Abstract
Background
Prostate cancer affects one-out-of-six men during their lifetime. Dietary factors are postulated to influence the development and progression of prostate cancer. Low-fat diets and flaxseed supplementation may offer potentially protective strategies.
Methods
We undertook a multi-site, randomized controlled trial to test the effects of low-fat and/or flaxseed-supplemented diets on the biology of the prostate and other biomarkers. Prostate cancer patients (n=161) scheduled at least 21 days before prostatectomy were randomly assigned to one of the following arms: 1) control (usual diet); 2) flaxseed-supplemented diet (30 g/day); 2) low-fat diet (<20% total energy); or 4) flaxseed-supplemented, low-fat diet. Blood was drawn at baseline and prior to surgery and analyzed for prostate specific antigen (PSA), sex hormone binding globulin, testosterone, insulin-like growth factor-1 and binding protein-3, c-reactive protein, and total and low density lipoprotein cholesterol. Tumors were assessed for proliferation (Ki-67, the primary endpoint) and apoptosis.
Results
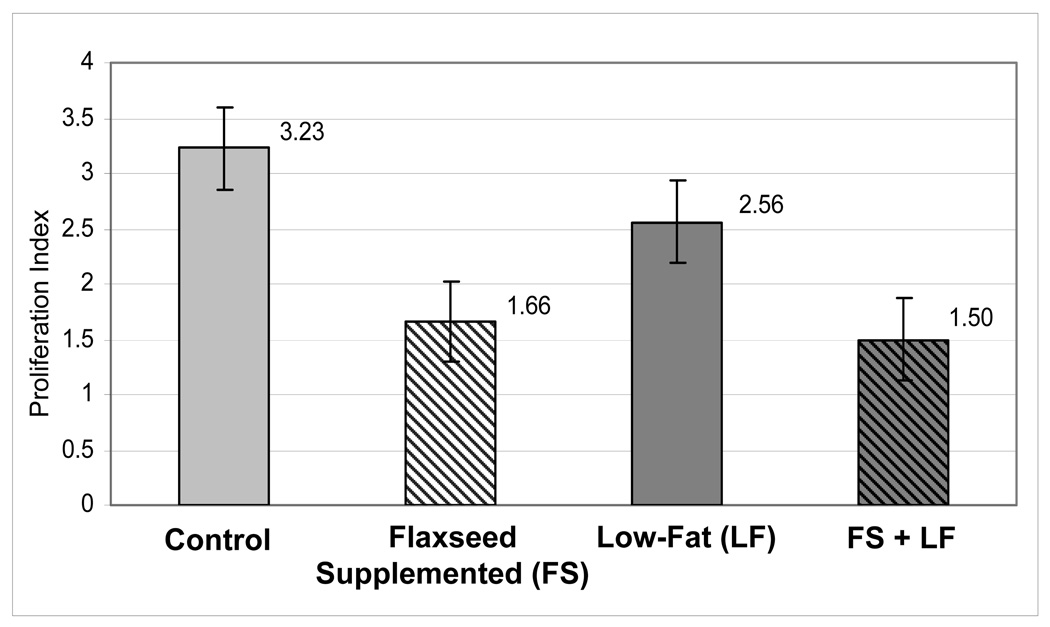
Men were on protocol an average of 30 days. Proliferation rates were significantly lower (P < 0.002) among men assigned to the flaxseed arms. Median Ki-67 positive cells/total nuclei ratios (x100) were 1.66 (flaxseed-supplemented diet) and 1.50 (flaxseed-supplemented, low-fat diet) vs. 3.23 (control) and 2.56 (low-fat diet). No differences were observed between arms with regard to side effects, apoptosis, and most serological endpoints; however, men on low-fat diets experienced significant decreases in serum cholesterol (P=0.048).
Conclusions
Findings suggest that flaxseed is safe, and associated with biologic alterations that may be protective for prostate cancer. Data also further support low-fat diets to manage serum cholesterol.
Introduction
This year in the U.S., approximately 186,320 men will be diagnosed with prostate cancer and 28,660 will die from it (1). Diet is presumed to play a major role in prostate cancer, yet few studies have prospectively explored the efficacy of dietary interventions in either the preventive or complementary care settings (2.3). While several dietary factors may be important for prostate cancer (2,3), we undertook a randomized controlled trial to determine the effects of flaxseed supplementation and a low-fat diet on the biology of prostate cancer and associated biomarkers, since our previous studies (4–8), and the work of others suggested potential benefit (9–12).
Flaxseed, an oilseed commonly consumed in the Middle Ages as a component of breads and cereals, has largely vanished from the modern-day food supply because of its abbreviated shelf-life (13). Given its unique nutrient profile, however, flaxseed has gained recent attention as a potential functional food (14,15). First, flaxseed is an exceptionally rich source of dietary lignan, possessing over 800-fold the amount in most other foods (13,14). Previous research suggests that lignan demonstrates anti-mitotic, anti-angiogenic, anti-oxidant and phytoestrogenic effects (9,11,15). Furthermore, lignan has been shown to reduce testosterone (total and free), and 5α-reductase, the enzyme which converts testosterone to its most active form, dihydrotestosterone (9,11). Such effects may be important for prostate cancer, a hormonally-driven neoplasm (2,3). Additionally, flaxseed is a rich source of plant-based omega-3 fatty acids (ω3FA), which have been shown to increase natural killer cell activity, alter tyrosine kinase cell signaling pathways, inhibit cell membrane synthesis, affect cell receptor status, and influence the eicosanoid milieu (i.e., suppressed production of prostaglandins E2 and I2, and 5-hydroxyeicosatetraenoic acid via cyclooxygenase and lipoxygenase pathways)(16). Despite the favorable effects of ω3FAs, the role of α-linolenic acid (ALA), the predominant fatty acid in flaxseed, is unclear (17). Some reports link ALA to decreased risk of prostate cancer or find no association with risk (18–20), while others suggest increased risk, though such findings come largely from observational studies where food sources of ALA were predominantly meat, dairy products, and salad dressings (not flaxseed) (21,22). It has been suggested that the metabolism of ALA may vary depending upon the concurrent intake of ω6FAs, i.e., that biochemical conversion of ALA to longer chained ω3FAs, eicosapentanoic (EPA) and docosahexanoic acids (DHA) is enhanced if ALA is consumed simultaneously with a reduced intake of ω6FAs, as in low-fat diets (23). Given this rationale, our pilot studies of flaxseed-supplementation have always employed concurrent dietary fat restriction (5,6). However, low-fat diets have independently been associated with reduced risk of prostate cancer (10,12), though results have been inconsistent (24,25). Thus, there was a need to disentangle the potential effects of flaxseed supplementation and dietary fat restriction using a rigorous randomized controlled approach, and to determine whether these effects operate independently or synergistically. Herein, we report the results of a NCI-funded Phase II randomized clinical trial (NCT00049309) that employed a pre-surgical model to assess the impact of flaxseed supplementation and/or dietary fat restriction on the biology of prostate cancer and associated biomarkers.
PATIENTS AND METHODS
Study Overview
A detailed description of the methods used in this trial are reported elsewhere (26). In brief, the trial employed a 2×2 factorial design, with the presence or absence of the two factors, flaxseed supplementation and dietary fat restriction, defining the following four treatment arms: 1) control (usual diet); 2) flaxseed-supplemented diet; 3) low-fat diet; and 4) flaxseed-supplemented, low-fat diet. The specific aims were to determine: 1) differences between study arms in tumor proliferation, as assessed by Ki-67 staining of prostatectomy specimens (primary aim), as well as rates of apoptosis (secondary aim); 2) differences between study arms with regard to change in serum PSA, total testosterone, sex hormone binding globulin (SHBG), insulin-like growth factor (IGF)-1 and IGF binding protein-3 (IGFBP-3), c-reactive protein (CRP), and serum lipids (secondary aim); 3) the effects of flaxseed supplementation and/or a low-fat diet on nutritional biomarkers, i.e., levels of lignans in the urine and seminal fluid, and fatty acid profiles of erythrocytes and prostatic tissue; and 4) to explore associations between dietary change, and changes in nutritional biomarkers, hormonal intermediates, and study endpoints (secondary aim).
Study Population
Patients with biopsy-confirmed prostatic carcinoma electing prostatectomy as their primary treatment, and at least 21 days from scheduled surgery were enrolled from Duke University Medical Center (DUMC), the Durham Veteran’s Administration Medical Center, and five sites within the University of Michigan Community Clinical Oncology Program Research Base. Only mentally-competent, English-speaking- and -writing men with telephone access were considered since evaluative surveys and intervention delivery relied on telephone counseling and written materials. Other exclusion criteria were: 1) recent flaxseed-use and/or adherence to a diet ≤ 30% of kcal from fat (patients’ diets were screened using the NCI Percent Energy from Fat Screener)(27); 2) dietary supplements started within the past 3-months (exception: standard multi-vitamin and mineral preparations); 3) current antibiotic-use (antibiotics reduce the intestinal microflora that convert dietary lignans to biologically-active mammalian-based lignans) (13,28); or 4) history of hormonal or other neoadjuvant therapies. The study was approved by the institutional review boards at each center. All participants provided written informed consent.
