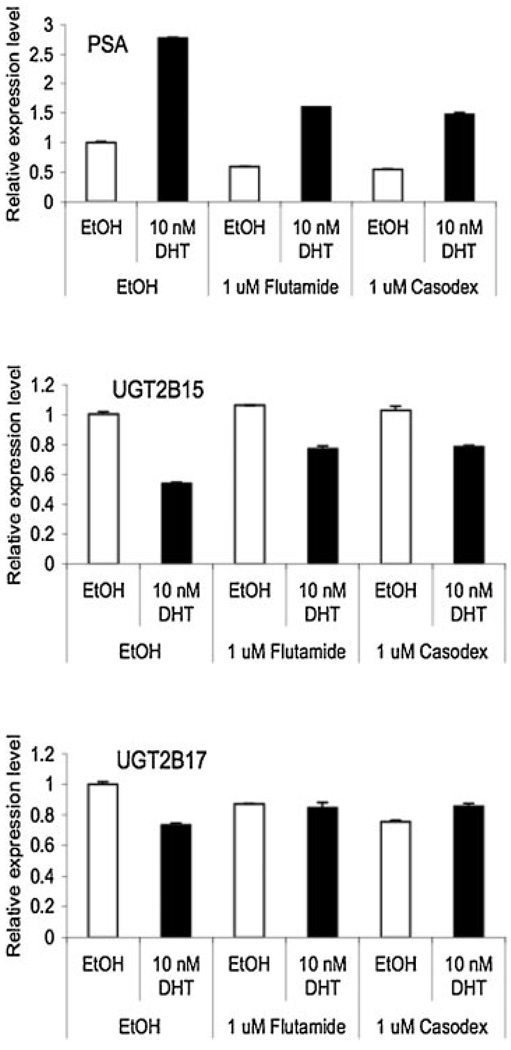
To corroborate the impact of DHT on the expression of UGT2B15 and B17, we further examined the effects of antiandrogens, flutamide and bicalutamide (Casodex) that block AR activity, on the DHT induced down-regulation of UGT2B15 and B17 expression in LNCaP cells. As shown in Figure 3, both flutamide and bicalutamide can suppress approximately 40–50% of basal and DHT-induced PSA mRNA expression (Fig. 3, top panel). In the absence of antiandrogens, DHT down-regulates UGT2B15 and UGT2B17 mRNA expression by 45% and 25% respectively. However, treatment with antiandrogens partially inhibited the DHT-induced down-regulation of UGT2B15 mRNA expression, and completely blocked the effect of DHT on UGT2B17. These data strongly supported the hypothesis that the DHT-induced down-regulation of UGT2B15 and B17 expression is mediated by AR.
Fig. 3.
The effect of anti-androgens on the DHT-induced down-regulation of UGT2B15/B17 expression. LNC aP cells were treated with ethanol vehicle,DHT (10nM), anti-androgens(Flutamide, 1µM; Casodex or bicalutamide, 1 µM), or a combination of DHT and anti-androgens for 24 hr and then total RNAs were isolated. The expression levels of PSA, UGT2B15, and UGT2B17 mRNAs were analyzed by real-time RT-PCR. Values represent the fold differences relative to those in cells without any drug treatments (which were set as 1.0).
Interaction of AR With the Promoter Region of UGT2B15 and B17 Genes
These biochemical studies strongly suggested that AR controls the expression of UGT2B15 and B17 genes. To provide direct evidence for UGT2B15 and B17 genes being primary androgen-responsive genes, we performed Chromatin immuno-precipitation (CHIP) analysis demonstrating direct interaction of AR with the UGT2B15 and B17 gene locus in LNCaP and LNCaP-abl cells (Fig. 4 and Fig. 5).
Fig. 4.
Chromosomal locations of AR binding sites at the UGT2B15/B17 gene locus. UGT2B15 and B17 are two closely located genes at chromosome 4 (chr4). Numbers indicate the nucleotide positions on chr4. Physical maps of UGT2B15 and UGT2B17 genes are indicated by the arrowed-gray lines with gray vertical boxes, which represent exons and arrows indicate the transcription direction. Two black bars located on the top of the 5′ end of each gene represent the location of AR binding sites mapped in LNCaP and LNCaP-abl cell lines by ChIP-on-chip analysis.
