Structured Abstract/Summary
Background
The cytokine network theory for psoriasis postulates a key role for TNFa in mediating inflammation and altered epidermal differentiation.
Objective
This study defines responses following administration of adalimumab, a TNFα inhibitor, in pre-psoriatic skin (PN) and lesional psoriatic plaques (PP) skin.
Methods
PN and PP skin before and after treatment were biopsied at days 2, 7, 28 and 84 (n=6 different patients). Cryosections were immunohistochemically stained to detect TNFα and other relevant markers in epidermal and dermal compartments. Detection of apoptosis utilized antibody specific for activated caspase 3. Semiquantitative assessments and statistical analysis was performed for each staining profile.
Results
TNFa+ cells were increased in PP skin. PP skin was also characterized by a 4-fold increase in number of CD68+ macrophages as well as 8-fold increase in CD11c+ dermal dendritic cells (DCs) compared to PN skin. By two-color immunofluorescence staining, both CD68+ cells as well as CD11c+ cells expressed TNFa. Following initiation of adalimumab therapy, CD11c+ cells, significantly decreased in PP skin at days 7, 28, and 84, while CD68+ and CD14+ cells decreased at days 28 and 84. Other markers for DCs (CD83, CD86) showed decreases at days 7, 28, and 84. Reduction in DCs, macrophages or T cells was not accompanied by increased activated caspase 3 positive cells. When a keratinocyte terminal differentiation marker was examined, adalimumab triggered rapid restoration of loricrin expression (beginning on day 2), with loss of aberrant differentiation marker, keratin 17 (K17).
Conclusion
Adalimumab impacts dermal-based immunocytes, and the epidermal compartment also responds by restoration of normal differentiation without detectable apoptosis.
Introduction
Psoriasis is a common, chronic inflammatory skin disease characterized by erythematous scaly plaques [1]. Within a psoriatic plaque (PP skin), there are complex alterations in epidermal keratinocyte growth and differentiation [2], accompanied by an inflammatory cell infiltrate consisting of T lymphocytes, dendritic cells and macrophages [3–6]. A cytokine network model for the immunopathogenesis of psoriasis was proposed in 1991 in which TNFα producing mononuclear cells were given center stage in orchestrating multi-cellular interactions by which pre-psoriatic (PN) skin was converted to PP skin [7].
Numerous clinical trials have demonstrated targeting TNFα produces significant improvement in the majority of patients suffering from psoriasis [8–10]. Despite clinical success in psoriasis, the mechanism by which targeting TNFα produces a reversion of PP skin to PN skin is unclear. Previous studies using the soluble receptor for TNF blockade (etanercept) found a rapid down-modulation of multiple pro-inflammatory pathways in PP skin [11], and apoptosis of dermal dendritic cells after 1 month of therapy [12]. In another study, a 48 hr time interval post TNFα blockade using infliximab revealed a decrease in macrophages in skin and joints of psoriatic patients without evidence of apoptosis [13]. However, the precise effect of anti-TNFα therapy on distinct immunocyte subsets using a broader panel of markers and numerous time points has not been previously reported; nor has a link between the presence of TNFα positive immunocytes and the aberrant epidermal differentiation program within PP skin been established. It should be noted that PP skin is not only a site of intense chronic inflammation, but there are also many alterations in the epidermal keratinocyte differentiation program within lesional skin.
As regards epidermal differentiation programs and PP skin, Mansbridge and colleagues originally proposed that an aberrant keratinocyte differentiation pathway was triggered in PP skin [14–17]. Furthermore, certain proteins that play a key role in terminal differentiation and barrier function such as loricrin are reduced in PP skin [18,19]. It is possible that the altered barrier function of PP skin actually perpetuates the chronic inflammation, leading to a vicious cycle such that immunocytes produce cytokines (including TNFα), which in turn produce aberrant epidermal differentiation, leading to barrier function abnormalities that also contribute to the inflammatory process [20,21].
Given the interplay between inflammation and aberrant epidermal differentiation in PP skin [22], we designed this study to probe not only how a TNFα inhibitor such as adalimumab influences immunocyte subsets, but also to determine the effects of a TNFα inhibitor on epidermal keratinocyte differentiation [23]. We were particularly interested in this epidermal response, as we previously had observed that adalimumab rapidly restored epidermal Langerhans cell density within PP skin [24]. To address a potential mechanistic link to changes in the number of viable cells in both epidermal and dermal compartments following adalimumab therapy, an assessment of cells induced to undergo apoptosis was performed by immunostaining to detect activated caspase 3. While some investigators attempt to detect apoptosis in tissue sections by the use of transferase-mediated uridine nick-end labeling (TUNEL) assays, we and others have previously noted significant limitations in the use of this technique for rapidly proliferating tissues such as psoriatic lesions [25–28]. Thus, we have relied on detection of activated caspase 3 that has been identified as a newer and more sensitive and specific marker for detecting apoptotic events in human skin samples [29].