Baseline Measures
Participants received instructions and supplies to collect a chilled 24-hour urine and ejaculate sample. Men also were asked to complete the NCI Diet History Food Frequency Questionnaire (29), and the Aerobics Center Longitudinal Study Physical Activity Questionnaire (30). A baseline visit after a 12-hour fast was scheduled at least 14-days post-biopsy and at least 3-days post-digital rectal exam (31). At this visit, heights and weights were measured (26), surveys were reviewed, sociodemographic and medical history information was recorded, and biological samples were collected.
Blood was drawn via venipuncture (21.5 cc) and configured into plasma and sera, and cryovials were prepared and stored at −70° C until completion of the study, whereupon samples were batch-analyzed at a commercial laboratory (LabCorp, Inc., Burlington, NC) via immunochemiluminometric assay for PSA, total testosterone, SHBG, total and low density cholesterol, CRP, IGF-1 and IGFBP-3 (26). These tests were selected since previous studies suggest that these biomarkers are associated with prostate cancer growth and/or are influenced by a low-fat diet or flaxseed supplementation (25,26). Erythrocytes were washed repeatedly with saline and stored at −70 °C until study completion whereupon erythrocyte membranes were batch-analyzed via capillary gas chromatography for fatty acid composition (32,33).
Start and stop times for 24-hour urine collections were recorded, and samples were measured for volume and aliquotted. Creatinine was measured using kinetic methods (Duke University Health Systems Clinical Laboratories and LabCorp, Inc.) to confirm 24-hour collection and to use as a benchmark for expressing lignan excretion (marker of dietary adherence to the flaxseed-supplemented regimen). Remaining cryovials were stored at −70 °C until completion of the study whereupon they were batch-analyzed. Urinary lignans were hydrolyzed and quantified via high performance liquid chromatography (HPLC) using techniques described previously (34,35).
Collection times and volumes of ejaculate samples were noted. Samples were held at room temperature until liquefaction was complete and centrifuged at 2500 rpm for 10 minutes. The resulting supernatant (seminal fluid) was pipetted-off, evenly aliquotted into two 2 cc Teflon-stoppered cryovials, and stored at −70 °C until completion of the study whereupon it was batch-analyzed. Seminal fluid lignans were assessed using the same HPLC methods described above (34,35). As in the urine, converted plant-lignans are expressed in the seminal fluid and have been associated with reduced risk for prostate cancer in previous studies (9,34).
Randomization and Interventions
After all baseline data and biospecimens were collected, the Duke Clinical Trials Office (located off-site) randomly assigned participants using stratification variables of race (black vs. non-black) and biopsy Gleason sum (< 7 vs. ≥ 7) to one of the following arms:
Control
Men in this arm were asked to continue their usual diet.
Flaxseed-Supplementation (FS)
Men assigned to this arm were provided with ample ground flaxseed to last until their date of surgery. To reduce the variability in nutrient composition that could occur between crops, the flaxseed used for this study was obtained from ENRECO, Inc. (Manitowoc, WI) in one lot (150 kg), and was analyzed for nutrient content at two time points during the study period. Given its propensity for rancidity (13), the flaxseed was stored in whole grain form under cold storage (4 °C), and ground and packaged in daily dose (30g) sealed opaque packets as needed; the dose of 30 g (~3 rounded Tablespoons) was chosen based on positive effects observed with an identical dose in our pilot studies among men with prostate cancer, as well as a similar dose, i.e., 25 g used successfully by Thompson et al. in a clinical trial among women with breast cancer (36). Starter kits with stepped doses of ground flaxseed were provided, i.e., 10g for days 1–3, 20g for days 4–6, and 30g for day 7 and beyond; a stepped-dose was used to accustom the gut to the considerable fiber load imposed by the flaxseed (~9g of fiber/30g dose). Men receiving flaxseed also were instructed to drink at least 64 oz of fluids/day to reduce potential risk of colonic impaction or dehydration resulting from the increased fiber load (13,37), and to keep their flaxseed packets under refrigeration (to retard spoilage). Participants in this arm also were provided with logs to record their daily intakes of flaxseed to the nearest quarter of a packet, and to return any unused packets at follow-up. These procedures were adapted from pill counts which provide a valid measure of adherence in pharmacologic trials (including fiber supplement trials)(37,38).
Low-fat Arm (LF)
Men randomized to this arm were instructed by registered dietitians on a diet with ≤ 20% of energy from dietary fat. Fat gram “budgets” were individually calculated using the following formula: ideal body weight (lb) × 15 × 0.2 kcal from fat/9 kcal/gram. Men were provided with fat gram counters (T-Factor 2000, W.W. Norton, Inc., NYC) and instructed to record all foods consumed with corresponding fat gram counts, and to tally their number of fat grams daily. Participants also received written and verbal instruction on meal planning, food preparation, shopping, and dining.
Flaxseed-supplemented, Low-fat Diet (FS+LF) Arm
Men in this arm received instruction and supplies for both of the diet regimens described above.
Men in all arms were contacted weekly by study staff to maintain contact, assess and reinforce adherence, and answer any diet-related questions. Additionally, participants’ wives or partners were encouraged to attend the baseline appointment.
End-of-Study Measures
Follow-up visits were conducted within three days of surgery. All baseline measures (except height) were repeated, and changes in health status and medication-use were assessed. Potentially relevant side effects, i.e., nausea, vomiting, diarrhea, decreased libido or impotence, and allergy were collected using the NCI Common Toxicity Criteria (version 3.0) (39). Men assigned to diet-modified arms also were asked to report the number of days/week they adhered to their assigned diet regimen and the average amount of flaxseed consumed, if appropriate. Upon prostatectomy, prostatic tissue was retrieved from defined central and peripheral zone regions using a 3 mm biopsy punch. Tissue samples were flash-frozen in liquid nitrogen and stored at −70 °C until study completion.
Histopathologic Outcomes
The primary study pathologist (RV), blinded with regard to study condition, reviewed clinical pathology reports and all slides for each case; he then chose one slide and one block per case for determination of proliferation and apoptosis. Slides (and blocks) were chosen based on the presence of adequate tumor, as well as benign tissue, and the histological grade of tumor on the slide was representative of the entire tumor in the specimen. Proliferation counts were assessed using the antibody MIB-1 for Ki-67 hybridoma clone at a dilution of 1:200 (Biocare, Walnut Creek, CA)(40). This marker has validated-use in nutrition intervention trials (41) and has been endorsed by the Prostate Cancer Chemoprevention Trial Consensus Panel as an accurate and reproducible measure (41). The labelled strepto-avidin/biotin/peroxidase/diaminobenzidine tetrachloride (DAB) method (Biocare, Walnut Creek, CA) was used with antigen retrieval by pressure-cooking in citrate buffer (DAKO, Inc., Carpintera, CA). Slides were counterstained with hematoxylin, and tonsillar tissue with lymphoid hyperplasia served as a control. Prepared slides were independently reviewed by the primary and secondary study pathologists (RV, JFM), both of whom were blinded to study condition. The following method was used: 1) at low magnification, a random starting point in the tumor was chosen; and 2) at high magnification, sequentially-encountered tumor cell nuclei were evaluated for Ki-67 positivity. The result was reported as the ratio of positive nuclei divided by the total number evaluated × 100.
The degree of apoptosis was measured using the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-nick end-labeling (TUNEL) method [TdT-FragEL kit (with manufacturer control), Ongogene, Boston, MA](42). Pretreated slides were incubated in biotin-labeled/unlabeled deoxynucleotides containing TdT at 37°C for 1.5 hours. The biotinylated nucleotides were detected using a streptoaviden-horseradish peroxidase conjugate and then reacted with diaminobenzidine, forming an insoluble, brown precipitate at the site of DNA fragmentation. Using light microscopy and assessing the degree of nuclear staining against a methyl green counterstain, labelled nuclei were then evaluated. Because preliminary results with the TUNEL stain indicated a very low number of positive nuclei, formal counts were not done. Instead, many microscopic fields (well over 1000 cells) were examined, and staining for TUNEL was ranked by the study pathologists as follows: score of “0” for no positive cells to rare cells, “1” for occasional positive cells, and “2” for frequent positive cells. Averaged values of the two pathologists’ scores were then used in statistical analyses (see reference 26 for greater detail and issues regarding histopathologic assessments and analysis).