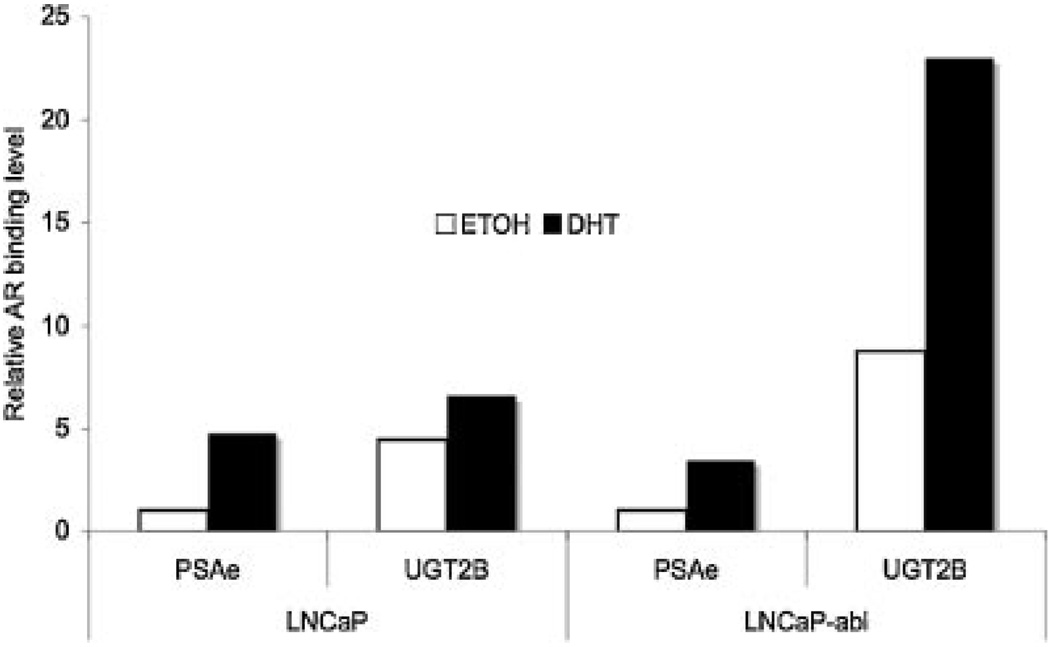
Fig. 5.
CHIP analysis of the interaction of AR with the UGT2B15/B17 genelocus in LNCaP and LNCaP-abl celllines. Cells were treated with vehicle or 100 nMDHT for 16 hr, and then subjected to ChIP analyses using antibodies against AR following a previously established protocol [24,25].Immuno-precipitated DNAs derived from the UGT2B15/B17 locus were quantified by real-time PCR using primers spanning the 5′ end of the exon 1 of the UGT2B15/B17 genes, as described in Materials and Methods Section. The level of PSA enhancer (PSAe) bound with AR was simultaneously determined as a positive control, using the previously described primer set. In each experiment, the amount of input chromatin was measured by PCR amplification with the same set of primers and the amount of immuno-precipitated DNA was adjusted for the relative amount of input chromatin. Values represent the relative amount of AR bound material comparing to the level of AR bound PSAe in the absence of DHT, which was arbitrarily set as 1. Each value represents the mean from two independent experiments.