We defined three primary aims for the present study: to carefully determine temporal effects of adalimumab on various subsets of immunocytes, including DCs, macrophages, and T cells; to evaluate the epidermal keratinocyte response to adalimumab; and to define a role for the induction of apoptosis in both immunocytes and keratinocytes within PP skin following adalimumab therapy. Based on the data presented, targeting TNFα using adalimumab produces rapid reductions in the numbers of DCs and macrophages (followed by lowered T cell counts) infiltrating PP skin; accompanied by rapid restoration of terminal differentiation by lesional epidermal keratinocytes; and both prominent cellular and molecular events in the epidermis and dermis do not reflect a conspicuous induction of apoptosis. These results reveal a very consistent and rapid series of events following adalimumab therapy that contribute to our understanding of the mechanism of action by which adalimumab reverses the many and diverse clinical and pathological features contributing to the maintenance of PP skin.
Materials and Methods
Patient study
Adalimumab was studied in a phase II, 12-week, randomized, double-blind, placebo-controlled, multicenter efficacy and safety study designed to demonstrate its effectiveness following repeated subcutaneous injections in the treatment of moderate to severe psoriasis. Adalimumab (Humira®, D2E7; Abbott Laboratories, Abbott Park, IL, U.S.A.) was administered as previously described (with IRB approval and informed consent) [24], and the same tissue samples were used for cryostaining followed by new analysis of different markers. The focus of this study was the procurement of sequential punch biopsies obtained from patients before and during treatment. Thirteen different individuals completed the treatment protocol with all biopsies performed by the investigators, comprised of seven individuals who received adalimumab and six who served as controls, receiving placebo injections as described in the following section. Subjects were randomly assigned to one of three study arms: two active treatment groups and one placebo group. Subjects assigned to the first treatment arm (n=4) received adalimumab 80 mg at week 0, followed by 40 mg every alternate week starting at week 3. Subjects assigned to the second arm (n=3) received adalimumab 80 mg at week 0 and at week 1, followed by 40 mg weekly starting at week 2. The third control arm included placebo injections.
Tissue samples
As described above, patients from two different enrollment sites participated in a biopsy sub study. Two punch biopsies (6 mm) each of psoriatic lesions (PP) and symptomless skin (PN) were taken at baseline. Two biopsies of PP skin only were also obtained at 48 hrs following the first dose, and at day 7, day 28 and day 84. Biopsies of PP skin were taken within a lesion, 1 cm from the edge of the plaque border. Biopsies of PN skin were taken 2 cm beyond the plaque border. Because of tissue shortages, only 6 out of the 7 clinical responders were analyzed during this current investigation compared to the previous study [23]. All patients in this treatment group were considered to be clinical responders (>75% improvement in Psoriasis Area and Severity Index (PASI) compared with baseline), irrespective of the dose of adalimumab administered, and hence subjects were pooled providing a sample size of six (n=6) for treated patients. None of the placebo control subjects experienced significant clinical improvement [23]. One of each pair of biopsies were either cryopreserved by immediate immersion in Optimal Cutting Temperature (OCT®, Sakura Finetek, Torrance, CA, U.S.A.) mounting medium with freezing in a slurry of methanol and dry ice, or snap-frozen using isopropanol in liquid nitrogen. Tissues were sectioned at 5 μm thickness. Both tissues and sections were stored at − 80°C.
Immunohistochemistry and Two-Color Immunofluorescence
Sections stored at − 80°C were equilibrated to room temperature for 10 min and immediately fixed in 100% acetone, − 20°C for 10 min Primary antibodies were diluted in 0.1% BSA in DPBS with Mg and Ca as follows: CD11c 1:50 (BD Biosciences, San Diego, CA), CD68 1:80 (Dako USA, Carpenteria, CA), TNFα 1:10 (R&D Systems, Minneapolis, MN), CD14 1:100 (Biomeda, Foster City, CA), CD83 1:20 (Santa Cruz Biotechnology, Santa Cruz, CA), CD86 1:80 (BD Biosciences), and CD3 1:10 (BD Biosciences). Primary antibodies were incubated at room temperature for 1 hour, followed by wash with FA buffer. Immunostaining was accomplished using appropriate ABC Vectastain Kit (Vector Laboratories, Burlingame, CA) per manufacturer’s instructions. Slides were then exposed to 3-amino-4-ethylcarbazole to produce a positive red reaction product, and counterstained with hematoxylin (Gill III Formula, Surgipath, Richmond, IL). Positive cells were counted by independent observers in three high power fields (40x magnification), and the mean values were calculated for each tissue sample.