Statistical Design and Analysis
This trial employed a 2×2 factorial design, with the presence or absence of flaxseed supplementation and low-fat, generating the four treatment arms. Thus, there were two primary tests, one for flaxseed supplementation and one for low-fat. The primary statistical outcome variable was proliferation rate. Our preliminary studies suggested that the combination of flaxseed supplementation with a low-fat diet resulted in log proliferation rates that were on average 33% lower than the rates observed among control subjects, or an effect size of approximately 0.56. Here, effect size was defined as the absolute value of the ratio of the differences of the two means to the (common) standard deviation. For a sample size of 128, the asymptotic power of the two-sided, two-sample t-test, at a level of 0.05, is 0.90 for detecting an effect size of 0.50, assuming that the proliferation indices in both arms are log-normal with common variance. The accrual target was set at 160 patients (40/arm) to account for attrition and the possibility of a weak negative interaction between flaxseed supplementation and low-fat factors with respect to proliferation rate. While power calculations were based upon the primary endpoint (proliferation index), data from our pilot study suggested that there also would be comparable power to detect differences between arms with respect to secondary outcomes. No adjustment was made for multiple comparisons since these analyses were considered exploratory.
Analyses were based on the intent-to-treat principle, and all participants were included in the arm to which they were randomized. For the primary hypotheses, the analysis population was restricted to those patients from whom cell counts (numerator and denominator) were available from both readers. Kruskal-Wallis tests were performed on the number of evaluated cells to insure that denominators did not differ between study arms; they did not (p =0.90). For each study participant, the proliferation “score” was defined as the log of the average of the proliferation index from each reader.
Per protocol, the association between the log-transformed proliferation score and flaxseed supplementation, and the log-transformed score and low-fat were to be tested using a two-sample t-test. For cases where the score was zero, the minimum non-zero score was imputed. We also used a Generalized Estimating Equation approach (Poisson variance function) as well as a Generalized Linear Mixed Effect approach (Poisson distribution conditional on the random effects) to model the proliferation counts as a function of the experimental factors and co-variables while accounting for the variability between the readers and among the patients. However, the results from these approaches added little to the simpler approaches. Analyses revealed no evidence that the low-fat diet was associated with the proliferation score; however, there was strong statistical evidence that flaxseed supplementation was associated with the score. Thus, to follow-up further on the flaxseed supplementation result, we employed a battery of sensitivity analyses. Unstratified and stratified (by low-fat) two-sample Wilcoxon tests were used to assess sensitivity with respect to the assumption of normality, the log-transformation and the imputation for ratios of zero. For two patients, the readings from one of the pathologists were not available. Both of these patients were from the flaxseed-supplemented arms. For these missing data, we imputed the maximum score for the reader to generate a worst case scenario unfavorable to the flaxseed supplementation effect. These results supported our initial finding that flaxseed supplementation is associated with the score.
Following the protocol, standard linear regression models were used to analyze the effect of baseline variables such as race, age, BMI, and biopsy Gleason sum in a multivariate model. For all other univariate analyses, the association between continuous outcomes and factors were tested using t-tests, while the association between frequency outcomes and factors were tested using chi-square tests for contingency tables.
RESULTS
Patients
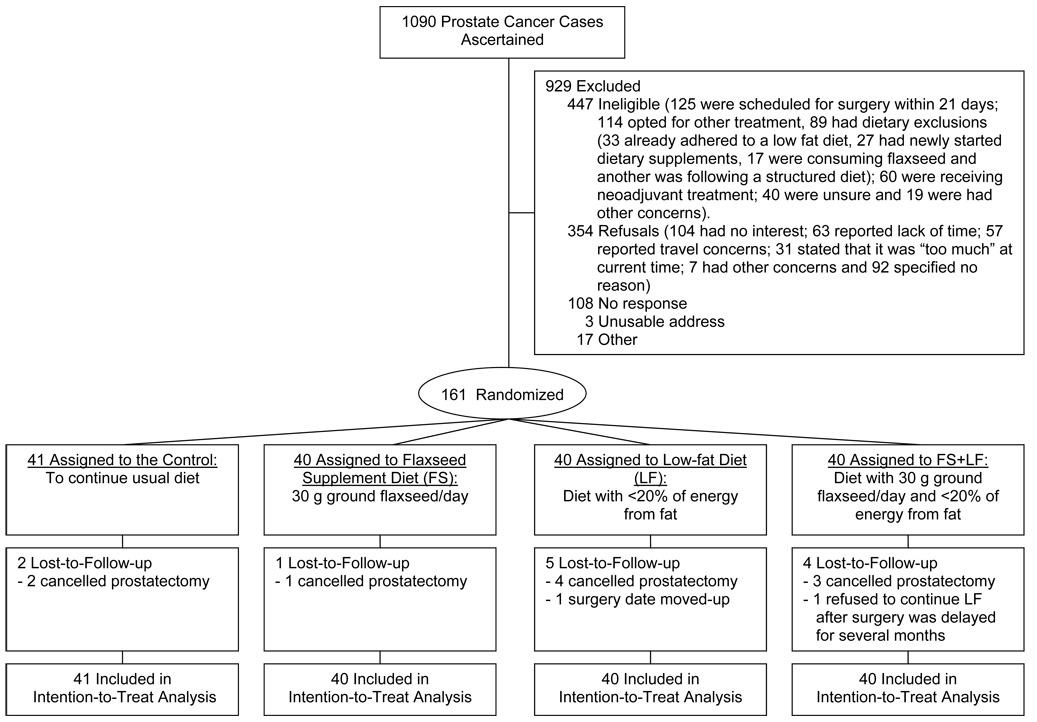
Between July 5, 2002, and April 17, 2006, a total of 1,090 men with histopathologically-confirmed prostatic carcinoma were screened and 161 were randomly assigned to one-of-four treatment arms (Fig. 1). Leading reasons for non-accrual were ineligibility due to selection of other treatment besides prostatectomy or because surgery was scheduled within a 21-day window (see reference 26 for details). Cancelled or rescheduled prostatectomy was the sole reason for attrition; drop-out rates were 7.5%. No age or race differences were observed between participants and non-participants, and study completers and those lost to follow-up. No differences in attrition were observed between study arms.
Fig. 1.
CONSORT tria flow diagram
Table 1 provides the baseline characteristics of the study sample. The trial was successful in accruing a racially representative sample, though the proportion of college-educated participants was higher than the population-at-large, i.e., 42% vs. 31% (43). Data on PSA and biopsy Gleason sum suggested that most participants had earlier-stage disease, with roughly two-thirds having biopsy Gleason sums ≤6. As in the general population, a majority of these middle-aged men were overweight or obese, and substantial numbers had cardiovascular disease or diabetes, and regularly took medications associated with these conditions. No differences existed between study arms with regard to any of these variables.
Table 1.