We first searched the database of whole genome AR binding sites that were generated in LNCaP and LNCaP-abl cells by chromatin immunoprecipitation (ChIP) combined with tiled DNA oligonucleotide microarray analysis (ChIP-on-chip; Wang et al. data not shown). We found that AR binding sites were located within the ∼1.0 to 1.35 kb region spanning the 5′ end of the UGT2B15 and B17 genes in LNCaP and LNCaP-abl cell lines, as shown in Figure 4. To validate the AR binding sites located at the UGT2B15 and B17 gene locus, we performed standard anti-AR ChIP followed by real-time PCR in LNCaP and LNCaP-abl cell lines (Fig. 5). UGT2B15 and B17 genes are highly conserved and the high degree homology extends to the 5′ flanking region (up to —1662 in UGT2B15 [12,26,27]). Due to the high degree of homology in the 5′ region end and the promoter region of the UGT2B15 and B17 gene, we were unable to design primers that allow to separately amplify the AR-bound DNA derived from the 5′ end of the UGT2B15 or that from the UGT2B17. Primers derived from the 5′ end of the first exon, a conserved region in UGT2B15 and B17, were used for the CHIP analysis. The PSA enhancer region (PSAe) was used as a positive control and the interaction of AR and PSAe was previously extensively characterized [24,25]. The amount of input extracts applied to each reaction was estimated by PCR amplification of individual target sequences in the extracts. All calculations were normalized or adjusted for the amount of input extracts used. As anticipated, treatment with DHT enhanced the binding of AR to PSAe in both the LNCaP and LNCaP-abl cell lines (at ∼4.7- and 3.5-fold, respectively). Compared to the binding of AR with PSAe, it appeared that in the absence of DHT, the level of AR binding to UGT2B15 and B17 promoter region was ∼4.5-fold and 8.7-fold higher, in LNCaP cells and in LNCaP-abl cells respectively than those binding to PSAe (Fig. 5, comparing the white bars). Treatment with DHT significantly enhanced the AR binding with UGT2B15 and B17 genes (by ∼1.5-fold in LNCaP cell line and ∼2.6-fold in LNCaP-abl cell line; Fig. 5). These results confirmed the association of AR with the promoter region of the UGT2B15 and B17 genes and indicated that even without androgen, AR can bind to the promoter region, suggesting the non-ligand bound AR might influence basal transcription of UGT2B15 and B17 genes. Based upon these data, we hypothesize that although the non-ligand bound AR can influence UGTB15/B17 transcription, addition of androgen enhances the binding of AR to AREs near the UGT2B15/B17 gene loci and simultaneously recruits co-regulator(s), that is, repressors, which subsequently leads to the down-regulation of the UGT2B15 and B17 expression.
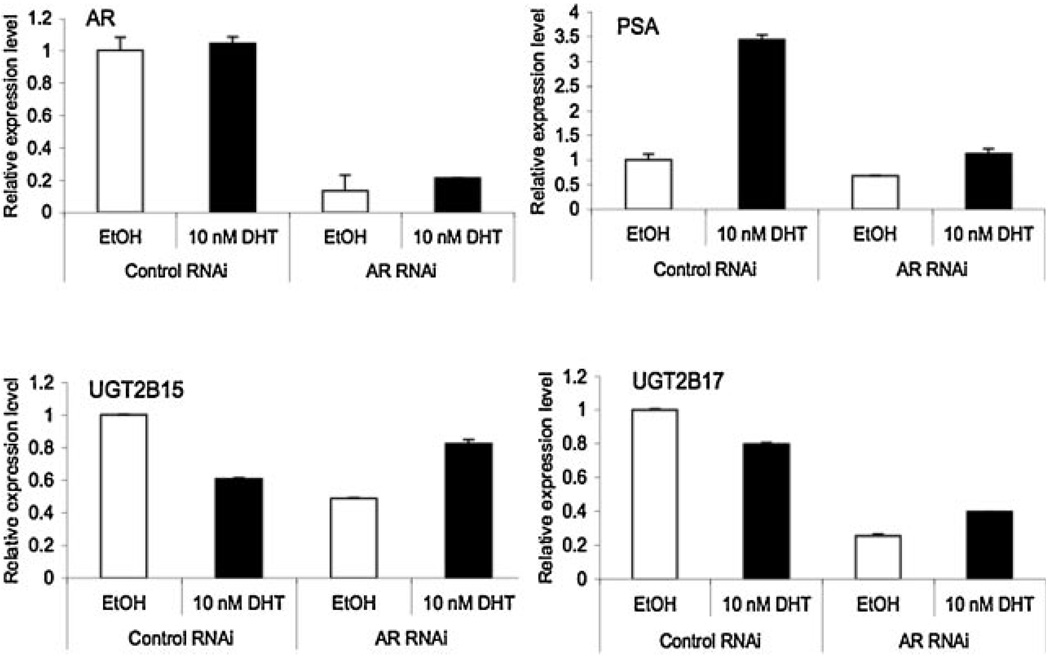
The Impact of AR RNAi on the Regulated Expression of UGT2B15 and B17 Genes
To further confirm the essentiality of AR in the basal level transcription of UGT2B15 and B17 and in their androgen induced down-regulation of gene expression, we determined the impact of AR RNAi, which knocks down the AR expression, on the mRNA levels of the UGT2B15, B17 and PSA (as a control) in the presence or absence of DHT treatment. The AR RNAi was transiently introduced into LNCaP cells, 48 hr post-transfection, cells were treated with or without DHT for another 24 hr and then mRNA levels of various genes were determined by qRT-PCR. As shown in Figure 6, introduction of the AR RNAi did not completely abolish but significantly reduced the AR mRNA level (to ∼15–20%), indicating the efficiency of the AR RNAi. We reproducibly