Two-color immunofluorescence procedure was performed the same as immunohistochemical staining protocol up to the secondary antibody, with mixing of the two primary antibodies at the above stated dilutions. After a 5 minute wash, sections were incubated with secondary antibodies: Alexa Fluor® 488 donkey anti-goat 1:400 and Alexa Fluor® 568 rabbit anti-mouse 1:1000 (Molecular Probes, Eugene, OR) for 30 mins. Samples were washed in FA buffer 5 mins and mounted with Slowfade® (Molecular Probes, Carlsbad, CA).
Detection of Epidermal Response
Tissue sections were immunohistochemically stained for loricrin 1:1750 (Convance, Berkeley, CA) and K17 1:20 (Vector Laboratories) using above protocol. Percent of loricrin was measured as linear horizontal positive expression over 400 μm of skin surface. K17 expression was graded according to the following scale: 0=no staining, 1=focal area <5% total, 2=skip areas between positive staining, 3=confluent horizontally plus <40% epidermis positive, 4=confluent horizontally plus >40% epidermis positive.
Detection of Apoptosis
The presence of cells undergoing apoptosis was determined by immunohistochemical detection of activated caspase 3 (R&D Systems, Minneapolis, MN). Activation of caspase 3 occurs at the intersection of both extrinsic as well as intrinsic apoptotic pathways, and we have previously utilized this approach to define apoptotic events in human skin samples, including psoriatic plaques. As a positive control, cultured human keratinocytes derived from neonatal foreskins were grown in 8-well Lab Tek chamber slides, exposed to UV-light (30mJ/cm2), and after 18 hrs were fixed and immunostained to detect activated caspase 3 as previously described [29].
Statistical Analysis
Differences in the number of various mononuclear cell subsets; and extent of keratin 17 and loricrin expression between baseline levels and at each treatment interval, were analyzed for significance using a one-tailed student’s t test. Differences were considered significant with p ≤ 0.05. An asterisk has been added to the relevant figures at the earliest time points at which statistically significant differences were noted compared to the untreated lesional skin sample. The data points presented represent mean ± standard error of mean (SEM).
Results
Clinical Response of PP Skin and PN Skin to Adalimumab Treatment
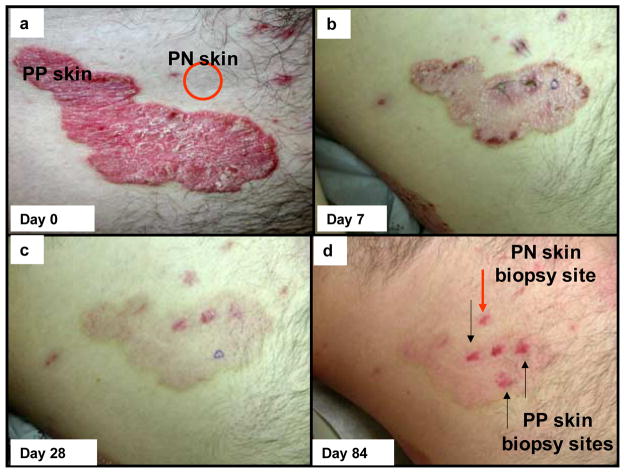
As previously noted [24], all patients receiving adalimumab were considered responders (reduction in PASI ≥ 75), whilst none of the placebo treated patients showed a clinical response. Figure 1 depicts a representative clinical series of a patient before and after adalimumab treatment. Note the only change in PN skin (red circle) was resolution of the biopsy site, but the thick, scaly, erythematous plaque on day 0 (Figure 1a) became less thick and scaly with diminished erythema on day 7 (Figure 1b), and was nearly normal in its appearance (only slight residual erythema) on days 28 and 84 (Figure 1c and d). While the PP skin healed without scarring following adalimumab treatment, note the residual erythema at the sides of the punch biopsies as highlighted on day 84 (Figure d). These clinical results are entirely consistent with a rapid and significant clinical response to adalimumab therapy, so we sought to probe potential cellular and molecular events to illuminate a potential mechanism of action for adalimumab in PP skin.
Figure 1. Clinical perspective of skin lesions on a psoriatic patient before and after adalimumab therapy.
Compared to adjacent symptomless (PN) skin, designated by red circle, the baseline appearance for untreated psoriatic plaques (PP skin) revealed well demarcated, erythematous, and scaly lesions (a). On day 7 after delivery of a single loading dose of adalimumab (80 mg, subcutaneous) the lesional skin is characterized by reduced thickness, erythema, and scaling (b; the sutures represent biopsy sites for PN and PP skin). On day 28 after continuation of adalimumab therapy, the target plaque is almost completely resolved with only slight residual erythema; a clinical trend continued on day 84 of therapy (c, d). Note the resolution of the inflammation and tissue remodeling of PP skin by adalimumab was not accompanied by any scar formation.