Characteristics of the study sample*
| Total (n = 161) | Controls (n = 41) | FS (n = 40) | LF (n = 40) | FS + LF (n = 40) | Significance | |
|---|---|---|---|---|---|---|
| Age (years): (Range: 36–73) | ||||||
| Mean ± sd | 59.2 ± 7.3 | 58.2 ± 6.8 | 60.2 ± 7.0 | 59.2 ± 8.0 | 59.3 ± 7.6 | N.S. |
| <65 | 74% (119) | 78% (32) | 73% (29) | 70% (28) | 75% (30) | N.S |
| 65+ | 26% (42) | 22% (9) | 27% (11) | 30% (12) | 25% (10) | |
| Race % (n) | ||||||
| White | 70% (112) | 68% (28) | 67% (27) | 70% (28) | 72% (29) | N.S. |
| African American | 26% (42) | 27% (11) | 28% (11) | 25% (10) | 25% (10) | |
| Other | 4% (7) | 5% (2) | 5% (2) | 5% (2) | 3% (1) | |
| Education % (n) | ||||||
| <High School/unknown | 9% (15) | 7% (3) | 8% (3) | 10% (4) | 13% (5) | N.S. |
| High School Grad/GED | 20% (32) | 22% (9) | 20% (8) | 18% (7) | 20% (8) | |
| Some College/Trade | 29% (46) | 32% (13) | 32% (13) | 30% (12) | 20% (8) | |
| College Grad/Post-grad | 42% (68) | 39% (16) | 40% (16) | 42% (17) | 47% (19) | |
| Current Smoker % (n) | 11% (18) | 15% (6) | 13% (5) | 5% (2) | 13% (5) | N.S. |
| BMI (kg/m2)* % (n) | ||||||
| Mean ± sd | 28.8 ± 4.1 | 28.8 ± 4.0 | 28.5 ± 3.9 | 29.5 ± 4.3 | 28.5 ± 4.5 | N.S. |
| 18.5 – 24.9 (normal weight) [% (n)] | 19% (31) | 20% (8) | 22% (9) | 15% (6) | 20% (8) | |
| 25 – 29.9 (overweight)[% (n)] | 46% (74) | 46% (19) | 45% (18) | 47% (19) | 45% (18) | |
| 30+ (obese) [% (n)] | 35% (56) | 34% (14) | 33% (13) | 38% (15) | 35% (14) | |
| Co-Morbidity [% (n)] | ||||||
| CVD | 35% (57) | 34% (14) | 28% (11) | 38% (15) | 43% (17) | N.S. |
| Diabetes | 17% (27) | 12% (5) | 23% (9) | 15% (6) | 18% (7) | |
| Medication-Use [% (n)] | ||||||
| Non-Steroidal Anti-inflammatory Agents (NSAIDS) | 38% (61) | 39% (16) | 33% (13) | 40% (16) | 40% (16) | N.S. |
| Statins | 25% (41) | 29% (12) | 20% (8) | 30% (12) | 23% (9) | |
| Thiazolidinediones | 8% (13) | 10% (4) | 10% (4) | 3% (4) | 10% (4) | |
| PSA at Diagnosis (ng/ml) % (n) | ||||||
| Mean ± sd | 5.7 ± 3.8 | 5.2 ± 2.7 | 5.2 ± 2.4 | 5.6 ± 5.0 | 6.8 ± 4.3 | N.S. |
| <4 | 23% (38) | 27% (11) | 23% (9) | 28% (11) | 18% (7) | |
| 4 to 10 | 70% (112) | 63% (26) | 72% (29) | 70% (28) | 72% (29) | N.S. |
| 10 to 20 | 6% (9) | 10% (4) | 5% (2) | 0% (0) | 8% (3) | |
| >20 | 1% (2) | 0% (0) | 0% (0) | 2% (1) | 2% (1) | |
| Biopsy Gleason Score | ||||||
| ≤5 (2,2/ 2,3/ 3,2) | 1% (4) | 0 | 5% (2) | 0 | 5% (2) | N.S. |
| 6 (3,3) | 63% (102) | 66% (27) | 60% (24) | 67% (27) | 60% (24) | |
| 7 (3,4) | 24% (39) | 22% (9) | 27% (11) | 27% (11) | 20% (8) | |
| 7 (4,3) | 6% (10) | 7% (3) | 5% (2) | 3% (1) | 10% (4) | |
| ≥ 8 (4,4/ 5,3/ 5,4) | 4% (6) | 5% (2) | 3% (1) | 3% (1) | 5% (2) | |
No participants were underweight (BMI <18.5)
Table 2 presents data on protocol duration, reported side-effects, self-rated adherence, biological markers of adherence, and the impact of dietary modification on body fluids and target tissues. The average duration on study was 30.7 days, with little variation among arms. By-in-large, there was a complete absence or only mild side effects reported. However, several participants reported symptoms of low libido or erectile dysfunction, though no differences were observed between study arms for this or any other side effects.
Table 2.
Days on protocol, side effects, self-rated adherence, biomarkers of adherence and nutrition-related changes in target tissues
| Controls (N=41) | Flaxseed Supplementation (FS) (N=40) | Low-Fat (LF) (N=40) | FS + LF (N=40) | P-Values* | ||
|---|---|---|---|---|---|---|
| Days on Protocol | ||||||
| Median (95% C.I.) | 30 (22–29) | 31 (25–32) | 30 (25–29) | 30 (24–30) | FS=.960 LF=.900 |
|
| Side Effects (grade 1/2/3 toxicity) | ||||||
| Nausea | 7%/ 0%/ 0% | 5%/ 0% / 0% | 21%/ 3% / 0% | 8%/ 0% / 0% | .467 | |
| Vomiting | 0% / 0% / 0% | 0% / 0% / 0% | 0% / 0% / 0% | 0% / 0% / 0% | NA | |
| Diarrhea | 5%/ 3% / 0% | 18%/ 0% / 0% | 6% / 0% / 0% | 14%/ 0% / 0% | .308 | |
| Libido/Impotence | 15%/ 5% / 10% | 8%/ 3% / 8% | 24%/ 0% / 6% | 11%/ 3% / 8% | .633 | |
| Allergy | 0% / 3% / 0% | 0% / 0% / 0% | 0% / 0% / 0% | 0% / 3% / 0% | .436 | |
| Flaxseed (% of 30 g dose taken) | ||||||
| [Median (95% C.I.)] | NA | 97.5 (97.5–100)% | NA | 100 (100–100)% | - | |
| Dietary Lignans μg/day | ||||||
| [Median (95% C.I.)] | Baseline | 253 (192–313) | 282 (217–320) | 263 (182–306) | 289 (223–310) | FS<.0001 |
| Follow-up | 257 (219–292) | 260935 (257229–268591) | 250 (227–329) | 262402 (258984–264174) | LF=.905 | |
| Urinary Lignans ng/mg creatinine | ||||||
| [Median (95% C.I.)] | Baseline | 257 (113–456) | 228 (147–609) | 308 (130–431) | 277 (197–447) | FS<.0001 |
| Follow-up | 239 (123–473) | 10566 (5187–15897) | 415 (194–754) | 10358 (4003–13434) | LF=.493 | |
| Seminal Fluid Lignans μg/ml | ||||||
| [Median (95% C.I.)]*** | Baseline | 182 (157–274) | 180 (162–266) | 202 (176–278) | 195 (139–244) | FS=.013 |
| Follow-up | 262 (139–423) | 430 (202–535) | 293 (91–410) | 362 (137–701) | LF=.797 | |
| Energy Intake | ||||||
| [Median (95% C.I.)] | Baseline | 2187 (1999–2503) | 2131 (1749–2572) | 1769 (1530–1955) | 1748 (1439–2392) | FS= .591 |
| Follow-up | 1959 (1591–2376) | 2143 (1715–2654) | 1689 (1423–1997) | 1536 (1370–1929) | LF=.068 | |
| % Calories from Fat | ||||||
| [Median (95% C.I.)] | Baseline | 34.6 (29.7–41.5) | 35.3 (32.5–37.9) | 36.4 (31.6–38.4) | 34.5 (32.4–38.6) | FS=.092 |
| Follow-up | 34.2 (29.7–39.7) | 34.0 (28.8–37.2) | 27.5 (25.2–31.7) | 24.8 (22.2–28.7) | LF<.0001 | |
| Dietary ω3 Fatty Acids (% kcal) | ||||||
| [Median (95% C.I.)] | ||||||
| α Linolenic (18.3) | Baseline | .074 (.067–.082) | .072 (.062–.078) | .077 (.064–.087) | .076 (.064–.084) | FS<.0001 |
| Follow-up | .076 (.067–.090) | .315 (.260–.392) | .064 (.059–.074) | .433 (.334–.474) | LF=.469 | |
| Eicosopentanoic acid (20:5) | ||||||
| Baseline | .000 (.000–.001) | .001 (.001–.002) | .001 (.001–.002) | .001 (.001–.002) | FS=.701 | |
| Follow-up | .001 (.001–.002) | .001 (.001–.002) | .002 (.001–.002) | .002 (.001–.005) | LF=.005 | |
| Docosahexanoic acid (22:6) | ||||||
| Baseline | .000 (.000–.001) | .001 (.000–.001) | .001 (.000–.001) | .001 (.001–.001) | FS=.163 | |
| Follow-up | .001 (.001–.001) | .001 (.000–.001) | .001 (.001–.001) | .001 (.001–.001) | LF=.006 | |
| Dietary ω6 Fatty Acids (% kcal) | ||||||
| [Median (95% C.I.)] | ||||||
| Linoleic acid (18.2) | Baseline | .745 (.623–.867) | .705 (.632–.772) | .755 (.645–.876) | .799 (.704–.870) | FS=0.780 |
| Follow-up | .782 (.688–.867) | .733 (.679–.803) | .582 (.519–.669) | .616 (.587–.686) | LF<.0001 | |
| Arachidonic acid (20:4) | ||||||
| Baseline | .006 (.005–.007) | .006 (.005–.007) | .006 (.005–.007) | .006 (.005–.007) | FS=.036 | |
| Follow-up | .006 (.005–.007) | .005 (.004–.007) | .006 (.005–.007) | .005 (.004–.006) | LF=.180 | |
| Dietary ω3/6 Fatty Acid Ratio | ||||||
| [Median (95% C.I.)] | Baseline | .11 (.10–.12) | .10 (.09–.11) | .11 (.10–.12) | .11 (.10–.11) | FS<.0001 |
| Follow-up | .10 (.10–.12) | .46 (.36–.60) | .11 (.11–.12) | .71 (.52–.82) | LF=.0102 | |
| Erythrocyte ω3 Fatty Acids (%) | ||||||
| [Median (95% C.I.)] | ||||||
| α Linolenic (18.3) | Baseline | .000 (.000–.000) | .000 (.000–.000) | .000 (.000–.000) | .000 (.000–.000) | FS=.520 |
| Follow-up | .000 (.000–.000) | .000 (.000–.000) | .000 (.000–.000) | .000 (.000–.000) | LF=.290 | |
| Eicosopentanoic acid (20:5) | ||||||
| Baseline | .633 (.539–.765) | .723 (.581–.941) | .651 (.440–.890) | .664 (.613–.793) | FS=.005 | |