noticed that addition of 10 nM DHT slightly increased the AR mRNA, even in the presence of the AR RNAi (Fig. 6, panel AR). Knocking down AR expression decreased the PSA mRNA level by 30% in the absence of DHT stimulation and it also significantly blocked the efficiency of DHT stimulated induction of PSA expression (Fig. 6, panel PSA). Down-regulation of AR in the absence of androgens also significantly reduced the UGT2B15 mRNA to 60% and reduced the UGT2B17 mRNA to 25%, compared to those without AR RNAi (Fig. 6, panels UGT2B15 and UGT2B17). In addition, DHT failed to further down-regulate UGT2B15 and B17 in AR RNAi-transfected cells (Fig.6, panels UGT2B15 and UGT2B17). We also reproducibly observed a relatively higher level of expression of UGT2B15 and B17 in AR RNAi transfected and DHT treated cells, compared to those AR RNAi transfected but without DHT treatment. This increased expression of UGT2B15 and B17 could be due to the slightly increased AR expression in AR RNAi transfected cells that were treated with DHT. In sum, our results suggested that AR is required for the basal expression of UGT2B15 and B17 and is also involved in the DHT-induced down-regulation of UGT2B15 and B17.
Fig. 6.
The impact of ARRNAion UGT2B15 and B17 expression. LNCaP cells were transfected with control and ARRNAi, respectively. Forty eight hours after transfection, cells were treated with ethanol vehicle or 10 nM DHT for another 24hr, and then total RNAs were isolated. AR, PSA, UGT2B15, and UGT2B17 mRNA expression were analyzed by real-time RT-PCR. Values represent the fold differences in gene expression relative to control. Each value represents the mean from two independent experiments.
DISCUSSION
UGT2B15 and B17 are highly homologous to each other and are expressed in human prostate and several AR positive prostate cancer cell lines [12,14,26–28]. These two enzymes are critical for the inactivation of DHT and its metabolites 3α-DIOL and ADT. Glucuronidation is a significant mediator of the androgen response in prostate cells [17]. It has been intriguing to observe that androgens rapidly down-regulate the glucuronidation activity and the expression of UGT2B15 and B17 in LNCaP cells, perhaps as a means of facilitating their biological activities [18–20].
We demonstrate herein several pieces of independent evidence indicating that AR modulates the expression of UGT2B15 and B17 in prostate cancer cells. First, the UGT2B15 and B17 genes are only expressed in prostate cancer cell lines that express AR. Second, the down-regulation of UGT2B15 and B17 by androgen is time and dose dependent and this process can be blocked by anti-androgens. Third, antiAR CHIP analysis confirmed the association of AR with the promoter regions of UGT2B15 and B17 genes. Finally, knocking down AR expression by AR RNAi reduced the expression of UGT2B15 and B17 in the absence of androgens, and also blocked the androgen-induced down-regulation of UGT2B15 and B17 genes. Based on these results, we hypothesize that in LNCaP cells, AR is required for the basal transcription of the UGT2B15 and B17 genes; though an AR-DHT complex enhances the binding of AR to the UGT2B15 and B17 gene locus, recruiting co-repressors leading to the down-regulation of UGT2B15 and B17 gene expression. Currently, we are engaged in the investigation of putative co-repressors and chromatin markers that potentially involved in the mechanistic control of UGT2B15/B17 expression, specifically on how their expression is up-regulated by AR in the absence of ligand and then repressed by the presence of ligand.