Location of TNFα Positive Cells in PP Skin and PN Skin
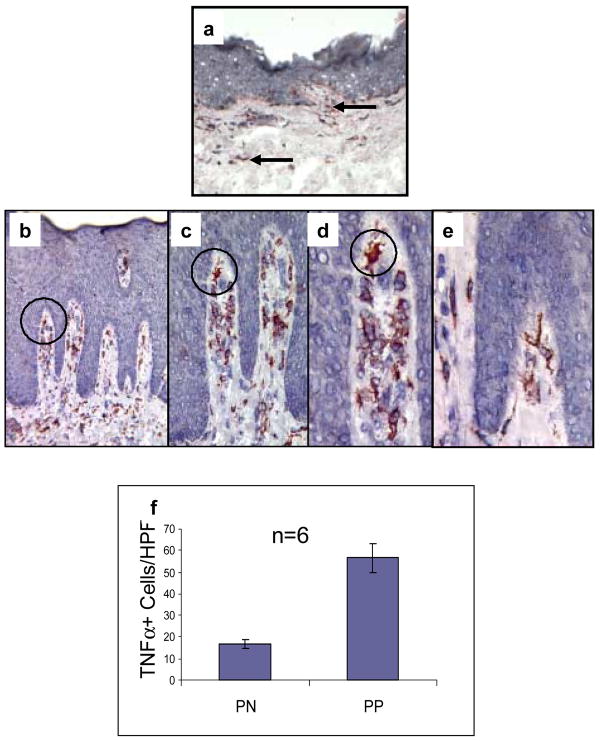
In 1991, using a polyclonal rabbit antisera, we demonstrated the localization of TNFα within mononuclear cells in PP skin [7], but did not specifically identify the immunocyte subset by two-color immunohistochemical analysis. Subsequently, several groups confirmed our original observation describing increased immunoreactivity and bioactivity of TNFα in PP skin [30,31], which appears to be regulated by a post-transcriptional mechanism [31]. In the current study we decided to utilize a commercially available antibody to localize TNFα in a cohort of tissue samples derived from patients before and after adalimumab therapy; and to complement this immunostaining profile by two-color staining in order to better define the type of mononuclear cell containing TNFα in both PN and PP skin. In addition, we were interested in determining what would happen to the tissue density of these mononuclear cells within PP skin when patients were treated with adalimumab, since it was unknown whether a change in TNFα positive cells would occur as an early or late event following administration of an effective biological agent. Thus, tissue sections were immunostained before and after therapy. Compared to only rare and focal immunostaining for TNFα in dermal and epidermal mononuclear cells in PN skin (Figure 2a arrows), there were easily identifiable and strongly TNFα+ cells within PP skin (ranging around 50 positive cells per HPF Figure 2f). PP skin (Figure 2b) was characterized by confluent parakeratotic scale, loss of the granular cell layer, and elongation of rete ridges accompanied by mononuclear cell infiltrates. In PP skin, TNFα positive cells were primarily clustered around the dermal-epidermal junction, and within the papillary dermis in and around the superficial vascular plexus (Figures 2b–d with circles to highlight specific immunopositively stained cells). There were some scattered positive immunocytes in the epidermis. Epidermal keratinocytes were not shown to be immunoreactive with the anti-TNFα antibody when compared with the aforementioned immunocytes. The cells displaying immunoreactivity for TNFα (e.g. TNFα+ cells) appeared by light microscopy to exhibit both macrophage and dendritic cell morphological characteristics as can be appreciated particularly in Figure 2, panels d and e). To further define the type of mononuclear cell expressing TNFα, we performed additional studies as described in the next section.
Figure 2. TNFα expression in PN and PP skin.
Cryopreserved sections of PN skin (panel a) and PP skin (panels b–e) were immunostained to detect TNFα. Note the markedly increased number of TNFα + cells in PP skin (b) compared to PN skin (a). The majority of TNFα + cells in PP skin are located in the papillary dermis (b–e). Note that progressively increased magnification is provided in panels b–d using a black circle for orientation. Many of the TNFα + cells had a dendritic morphology (e), whereas others had a plump macrophage-like appearance (d). No epidermal keratinocyte immunoreactivity for TNFα was observed.
Quantitation of TNFα immunoreactive cells involving six different subjects (n=6) for PN skin (17±2 cells/HPF) and PP skin (57±7 cells/HPF) revealed significant differences between PN and PP skin (p<0.05) as depicted in (f).
These results indicate PP skin is characterized by an increased density of TNFα + mononuclear cells that infiltrate the papillary dermis and focally extend into the epidermal compartment.