| Follow-up | .651 (.533–.793) | .784 (.651–.890) | .475 (.372–.620) | .825 (.702–1.101) | LF=.705 | |
| Docosahexanoic acid (22:6) | ||||||
| Baseline | 5.95 (5.54–6.64) | 6.01 (4.73–6.80) | 5.62 (4.55–6.53) | 5.82 (5.00–6.32) | FS=.077 | |
| Follow-up | 6.03 (5.01–7.09) | 5.51 (4.05–6.72) | 4.92 (4.36–6.38) | 6.07 (5.43–7.08) | LF=.320 | |
| Erythrocyte ω6 Fatty Acids (%) | ||||||
| [Median (95% C.I.)] | ||||||
| Linoleic acid (18.2) | Baseline | 11.11 (10.30–11.72) | 11.04 (10.03–12.11) | 11.05 (9.99–11.85) | 11.09 (10.14–11.45) | FS=.544. |
| Follow-up | 10.46 (9.23–11.53) | 10.83 (9.73–11.53) | 9.71 (8.39–11.64) | 9.94 (9.32–10.83) | LF=.037 | |
| Arachidonic acid (20:4) | ||||||
| Baseline | .347 (.292–.371) | .368 (.293–.428) | .290 (.230–.419) | .336 (.279–.391) | FS=.650 | |
| Follow-up | .294 (.218–.337) | .358 (.259–.390) | .303 (.217–.357) | .324 (.269–.380) | LF=.250 | |
| Erythrocyte ω3/6 Fatty Acid Ratio | ||||||
| [Median (95% C.I.)] | Baseline | .60 (.49–.72) | .62 (.50–.71) | .55 (.44–.72) | .56 (.48–.66) | FS=.480 |
| Follow-up | .61 (.47–.78) | .54 (.42–.76) | .50 (.39–.76) | .64 (.59–.78) | LF=.090 | |
| Prostatic ω3 Fatty Acids (%) | ||||||
| [Median (95% C.I.)] | ||||||
| α Linolenic (18:3) | Follow-up | .000 (.000–.000) | .000 (.000–.000) | .000 (.000–.000) | .000 (.000–.000) | FS=.662 LF=.924 |
| Eicosopentanoic acid (20:5) | ||||||
| Follow-up | .216 (.148–.268) | .300 (.221–.385) | .195 (.133–.259) | .298 (.245–.428) | FS=.010 LF=.970 |
|
| Docosahexanoic acid (22:6) | ||||||
| Follow-up | 3.53 (3.11–4.27) | 3.68 (3.17–4.53) | 3.05 (3.34–4.21) | 3.94 (3.37–4.37) | FS=.580 LF=.130 |
|
| Prostatic ω6 Fatty Acids (%) | ||||||
| [Median (95% C.I.)] | ||||||
| Linoleic acid (18.2) | Follow-up | 9.50 (8.99–10.28) | 9.48 (8.47–10.66) | 9.37 (8.34–10.52) | 9.47 (8.75–10.35) | FS=.210 LF =.820 |
| Arachidonic acid (20:4) | ||||||
| Follow-up | .269 (.235–.307) | .247 (.221–.295) | .259 (.235–.274) | .241 (.231–.285) | FS=.230 LF =.760 |
|
| Prostatic ω3/6 Fatty Acid Ratio | ||||||
| [Median (95% C.I.)] | Follow-up | .40 (.32–.45) | .42 (.34–.50) | .42 (.34–.48) | .43 (.38–.47) | FS=.175 LF=.974 |
For factors that have both baseline and follow-up data, factorial testing was performed under intent-to-treat analysis on change scores and P-values are reported for both FS and LF conditions.
Average dose of flaxseed compared to amount prescribed and/or % of days adhered to <20% of kcal from fat
Note that seminal fluid was unavailable on several participants; the numbers of samples for baseline and follow-up amongst the study arms are as follows: control n=19 and n=13; FS n=17 and n=12; LFD n=22 and n=10; and FSLFD n=16 and n=10, respectively.
Dietary logs revealed good-to-excellent adherence. Adherence to flaxseed supplementation was supported by significantly higher lignan intakes and expression within urine and seminal fluid. Participants assigned to low-fat diets significantly reduced their fat intakes to 25–28% of Calories and had higher dietary intakes of EPA since they substituted fish for red meat in efforts to reduce overall fat consumption. However, the proportion of dietary ω3:ω6FAs was significantly higher among flaxseed-supplemented participants owing to significantly higher intakes of ALA. Both erythrocytes and prostatic tissue had significantly higher levels of EPA (not ALA) in the flaxseed-supplemented arms, suggesting that dietary ALA from flaxseed sources may be converted to longer chained ω3FAs in vivo. The prostatic tissue of flaxseed-supplemented participants also had significantly higher proportions of ω3FAs (ALA+EPA+DHA) to ω6FAs (arachidonic+linoleic acid).
Tumor proliferation rate (primary endpoint) was significantly lower in the flaxseed-supplemented arms (Table 3/Fig. 2). While the significance level varied depending upon the various methods used, findings always remained significant (P-values ranged from 0.0007 – 0.02)(25). Although the flaxseed supplementation effect appeared somewhat stronger among African-American patients and also among those with Gleason sums <7, these results were not statistically significant based on standard statistical interaction tests. In contrast, there was no statistical evidence suggesting an impact of the low-fat diet on proliferation. Furthermore, no differences among treatment arms were noted for apoptosis or Gleason sum.
Table 3.
Histopathologic, Serologic and Physiologic Outcomes
| Controls (N=41) | Flaxseed Supplementation (FS) (N=40) | Low-fat (LF) (N=40) | FS + LF (N=40) | P-Values* | ||
|---|---|---|---|---|---|---|
| Tumor Proliferation Rate (Ki-67) | Median (95% C.I.) | 3.23 (2.42–3.92) | 1.66 (1.13–2.64) | 2.56 (2.00–3.69) | 1.50 (1.05–2.65) | FS=.0013/LF=.661 |
| Tumor Apoptotic Rate (TUNEL) [%(n)] | 0 | 84% (33) | 74% (29) | 74% (26) | 89% (32) | |
| >0–1 | 13% (5) | 16% (6) | 14% (5) | 3% (1) | FS=.880/LF=.730 | |
| >1–2 | 3% (1) | 10% (4) | 12% (4) | 8% (3) | ||
| Gleason Sum [Median (95% C.I.)] | Biopsy | 6 (6 – 7) | 6 (6 – 6) | 6 (6 – 6) | 6 (6 – 7) | FS=.538/LF=.918 |
| Surgical | 7 (6 – 7) | 6 (6 – 7) | 6 (6 – 7) | 7 (6 – 7) | ||
| PSA (ng/ml) [Median (95% C.I.)]† | Baseline | 5.3 (3.7–5.8) | 6.2 (4.8–7.7) | 5.5 (4.6–6.7) | 5.9 (4.9–9.4) | FS=.286/LF=.764 |
| Follow-up | 4.9 (3.5–6.2) | 6.4 (5.0–7.0) | 5.6 (3.9–6.7) | 5.7 (4.9–8.6) | ||
| Testosterone (ng/dL) [Median (95% C.I.)]† | Baseline | 442 (386–458) | 424 (358–475) | 423 (387–476) | 414 (346–446) | FS=.120/LF=.394 |
| Follow-up | 372 (334–429) | 387 (351–430) | 377 (327–399) | 382 (346–448) | ||
| SHBG (nmol/L) [Median (95% C.I.)]† | Baseline | 31 (27–39) | 34 (25–41) | 33 (28–37) | 31 (28–35) | FS=.597/LF=.066 |
| Follow-up | 28 (22–37) | 31 (28–37) | 31 (26–35) | 32 (26–35) | ||
| Free Androgen Index [Median (95% C.I.)]‡ | Baseline | 13.3 (10.9–14.3) | 12.6 (11.5–14.2) | 13.2 (11.8–14.3) | 12.8 (11.9–14.7) | FS=.148/LF=.123 |
| Follow-up | 12.1 (11.0–13.0) | 12.0 (10.9–15.0) | 11.3 (10.2–13.0) | 11.8 (10.6–13.2) | ||
| IGF-1 (ng/ml) [Median (95% C.I.)]† | Baseline | 120 (106–133) | 124 (115–148) | 133 (109–150) | 129 (110–148) | FS=.174/LF=.370 |
| Follow-up | 112 (98–128) | 119 (107–133) | 123 (100–141) | 125 (113–139) | ||
| IGFBP-3 (mg/L) [Median (95% C.I.)]† | Baseline | 4.2 (3.6–4.5) | 4.1 (3.9–4.5) | 4.2 (4.0–4.5) | 4.1 (3.9–4.9) | FS=.859/LF=.853 |
| Follow-up | 3.7 (3.3–4.5) | 4.0 (3.5–4.3) | 3.8 (3.3–4.3) | 3.8 (3.6–4.3) | ||
| CRP (mg/L) [Median (95% C.I.)]† | Baseline | 1.5 (1.1–2.2) | 1.4 (1.0–2.7) | 1.6 (1.2–2.5) | 1.2 (0.9–2.5) | FS=.668/LF=.982 |
| Follow-up | 1.6 (1.1–2.4) | 1.8 (1.3–2.3) | 2.0 (1.1–3.2) | 1.1 (0.8–2.1) | ||
| Total Cholesterol(mg/dL)[Median(95% C.I.)]† | Baseline | 230 (212–252) | 217 (205–245) | 222 (206–240) | 218 (210–228) | FS=.174/LF=.048 |
| Follow-up | 196 (180–226) | 211 (118–220) | 182 (161–192) | 183 (168–206) | ||
| LDL Cholesterol (mg/dL)[Median(95% C.I.)]† | Baseline | 130 (113–144) | 124 (110–138) | 134 (122–146) | 123 (115–141) | FS=.621/LF=.032 |
| Follow-up | 111 (90–128) | 111 (89–121) | 105 (90–124) | 107 (93–121) | ||
| Body Mass Index (kg/m2)[Median (95% C.I.)] | Baseline | 28.9 (27.4–30.0) | 28.0 (26.8–29.8) | 29.0 (27.0–31.0) | 27.1 (25.8–30.4) | FS=.496/LF=.003 |
| Follow-up | 29.1 (27.2–30.0) | 27.6 (26.8–29.7) | 28.0 (26.5–29.5) | 26.9 (25.3–29.5) | ||
For factors that have both baseline and follow-up data, factorial testing was performed under intent-to-treat analysis on change scores and P-values are reported for both FS and LF conditions.