Increased testosterone uptake or synthesis has been thought to be a potential cause for the enhanced AR activity in the progression of prostate cancer to CRPC. This hypothesis was supported by the observation of the increased expression of HSD3B2, AKR1C3, SRD5A1, potentially enhancing intracellular conversion of adrenal androgens to testosterone and DHT, in CRPC. Following this rationale, we anticipate that the enhanced AR activity in CRPC should down regulate the UGT2B15 and B17 genes. However, in the LNCaP-abl androgen independent cells and also in CRPC, the expression level of UGT2B15 and B17 is up-regulated; and UGT2B15 and B17 genes in LNCaP-abl cells are no longer responsive to DHT stimulation. We postulated that the process of recruiting repressors might be hampered in LNCaP-abl cells and/or in CRPC via altered chromatin structure at UGT2B15 and B17 locus, altered conformation of DHT-AR, altered repressors or missing repressors. These hypotheses remain to be validated. The lack of androgen regulated PSA expression in LNCaP-abl cells is also suggestive of an altered co-regulator status or altered chromatin structures, given that both the AR and upstream AR binding sites are present in this cell line. In sum, our finding of AR being a necessary component for UGT2B15 and B17 gene expression explains the altered expression of UGT2B15 and B17 genes observed in conjunction with changes of AR or AR related function as reported [10,11,19–22,29,30]. The increased expression of UGT2B15 and B17, presumably facilitating androgen catabolism, in CRPC is apparently paradoxical to the relatively high levels of testosterone observed in androgen-independent prostate biopsy samples [6,31,32]. The biological significance of the role of UGT2B15 and B17 in the development of androgen independence in CRPC is not clear. Though their altered expression may not be a cause but may represent a consequence of the development of CRPC.
The ChIP-on ChIP analysis identified two AR binding sites located within 1.0 or 1. 35 kb upstream of the UGT2B15 and B17 genes and our regular ChIP confirmed the association of AR with the promoter regions of the UGT2B15 and B17 genes. Following the CHIP study, we searched for AREs in the promoter region of UGT2B15 and B17 genes by in silico analysis for sequence similarity with consensus AREs [20]. Putative AREs (sequences similar to AGTACATTT-GTTCT; http://bio.chip.org/mapper) can be found at −916 to −930 upstream region of the UGT2B15 and at −823 to −837 upstream region of the UGT2B17 gene. When comparing to the AGAACAnnnTGTTCT ARE consensus sequence, we found a putative ARE-AGAA-CttcagcTTCT of three mismatched nucleotides located at −923 to −937 upstream of UGT2B15 (or at −865 to −879 upstream of UGT2B17), a TGTTCT ARE half site at −768 to −773 upstream of UGT2B15 (or −710 to −715 upstream of the UGT2B17), and a AGAACA ARE half site at −201 to −206 upstream of UGT2B15 (or −142 to −148 upstream of the UGT2B17). The biological significance of these AREs and ARE half sites is currently unclear and will be characterized and presented separately. However, as noted by Wang et al., [25] the majority of AR binding regions mapped on chromosomes 21 and 22 in prostate cancer cells contains noncanonical AREs.
It has been reported that both estrogen and DHT can up regulate the expression of UGT2B15 gene in the estrogen receptor (ER) positive breast cancer cells via ER [21,22]. In addition, the expression of UGT2B15 gene can also be up-regulated by 17β-estradiol in the androgen independent LuCaP 35V as determined by RNA expression profiling [22]. It is possible that ER coordinates with AR and/or other co-regulators in the control of UGT2B15 gene expression and this possibility remains to be further investigated. Interestingly, it has been reported that steroid-induced AR-ERβ-Src complex triggers prostate cancer cell proliferation and a p21-activated kinase PAK6 can interact with both ER and AR [33,34].