Immunophenotypic Characterization of TNFα Positive Cells in Psoriatic Lesions
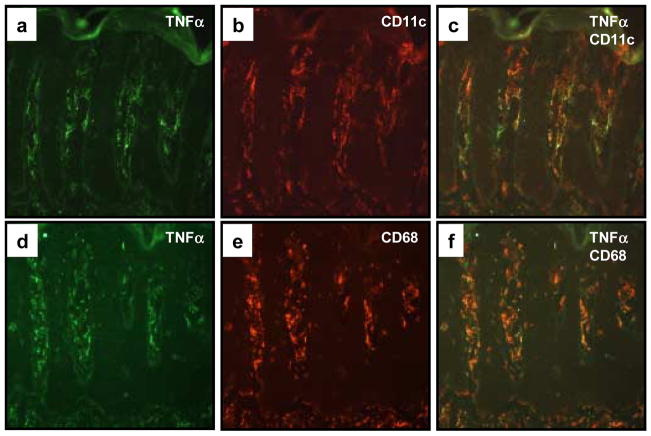
Since TNFα positive cells exhibited the aforementioned macrophage and dendritic morphology, we stained for macrophage lineage using CD68, and DC lineage using CD11c, as markers [32]. Using two-color immunofluorescence CD68+ and CD11c+ cells were shown to be the major producers of TNFα in PP skin (Figure 3). Strongly staining CD68+ cells were more often larger and centered in the papillary dermis, while strongly CD11c+ cell numbers were higher lining the dermal-epidermal junction. There was significant overlap between the populations, since there were very few TNF+/CD11c− and TNF+/CD68− cells.
Figure 3. Co-localization of TNFα expressing cells within CD11c+ dendritic cells and CD68+ macrophages.
Cryopreserved sections of PP skin were subjected to two-color immunofluorescence staining in which TNFα expressing cells were labeled green (FITC; a, d), and the CD11c+ (b) or CD68+ (e) mononuclear cells labeled red (rhodamine). Note the similar distribution patterns and morphological profiles, as well as dual fluorescence imaging that highlight the co-expression of TNFα in both CD11c+ (c) as well as CD68+ (f) cell subsets.
Adalimumab Treatment Reduces Density of TNFα Positive Immunocytes
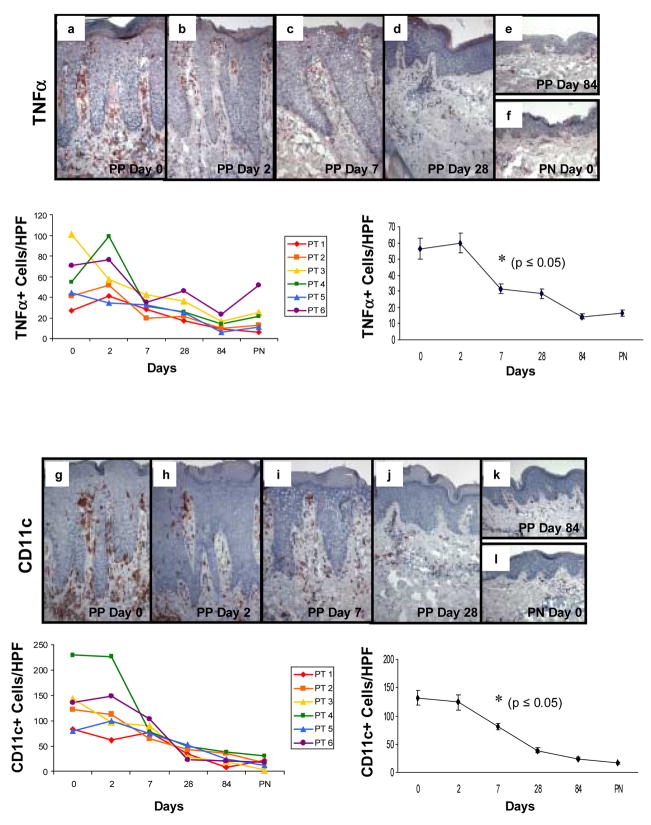
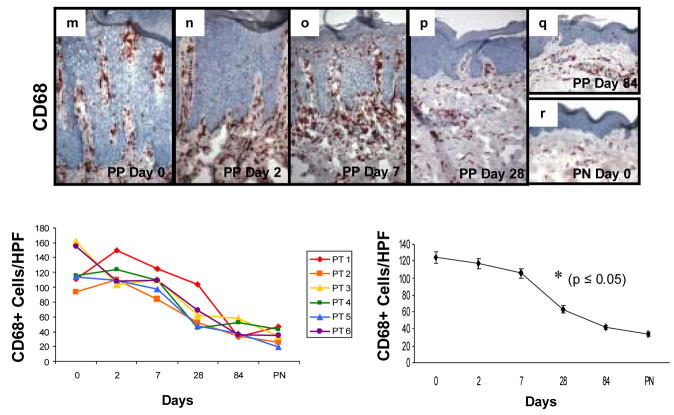
Immunohistochemical staining of sequential PP skin biopsies revealed decreases in TNFα+ mononuclear cells in all six different patients following adalimumab therapy that resembled PN skin by day 84 (Figure 4a–f). TNFα+ cells were counted and revealed a statistically significant decline at days 7, 28, and 84 when compared with untreated plaques. Below each immunostained profile of skin samples are panels depicting data points as follows: Left side panel displays individual values for each patient at each tissue sampling revealing relatively consistent trends amongst the treated individuals: Right side panel displays the overall values (mean ± SEM) for the treated group with the asterisk signifying the earliest time point at which a significant decrease (P<0.05) was noted following the onset of adalimumab treatment.