Intra-and inter- assay coefficients of variation, with accompanying means(sd) are as follows: PSA: 1.2%, 4.39(.05) ng/ml and 3.7%, 4.61(.17) ng/ml; Testosterone: 4.4%, 365.4(12.7) and 6.6%, 365.4(18.1); SHBG: 2.5%, 21(.52)nmol/L and 5.2%, 21(1.1)nmol/L; IGF-1: 3.8%, 169(6.9)ng/ml and 5.4%, 169(9.1)ng/ml; IGFBP-3: 4.2%, 3.59(.15)mg/L and 7.2%, 3.59(.26)mg/ml; CRP: 1.8%, 6.2(.07)mg/L and 2.9%, 6.2 (.1)mg/L; Total Cholesterol: 0.8%, 231(5.98)mg/dL and 210.1(5.44)mg/dL; and LDL Cholesterol: 1.1%, 166(3.9)mg/dL and 1.8% 166(4.3)mg/dL.
Free Androgen Index derived using the formula: Total Testosterone/SHBG
Fig. 2.
Median Tumor Proliferation Rates
Over the presurgical study period, serum PSA, testosterone, IGF-1 and IGFBP-3 decreased in all arms, with no differences in change observed between arms. Within the control arm these decreases were significant for testosterone and PSA (P-values < 0.05), and of borderline significance for IGF-1 (P = 0.0547). No between arm differences were observed in change scores for SHBG, free androgen index, and CRP. Participants in the low-fat arms experienced significant reductions in body mass index, and total and low density lipoprotein cholesterol.
Discussion
While other studies have employed presurgical models to test the effects of complementary therapies on prostate cancer (44,45), to our knowledge, this has been the largest effort to date . Not only does it demonstrate the feasibility of implementing complex prevention trials within the community setting, it also suggests that flaxseed is well-accepted, safe to use, and may affect tumor proliferation rates. These effects appear independent of dietary fat intake, though we lacked adequate power to detect interactions. Furthermore, mean decreases in fat intake to only 25–28.4% of total calories, instead of the prescribed level of <20%, may have jeopardized our ability to observe effects that may have accompanied better adherence. The low-fat diet has shown success in other studies, though these studies also included exercise and endpoints differed (10,12).
Our observation of lower proliferation rates with flaxseed supplementation is consistent with our previous in vitro work in LNCaP, DU-145 and PC-3 prostate cancer cell lines which also found inhibited cell growth with exposure to flaxseed-derived lignans (8). Lower cellular proliferation, and reduced tumor burden and urogenital weight also was found in our preclinical study using the transgenic adenocarcinoma mouse model which compared 5% flaxseed supplementation vs. an elemental control diet (AIN-76A) (7). Furthermore, the lower proliferation rates observed with flaxseed supplementation in the current study, parallel findings from our previous clinical studies, one which also used a presurgical model and found lower proliferation indexes among 25 patients assigned to a flaxseed-supplemented, low-fat diet as compared to historic controls matched on Gleason sum, PSA at diagnosis, disease laterality, race and age (5), and another which found reduced pre-post proliferation rates in the benign epithelium of patients with abnormal biopsies scheduled for rebiopsy (6). Reduced proliferation rates with flaxseed also have been observed by Thompson et al. who employed a presurgical model in breast cancer (n=32) and found a 34.2% reduction in the Ki-67 labelling index (p=.001) (36); the strength of this study is that the tumor proliferation rate in biopsy specimens serves as a strong baseline measure from which to assess change in tumors in the breast, whereas in prostate cancer the ability to assess change from biopsy to surgery is limited given its multi-focal and biologically-diverse nature. Animal studies by Thompson and colleagues also support reduced proliferation rates with flaxseed supplementation (46,47). Therefore, all published studies have observed lower or reduced proliferation rates with flaxseed supplementation, thus providing a consistent finding.
However, unlike previous reports, including one of our recent studies which found that flaxseed-derived lignans induced apoptosis in LNCaP cells via a mitochondrial-mediated case-dependent pathway (4–7,46,47) we did not observe differences in apoptosis between treatment arms. Indeed, we observed little variation in TUNEL scores in this study, since apoptosis was either absent or negligible in the majority of our samples. Reasons for this are unknown.
In contrast to our previous studies conducted among men with prostate cancer and those with abnormal biopsies that showed high grade prostatic intraepithelial neoplasia or foci of atypical cells (5,6), we did not observe differences in PSA change between the study arms. Curiously, all study arms experienced significant decreases in both PSA and testosterone during the presurgical period. While Nakashima et al. (48) report consistent decreases in testosterone among patients from pre-anesthesia to 7 days post-prostatectomy, there are no antecedent reports of decreases in testosterone or PSA during the presurgical period. A handful of reports exist; however, describing declines in testosterone with acute stress imposed in the laboratory setting or observed in community-dwelling subjects under a variety of situational factors (49). Therefore, the decreases in testosterone observed in this study may relate to the acute stress attendant with impending surgery-a decline in testosterone that then drives PSA downward. Further study is needed to support or refute this conjecture. The decreases in PSA noted within the control arm also point to the importance of a randomized controlled design during this period of time, and provide evidence that subjects are unable to serve as their own controls.
Our initial premise, that flaxseed exerts its effects through androgen and IGF pathways was unsupported, at least with respect to the biomarkers tested. Indeed, it is possible that other biomarkers assessed along these pathways might be responsible for the effects that we witnessed. For example, reductions in intracellular 5oc-reductase, effects on IGFBP-1 or -2, or other mechanisms may be at play, such as natural killer cell activity, vascular endothelial growth factor, etc (2,3). Eicosanoid-related pathways may hold particular promise since our data suggest that the ALA in flaxseed may be converted to EPA in both the erythrocytes and the target tissue. Therefore, membrane-mediated events that directly relate to the mechanical integrity of cell membranes or to signal transduction also warrant further exploration, as do mechanistic studies that build on recent work suggesting that ω3FAs may impact HER2 (erbB-2) oncogene expression and thus hold promise for both breast and prostate cancer (50). In addition to mechanistic studies, investigations also are needed to determine dose-response and effects among patients who manifest recurrent disease after surgery or those electing expectant management.
An unexpected finding of this study was despite the fact that ALA intakes were significantly higher among flaxseed-supplemented men, we did not find any evidence that this translated into higher levels of ALA in the erythrocytes or prostatic tissue. Instead, we found evidence that EPA levels were higher, thus suggesting that conversion of ALA to higher-chained ω3FAs may occur and may not be as rate-limiting as previously thought (23). Speculation exists as to whether ALA from various sources is metabolized differently or may be influenced by energy balance or temporal changes in the hormonal milieu, thus calling for further investigation. Therefore, more research is needed regarding ALA and prostate cancer, especially studies which control for salient risk factors and which can distinguish between markers of dietary intake or of energy balance, and those that are on the causal pathway (17). While erythrocyte levels of fatty acids provide a reliable measure of intermediate intake (51,52), in conducting further study, the use of other methods, such as radioisotope tracing to discern immediate effects on metabolism, as well as fatty acid analyses of fat biopsy tissue for longer term investigations would be of interest.