ACKNOWLEDGMENTS
This work was supported by a Prostate Cancer SPORT grant 2P50CA90381 (P.W.K.).
REFERENCES
- 1.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 2.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 4.Balk SP. Androgen receptor as a target in androgen independent prostate cancer. Urology. 2002;60:132–138. doi: 10.1016/s0090-4295(02)01593-5. [DOI] [PubMed] [Google Scholar]
- 5.Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, Reuter V, Gerald WL. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohler JL, Gregory CW, Ford OH, III, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 7.Gregory CW, Hamil KG, Kim D, Hall SH, Pretlow TG, Mohler JL, French FS. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res. 1998;58:5718–5724. [PubMed] [Google Scholar]
- 8.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 9.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of and rogen-refractory prostate cancer cells. Cancer Res. 2002;62:1008–1013. [PubMed] [Google Scholar]
- 10.Chang GT, Blok LJ, Steenbeek M, Veldscholte J, van Weerden WM, van Steenbrugge GJ, Brinkmann AO. Differentially expressed genes in androgen-dependent and -independent prostate carcinomas. Cancer Res. 1997;57(18):4075–4081. [PubMed] [Google Scholar]
- 11.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 12.Beaulie M, Levesque E, Hum DW, Belanger A. Isolation and characterization of a novel cDNA encoding a human UDP-glucuronosyltransferase active on C19 steroids. J Biol Chem. 1996;271:22855–22862. doi: 10.1074/jbc.271.37.22855. [DOI] [PubMed] [Google Scholar]
- 13.Tukey RH, Strassburg CP. Human UDP-glucuronosyltransfer-ases: Metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 14.Chouinard S, Pelletier G, Belanger A, Barbier O. Cellular specific expression of the androgen-conjugating enzymes UGT2B15 and UGT2B17 in the human prostate epithelium. Endocr Res. 2004;30(4):717–725. doi: 10.1081/erc-200044014. [DOI] [PubMed] [Google Scholar]
- 15.Belanger A, Brochu M, Lacoste D, Noël C, Labrie F, Dupont A, Cusan L, Caron S, Couture J. Steroid glucuronides: Human circulatory levels and formation by LNCaP cells. J Steroid Biochem Mol Biol. 1991;40(4–6):593–598. doi: 10.1016/0960-0760(91)90281-9. [DOI] [PubMed] [Google Scholar]
- 16.Roy AK. Regulation of steroid hormone action in target cells by specific hormone-inactivating enzymes. Proc Soc Exp Biol Med. 1992;199(3):265–272. doi: 10.3181/00379727-199-43356a. [DOI] [PubMed] [Google Scholar]
- 17.Chouinard S, Barbier O, Belanger A. UDP-glucuronosyltransfer-ase (UGT)2B15 and UGT2B17 enzymes are major determinants of the androgen response in prostate cancer LNCaP cells. J Bio Chem. 2007;282(46):33466–33474. doi: 10.1074/jbc.M703370200. [DOI] [PubMed] [Google Scholar]
- 18.Kanaya J, Takashima M, Koh E, Namiki M. Androgen-independent growth in LNCaP cell lines and steroid uridine diphosphate-glucuronosyltransferase expression. Asian J Androl. 2003;5(1):9–13. [PubMed] [Google Scholar]
- 19.Guillemette C, Hum DW, Belanger A. Regulation of steroid glucuronosyltransferase activities and transcripts by androgen in the human prostatic cancer LNCaP cell line. Endocrinology. 1996;137(7):2872–2879. doi: 10.1210/endo.137.7.8770908. [DOI] [PubMed] [Google Scholar]
- 20.Guillemette C, Levesque E, Beaulieu M, Turgeon D, Hum DW, Belanger A. Differential regulation of two uridine diphospho-glucuronosyltransferases, UGT2B15 and UGT2B17, in human prostate LNCaP cells. Endocrinology. 1997;138(7):2998–3005. doi: 10.1210/endo.138.7.5226. [DOI] [PubMed] [Google Scholar]