Figure 4. Expression and kinetic profile highlighting temporal reductions in density of TNFα +, CD11c+, and CD68+ cells in psoriatic patients before and after treatment with adalimumab.
Untreated PP skin (PP day 0) is characterized by an increased number of mononuclear cells expressing TNFα compared to PN skin (a and f), as well as increased number of CD11c+ cells (g and l), and CD68+ cells (m and r). Beneath each representative staining profile of untreated and treated PP skin and PN skin are panels depicting individual data points for each of the six treated subjects (left side), as well as a summary panel depicting mean +/− SEM for the group (n=6). For each treated subject, a color and symbol are provided to delineate a specific patient in figures 4 and 5.
Note the consistent and rapid reduction in TNFα expressing cells (panels b–e, s) which first became statistically significant at day 7 (asterisk; p<0.05), as does the reduction in density of CD11c+ cells (panels h–k, t); whereas the reduction in CD68+ cells does not achieve significance until day 28 (panels n–q; u). Note the parallel reduction in epidermal thickness that accompanies the diminution in the density of mononuclear cells at the indicated time points, consistent with our previous report [24].
Immunohistochemical staining of patient tissue samples also revealed decreased numbers of DCs (Figure 4g–l) using CD11c as a cell marker, as well as decreased numbers of macrophages using CD68 as a cell marker (Figure 4m–r). Immunohistological analysis and cell counts revealed a return to near baseline cell numbers within the treatment group for both DCs and macrophages by day 84 resembling PN skin (Figure 4k, l, and q, r). CD11c + cells were counted and revealed a statistically significant decline at days 7, 28, and 84 when compared with untreated plaques. Below each immunostained profile of skin samples are panels depicting data points as follows: Left side panel displays individual values for each patient at each tissue sampling revealing relatively consistent trends amongst the treated individuals: Right side panel displays the overall values (mean ± SEM) for the treated group with the asterisk signifying the earliest time point at which a significant decrease (p<0.05) was noted following the onset of adalimumab treatment.
CD68+ cells were counted and revealed a statistically significant decline at days 28 and 84 when compared with untreated plaques. Below each immunostained profile of skin samples are panels depicting data points as follows: Left side panel displays individual values for each patient at each tissue sampling revealing relatively consistent trends amongst the treated individuals: Right side panel displays the overall values (mean ± SEM) for the treated group with the asterisk signifying the earliest time point at which a significant decrease (p<0.05) was noted following the onset of adalimumab treatment.
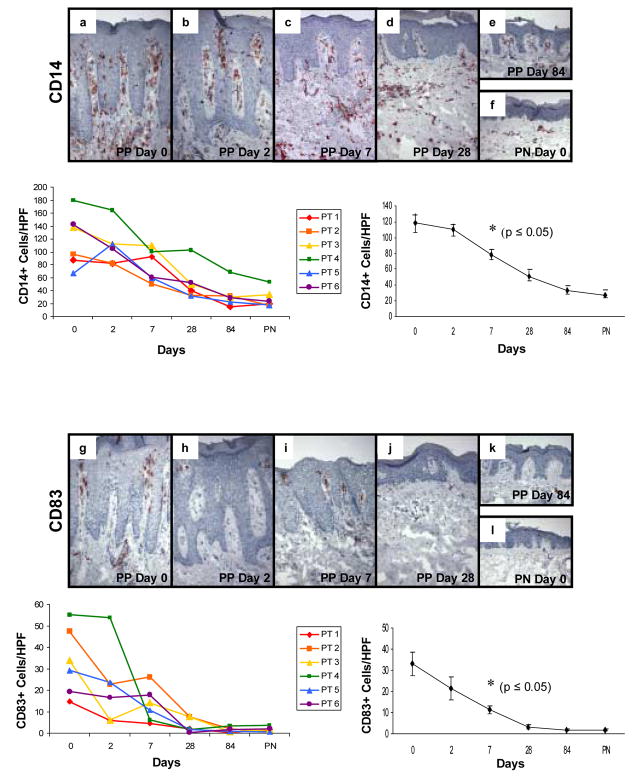
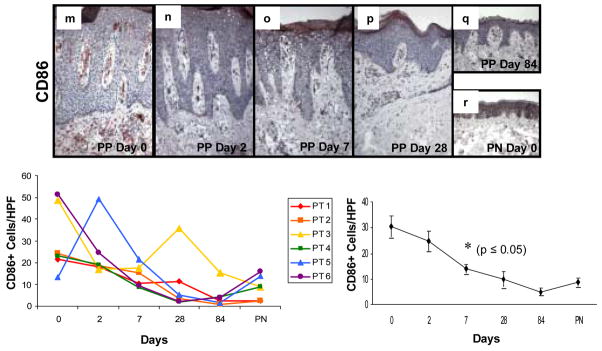