Additionally, this trial produced findings which again support the benefits of a low-fat diet in reducing serum lipids, and helping with weight management via a reduction in energy intake. While the reduction in dietary fat to 25–28.4% of total energy did not translate specifically into favorable outcomes in prostate cancer associated endpoints, since cardiovascular disease is a leading co-morbid factor among men with prostatic carcinoma (2), this study provides favorable findings for both interventions. However, unlike a low-fat diet which has proven benefit for cardiovascular disease (53), further studies are needed before we can definitively support flaxseed supplementation as a proven complementary therapy for prostate cancer. To date however, the evidence suggests that flaxseed is: 1) a good, low cost source of select vitamins and minerals, and fiber; 2) is well-accepted and safe to use; and 3) warrants further testing as a preventive or complementary therapy for prostate cancer.
Caution however is warranted in generalizing these findings. Limitations that are specific to the study design (i.e., lack of a placebo-control and lack of power to detect the potential impact of the low fat diet or interactions by study arm or race), the study sample (i.e., over-representation of more highly educated men) or inherent difficulties in conducting prostate cancer research (i.e., the multifocality of prostate cancer or small volume disease) may have influenced our findings. While overcoming these challenges may be difficult, e.g., the creation of a food product that could successfully mask a 30 g. dose of flaxseed, others such as, conducting further research to determine dose-response and additional mechanisms of action, as well as further studies aimed at determining the potential synergy between low fat and flaxseed regimens, or potentially stronger effects among African-Americans is of particular interest.
In summary, this pre-prostatectomy evaluation of the chemopreventive potential of two nutritional interventions utilizes surrogate endpoint biomarkers as primary endpoints. In our study, the modest sample size and short duration, together with the infrequency of cancer recurrence, precluded investigation of clinical cancer endpoints. Furthermore, the development and validation of molecular markers as modifiable surrogates for preferred clinical endpoints remains a work in progress. Although the strength of conclusions drawn from our data is limited by these factors, our study makes several important contributions to clinical intervention trial implementation in cancer prevention. Indeed, the down-regulation of Ki-67, a candidate surrogate for cell proliferation, in the flaxseed-treated arms is highly suggestive of an anti-carcinogenic effect on prostate cancer cells in vivo. Thus, this study serves to generate hypotheses for future larger trials in which flaxseed supplementation can be juxtaposed against prostate cancer recurrence, thereby testing the cancer preventive efficacy of the intervention, as well as contributing to the literature documenting the validity of Ki-67 as a surrogate endpoint biomarker. The current emphasis on biomarker development is thus well-served by this study. Therefore, this study contributes not only to development of nutritional preventive interventions for prostate cancer, but also exemplifies the successful implementation of a study model in which biomarker development is carried-out in a cancer prevention trial that targets accrual within the community setting.
ACKNOWLEDGEMENTS
Funding for this trial was provided by the National Institutes of Health (CA85740, CA07464830, and M01-RR-30). Flaxseed was donated by ENRECO, Inc.
We are grateful for the efforts of the following individuals who helped to facilitate this trial: Mary Jo Beck; Jennifer Blevins; Martha Boggs; Virginia Bolin; Luellen Bratt; Philipp Dahm, MD; Diane Dowdee; Luisa Eisen, Brian Evans, MD; Nicholas Fitzsimons, MD; Linda Hofbauer; Rita Joost; Kuravilla Kurian, PhD; Beth Lavasseur; Deborah Lee; Charles Marguet, MD; Peggy McGraw; Paige Miller; Alister Muir, PhD; Kristy McKiernan-Borawski, MD; Brian Murphy, MD; Regina Norris, MD; David Paulson, MD; Mary Beth Reardon; Allana Richmond; Charles Scales, MD; Candice Singletary; Courtney Streeter; Gregory Taylor, PhD; Wayne Terrell; Jody Thomas; Timothy Tseng, MD; Valeda Stull; Petra Wahnefried; and Jeremy Wiygul, MD. Finally, we are grateful for the support of Norwood and David Bryan, and Wesley Jones, MD.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Canby-Hagino ED, Thompson IM. Mechanisms of disease: Prostate cancer–a model for cancer chemoprevention in clinical practice. Nature Clin Pract Oncol. 2005;2:255–261. doi: 10.1038/ncponc0172. [DOI] [PubMed] [Google Scholar]
- 3.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 4.Chen L-H, Fang J, Li H, Demark-Wahnefried W, Lin X. Enterolactone induces apoptosis in human prostate carcinoma LNCaP cells via a mitochondrial-mediated, caspase-dependent pathway. Mol Cancer Ther. 2007;6:2581–2590. doi: 10.1158/1535-7163.MCT-07-0220. [DOI] [PubMed] [Google Scholar]
- 5.Demark-Wahnefried W, Price DT, Polascik TJ, Robertson CN, Anderson EE, Paulson DF, et al. A Pilot Study of Dietary Fat Restriction and Flaxseed Supplementation in Men with Prostate Cancer Pre-Surgery: Exploring Effects on Hormonal Levels, PSA and Histopathology. Urol. 2001;58:47–52. doi: 10.1016/s0090-4295(01)01014-7. [DOI] [PubMed] [Google Scholar]
- 6.Demark-Wahnefried W, Robertson CN, Walther PJ, Polascik TJ, Paulson DF, Vollmer RT. Pilot study to explore effects of low-fat, flaxseed-supplemented diet on proliferation of benign prostatic epithelium and prostate specific antigen. Urol. 2004;63:900–904. doi: 10.1016/j.urology.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Lin X, Gingrich JR, Bao W, Li J, Haroon ZA, Demark-Wahnefried W. The effect of flaxseed supplementation on prostatic carcinoma in transgenic mice. Urol. 2002;60:919–924. doi: 10.1016/s0090-4295(02)01863-0. [DOI] [PubMed] [Google Scholar]
- 8.Lin X, Switzer BR, Demark-Wahnefried W. Effect of mammalian lignans on the growth of prostate cancer cell lines. Anticancer Res. 2001;21:3995–4000. [PubMed] [Google Scholar]
- 9.Denis L, Morton MS, Griffiths K. Diet and its preventive role in prostatic disease. Eur Urol. 1999;35:377–387. doi: 10.1159/000019912. [DOI] [PubMed] [Google Scholar]
- 10.Link LB, Thompson SM, Bosland MC, Lumey LH. Adherence to a low-fat diet in men with prostate cancer. Urol. 2004;64:970–975. doi: 10.1016/j.urology.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 11.McCann MJ, Gill CI, McGlynn H, Rowland IR. Role of mammalian lignans in the prevention and treatment of prostate cancer. Nutr Cancer. 2005;52:1–14. doi: 10.1207/s15327914nc5201_1. [DOI] [PubMed] [Google Scholar]
- 12.Tymchuk CN, Barnard RJ, Heber D, Aronson WJ. Evidence of an inhibitory effect of diet and exercise on prostate cancer cell growth. J Urol. 2001;166:185–189. [PubMed] [Google Scholar]
- 13.Thompson LU. Flaxseed, lignans, and cancer. In: Cunnane SC, Thompson LU, editors. Flaxseed in human nutrition. Chicago, IL: AOCS Press; 1995. pp. 219–236. [Google Scholar]
- 14.Hall C, III, Tulbek MC, Xu Y. Flaxseed. Adv Food Nutr Res. 2006;51:1–79. doi: 10.1016/S1043-4526(06)51001-0. [DOI] [PubMed] [Google Scholar]
- 15.Webb AL, McCullough ML. Dietary lignans: potential role in cancer prevention. Nutr Cancer. 2005;51:117–131. doi: 10.1207/s15327914nc5102_1. [DOI] [PubMed] [Google Scholar]
- 16.Astorg P. Dietary N-6 and N-3 polyunsaturated fatty acids and prostate cancer risk: a review of epidemiological and experimental evidence. Cancer Causes Contr. 2004;15:367–386. doi: 10.1023/B:CACO.0000027498.94238.a3. [DOI] [PubMed] [Google Scholar]
- 17.Demark-Wahnefried W. Flaxseed and Prostate Cancer: Demon Seed or Seed of Salvation? Sem Prev Alternative Med. 2006;2:205–207. [Google Scholar]
- 18.Freeman VL, Meydani M, Yong S, Pyle J, Flanigan RC, Waters WB, et al. Prostatic levels of fatty acids and the histopathology of localized prostate cancer. J Urol. 2000;164:2168–2172. [PubMed] [Google Scholar]
- 19.Koralek DO, Peters U, Andriole G, Reding D, Kirsh V. A prospective study of dietary alpha-linolenic acid and the risk of prostate cancer (United States) Cancer Causes Control. 2006;17:783–791. doi: 10.1007/s10552-006-0014-x. [DOI] [PubMed] [Google Scholar]
- 20.Schuurman AG, van den Brandt PA, Dorant E, Brants HA, Goldbohm RA. Association of energy and fat intake with prostate carcinoma risk: results from The Netherlands Cohort Study. Cancer. 1999;86:1019–1027. [PubMed] [Google Scholar]
- 21.Brouwer IA, Katan MB, Zock PL. Dietary alpha-linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta-analysis. J Nutr. 2004;134:919–922. doi: 10.1093/jn/134.4.919. [DOI] [PubMed] [Google Scholar]
- 22.Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 23.Lands WE. Biochemistry and physiology of n-3 fatty acids. FASEB J. 1992;6:2530–2536. doi: 10.1096/fasebj.6.8.1592205. [DOI] [PubMed] [Google Scholar]
- 24.Kushi L, Giovannucci E. Dietary fat and cancer. Am J Med. 2002;113(Suppl 9B):63S–70S. doi: 10.1016/s0002-9343(01)00994-9. [DOI] [PubMed] [Google Scholar]
- 25.Shike M, Latkany L, Riedel E, Fleisher M, Schatzkin A, Lanza E, et al. Lack of effect of a low-fat, high-fruit, -vegetable, and -fiber diet on serum prostate-specific antigen of men without prostate cancer: results from a randomized trial. J Clin Oncol. 2002;20:3592–3598. doi: 10.1200/JCO.2002.02.040. [DOI] [PubMed] [Google Scholar]
- 26.Demark-Wahnefried W, George SL, Switzer BR, et al. Overcoming challenges in designing and implementing a phase II randomized controlled Trial using a presurgical model to test a dietary intervention in prostate cancer. Clin Trials. 2008;5:262–272. doi: 10.1177/1740774508091676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Cancer Institute. Percent Calories from Fat Screener. http://www.riskfactor.cancer.gov/diet/screeners/fat. Accessed November 13, 2007.