- 21.Harrington WR, Sengupta S, Katzenellenbogen BS. Estrogen regulation of the glucuronidation enzyme UGT2B15 in estrogen receptor-positive breast cancer cells. Endocrinology. 2006;147(8):3843–3850. doi: 10.1210/en.2006-0358. [DOI] [PubMed] [Google Scholar]
- 22.Coleman IM, Kiefer JA, Brown LG, Pitts TE, Nelson PS, Brubaker KD, Vessella RL, Corey E. Inhibition of androgen-independent prostate cancer by estrogenic compounds is associated with increased expression of immune-related genes. Neoplasis. 2006;8(10):862–878. doi: 10.1593/neo.06328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao BY, Yeh SD, Lee YF. 1alpha, 25-dihydroxyvitamin D3 inhibits prostate cancer cell invasion via modulation of selective proteases. Carcinogenesis. 2006;27(1):32–42. doi: 10.1093/carcin/bgi170. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Carroll JS, Brown M. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell. 2005;19(5):631–642. doi: 10.1016/j.molcel.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Li W, Liu XS, Carroll JS, Jänne OA, Keeton EK, Chinnaiyan AM, Pienta KJ, Brown M. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27:380–392. doi: 10.1016/j.molcel.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turgeon D, Carrier JS, Lévesque E, Beatty BG, Bélanger A, Hum DW. Isolation and characterization of the human UGT2B15 gene, localized within a cluster of UGT2B genes and pseudogenes on chromosome 4. J Mol Biol. 2000;295:489–504. doi: 10.1006/jmbi.1999.3374. [DOI] [PubMed] [Google Scholar]
- 27.Beaulieu M, Levesque E, Tchernof A, Beatty BG, Belanger A, Hum DW. Chromosomal localization, structure, and regulation of the UGT2B17 gene, encoding a C19 steroid metabolizing enzyme. DNA Cell Biol. 1997;16(10):1143–1154. doi: 10.1089/dna.1997.16.1143. [DOI] [PubMed] [Google Scholar]
- 28.Chouinard S, Pelletier G, Belanger A, Barbier O. Isoform-specific regulation of uridine diphosphate-glucuronosyltransferase 2B enzymes in the human prostate: Differential consequences for androgen and bioactive lipid inactivation. Endocrinology. 2006;147:5431–5442. doi: 10.1210/en.2006-0229. [DOI] [PubMed] [Google Scholar]
- 29.Febbo PG, Richie JP, George DJ, Loda M, Manola J, Shankar S, Barnes AS, Tempany C, Catalona W, Kantoff PW, Oh WK. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2005;11(14):5233–5240. doi: 10.1158/1078-0432.CCR-05-0299. [DOI] [PubMed] [Google Scholar]
- 30.Fromont G, Chene L, Vidaud M, Vallancien G, Mangin P, Fournier G, Validire P, Latil A, Cussenot O. Differential expression of 37 selected genes in hormone-refractory prostate cancer using quantitative taqman real-time RT-PCR. Int J Cancer. 2005;114(2):174–181. doi: 10.1002/ijc.20704. [DOI] [PubMed] [Google Scholar]
- 31.Geller J, Albert JD, Nachtsheim DA, Loza D. Comparison of prostatic cancer tissue dihydrotestosterone levels at the time of relapse following orchiectomy or estrogen therapy. J Urol. 1984;132:693–696. doi: 10.1016/s0022-5347(17)49829-6. [DOI] [PubMed] [Google Scholar]
- 32.Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004;10:7121–7126. doi: 10.1158/1078-0432.CCR-04-0913. [DOI] [PubMed] [Google Scholar]
- 33.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbon-danza C, Auricchio F. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SR, Ramos SM, Ko A, Masiello D, Swanson KD, Lu ML, Balk SP. AR and ER interaction with a p21-Activated Kinase (PAKT6) Mol Endocrinol. 2002;16(1):85–99. doi: 10.1210/mend.16.1.0753. [DOI] [PubMed] [Google Scholar]