To further characterize the effect of adalimumab on mononuclear subsets, additional immunostaining was performed using CD14 to detect predominantly macrophage-like cells (Figure 5a–f), CD83 to detect DCs (Figure 5g–l), and CD86 to assess those DCs expressing a critically important co-stimulatory molecule CD86 (Figure 5m–r). CD86 was of particular relevance because a reagent that blocks engagement of CD28 with its ligands CD80 and CD86 (e.g. CTLA4Ig) has been observed to improve PP skin in a clinical trial. [33] The adalimumab therapy induced reduction in CD14+ mononuclear cells which first became statistically significant at day 7 (Figure 5, panels beneath immunostaining profiles depicting individual patient values as well as pooled data summation); accompanied by a very consistent decrease in the number of CD83+ DCs, also becoming significant at day 7 (Figure 5, panels beneath immunostaining profiles depicting individual patient values as well as pooled data summation). Simultaneously, the number of mature DCs expressing C86 following adalimumab therapy also became significant at day 7 (Figure 5, panels beneath immunostaining profiles depicting individual patient values as well as pooled data summation).
Figure 5. Expression and kinetic profile highlighting temporal reductions in density of CD14+, CD83+, and CD86+ cells in psoriatic patients before and after treatment with adalimumab.
Untreated PP skin (PP day 0) is characterized by an increased number of mononuclear cells expressing CD14+ compared to PN skin (a and f), as well as increased number of DCs expressing maturation markers CD83 (g and l) and CD86 (m and r). Beneath each representative staining profile of untreated and treated PP skin and PN skin are panels depicting individual data points for each of the six treated subjects (left side), as well as a summary panel depicting mean +/− SEM for the group (n=6). The reduction in these markers initially became statistically significant at day 7 (asterisk; p<0.05 comparing untreated to treated samples).
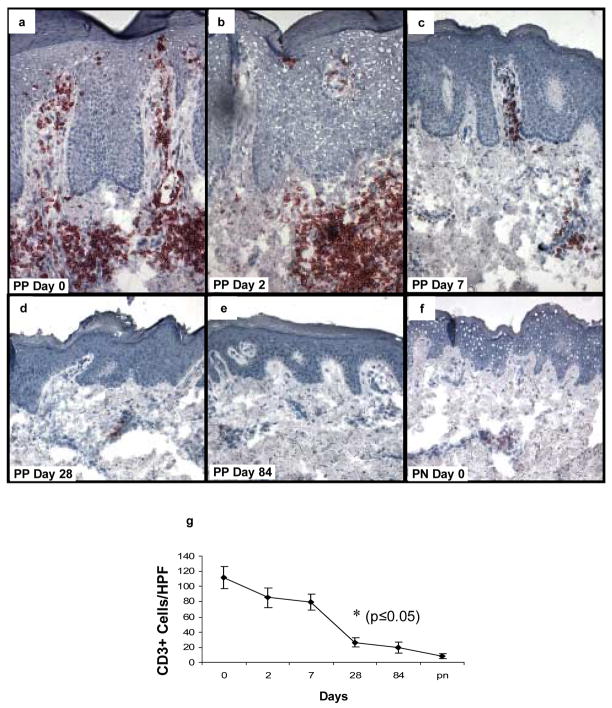
Besides an effect on the number of mononuclear cells including DCs and macrophages, adalimumab treatment also produced a reduction in the number of CD3+ T cells in the six treated patients (Figure 6). In untreated PP skin, T cells are predominantly located around the superficial vascular plexus with focal infiltration of the epidermis (Figure 6a). Following initiation of adalimumab treatment, the number of T cells present declined on days 2 and 7 (Figure 6b, c), but did not achieve statistical significance until day 28 of therapy, at which time both day 28 and 84 biopsies resembled the distribution seen in PN skin (Figure 6d–f).
Figure 6. Expression and kinetic profile highlighting temporal reductions in density of CD3+ T cells in psoriatic patients before and after treatment with adalimumab.
Untreated PP skin is characterized by influx of CD3+ T cells, located both in the upper dermis with focal extension into the epidermis. Following treatment with adalimumab, the number of T cells begins to decline on days 2 and 7 (b, c), with a more pronounced reduction on days 28 and 84 (d, e) where they resemble the density in PN skin (f). The earliest time point with statistically significant reduction in CD3+ cell counts compared to untreated samples is day 28 (g; n=6; asterisk, p<0.05).