- 28.Lampe JW, Atkinson C, Hullar MA. Assessing exposure to lignans and their metabolites in humans. J AOAC Int. 2006;89:1174–1181. [PubMed] [Google Scholar]
- 29.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 30.Kohl HW, Blair SN, Jr, Paffenbarger RS, Macera CA, Kronenfeld JJ. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol. 1988;127:1228–1239. doi: 10.1093/oxfordjournals.aje.a114915. [DOI] [PubMed] [Google Scholar]
- 31.Polascik TJ, Oesterling JE, Partin AW. Prostate specific antigen: a decade of discovery–what we have learned and where we are going. J Urol. 1999;162:293–306. doi: 10.1016/s0022-5347(05)68543-6. [DOI] [PubMed] [Google Scholar]
- 32.Pomfret EA, daCosta KA, Schurman LL, Zeisel SH. Measurement of choline and choline metabolite concentrations using high-pressure liquid chromatography and gas chromatography-mass spectrometry. Anal Biochem. 1989;180:85–90. doi: 10.1016/0003-2697(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 33.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 34.Morton MS, Chan PS, Cheng C, Blacklock N, Matos-Ferreira A, Abranches-Monteiro L, et al. Lignans and isoflavonoids in plasma and prostatic fluid in men: samples from Portugal, Hong Kong, and the United Kingdom. Prostate. 1997;32:122–128. doi: 10.1002/(sici)1097-0045(19970701)32:2<122::aid-pros7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 35.Gamache PH, Acworth IN. Analysis of phytoestrogens and polyphenols in plasma. Proc Soc Exp Biol Med. 1998;217:274–280. doi: 10.3181/00379727-217-44232. [DOI] [PubMed] [Google Scholar]
- 36.Thompson LY, Chen JM, Li T, Strasser-Weippl K, Goss PE. Dietary flaxseed alters tumor biological markers in postmenopausal breast cancer. Clin Cancer Res. 2005;11:3828–3835. doi: 10.1158/1078-0432.CCR-04-2326. [DOI] [PubMed] [Google Scholar]
- 37.Stasse-Wolthuis M, Hautvast JG, Hermus RJ, Katan MB, Bausch JE, Rietberg-Brussaard JH, et al. The effect of a natural high-fiber diet on serum lipids, fecal lipids, and colonic function. Am J Clin Nutr. 1979;32:1881–1888. doi: 10.1093/ajcn/32.9.1881. [DOI] [PubMed] [Google Scholar]
- 38.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Therapeut. 1999;21:1074. doi: 10.1016/S0149-2918(99)80026-5. [DOI] [PubMed] [Google Scholar]
- 39.National Cancer Institute. Common Terminology Criteria for Adverse Events (version 3) http://ctep.cancer.gov/reporting/ctc_v30.html (accessed November 28, 2007)
- 40.Bostwick DG, Montironi R, Nogle R, Pretlow T, Miller G, Wheeler T, et al. Current and proposed biologic markers in prostate cancer. J Cell Biochem. 1992;16:65–67. doi: 10.1002/jcb.240501214. [DOI] [PubMed] [Google Scholar]
- 41.Kucuk O. Chemoprevention of prostate cancer. Cancer Metastasis Rev. 2002;21:111–124. doi: 10.1023/a:1020809806121. [DOI] [PubMed] [Google Scholar]
- 42.Tateyama H, Tada T, Hattori H, Murase T, Li WX, Eimoto T. Effects of prefixation and fixation times on apoptosis detection by in situ end-labeling of fragmented DNA. Arch Pathol Lab Med. 1998;122:252–255. [PubMed] [Google Scholar]
- 43.US Bureau of Census. Educational Attainment. 2000 http://www.census.gov/prod/2003pubs/c2kbr-24.pdf (accessed June 9, 2008)
- 44.Kucuk O, Sarkar FH, Sakr W, et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2001;10:861–868. [PubMed] [Google Scholar]
- 45.Urban D, Myers R, Manne U, et al. Evaluation of Biomarker Modulation by Fenretinide in Prostate Cancer Patients. Eur Urol. 1999;35:429–438. doi: 10.1159/000019875. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Power KA, Mann J, Cheng A, Thompson LU. Flaxseed alone or in combination with tamoxifen inhibits MCF-7 breast tumor growth in ovariectomized athymic mice with high circulating levels of estrogen. Expt Biol Med. 2007;232:1071–1080. doi: 10.3181/0702-RM-36. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Hui E, Ip T, Thompson LU. Dietary flaxseed enhances the inhibitory effect of tamoxifen on the growth of estrogen-dependent human breast cancer (MCF-7) in nude mice. Clin Cancer Res. 2004;10:7703–7711. doi: 10.1158/1078-0432.CCR-04-1130. [DOI] [PubMed] [Google Scholar]
- 48.Nakashima A, Koshiyama K, Uozumi T, Monden Y, Hamanaka Y. Effects of general anaesthesia and severity of surgical stress on serum LH and test osterone in males. Acta Endocrinol. 1975;78:258–269. doi: 10.1530/acta.0.0780258. [DOI] [PubMed] [Google Scholar]
- 49.Zitzmann M, Nieschlag E. Testosterone levels in healthy men and the relation to behavioural and physical characteristics: facts and constructs. Eur J Endocrinol. 2001;144:183–197. doi: 10.1530/eje.0.1440183. [DOI] [PubMed] [Google Scholar]
- 50.Osman I, Mikhail M, Shuch B, Clute M, Cheli CD, Ghani F, et al. Serum levels of shed Her2/neu protein in men with prostate cancer correlate with disease progression. J Urol. 2005;174:2174–2177. doi: 10.1097/01.ju.0000181205.23233.65. [DOI] [PubMed] [Google Scholar]
- 51.Poppitt SD, Kilmartin P, Butler P, Keogh G. Assessment of erythrocyte phospholipid fatty acid composition as a biomarker for dietary MUFA, PUFA or saturated fatty acid intake in a controlled cross-over intervention trial. Lipids Health Dis. 2005;4:30–40. doi: 10.1186/1476-511X-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007;86:74–81. doi: 10.1093/ajcn/86.1.74. [DOI] [PubMed] [Google Scholar]
- 53.Yu-Poth S, Zhao G, Etherton T, Naglak M, Jonnalagadda S, Kris-Etherton PM. Effects of the National Cholesterol Education Program's Step I and Step II dietary intervention programs on cardiovascular disease risk factors: a meta-analysis. Am J Clin Nutr. 1999;69:632–646. doi: 10.1093/ajcn/69.4.632. [DOI] [PubMed] [Google Scholar]