Lack of Apoptosis in Immunocytes within Psoriatic Plaques Following Adalimumab
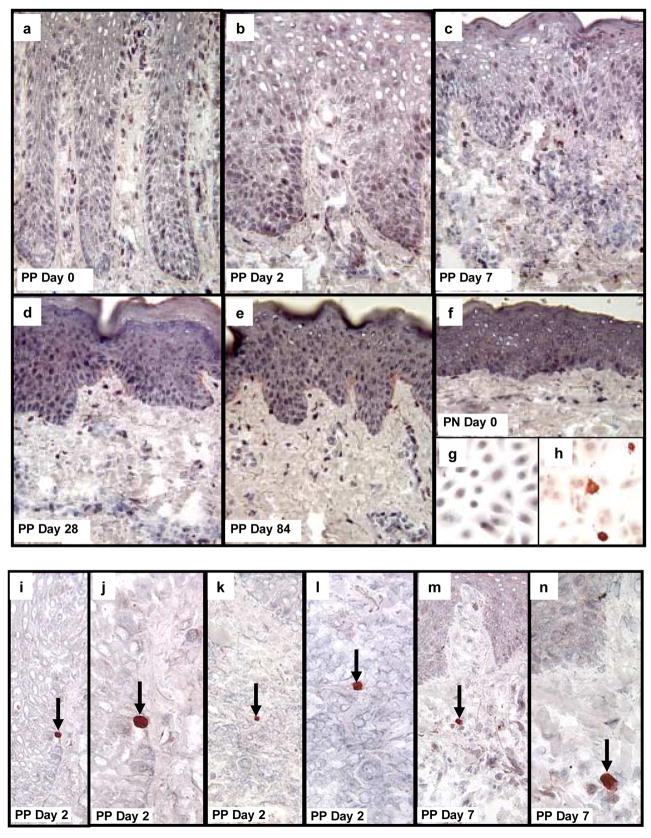
No cells were detected undergoing apoptosis (e.g. activated caspase 3 positive) in PN or untreated PP skin (Figure 7a, f), consistent with a previous report [13]. Moreover, there was no detection of a large number of cells expressing activated caspase 3 in any of the treated tissue samples in either epidermal or dermal compartments at days 2, 7, 28, 84, for any of the patients treated with adalimumab (Figure 7b–e). While normal cultured KCs do not spontaneously express activated caspase 3 (Figure 7g), following UV-light exposure strong cytoplasmic immunoreactivity was identified verifying our staining procedures (Figure 7h). Occasional activated caspase 3 positive cells were identified by their bright red appearance in scattered tissue sections of PP skin including rare epidermal KCs, lymphocytes, and macrophages (Figure 7g–l). These rare but intensely activated caspase 3 cells served as internal staining controls. It should also be noted that the nucleus within these positive cells displayed condensed chromatin, consistent with cells undergoing apoptosis. Thus, the mechanism by which reduction in the number of immunocytes located within the dermis does not appear to involve induction of apoptosis. In line with this conclusion, no cellular debris or other morphological evidence for apoptosis, or other forms of cell death, were visible by light microscopy in any of the treated tissue samples examined. In the next section, we move from the dermal compartment to explore molecular events within the epidermal compartment.
Figure 7. Detection of activated caspase 3 as a marker for cells undergoing apoptosis in psoriatic patients before and after treatment with adalimumab.
Untreated PP and PN skin are characterized by the absence of cells in either the epidermis or dermis undergoing apoptosis because no activated caspase 3 positive cells are present in the tissue samples (a, f). Moreover, following treatment with adalimumab, the reduction in epidermal thickness as well as the decreased density of immunocytes (dendritic cells, macrophages, T cells) was also not accompanied by and prominent increase in detectible cells expressing activated caspase 3 (b–e). As a positive control, cultured human keratinocytes were immunostained before (g), and after, (h) UV-light to confirm detection of activated caspase 3. Note the strong cytoplasmic immunostaining for activated caspase 3 in keratinocytes undergoing apoptosis following UV-light (h).
In skin tissue sections, occasional, rare scattered cells expressing bright red anti-activated caspase 3 reaction product was detected in epidermal keratinocytes (i, j; arrows depict basal layer keratinocyte undergoing apoptosis in PP skin 2 days after adalimumab treatment. A rare dermal lymphocyte expressing activated caspase 3 was also identified at day 2 in treated PP skin (k, l; arrows depict apoptotic lymphocyte). A rare macrophage-like cell expressing activated caspase 3 is shown in (m, n) on day 7 in treated PP skin.
Epidermal Response to Adalimumab
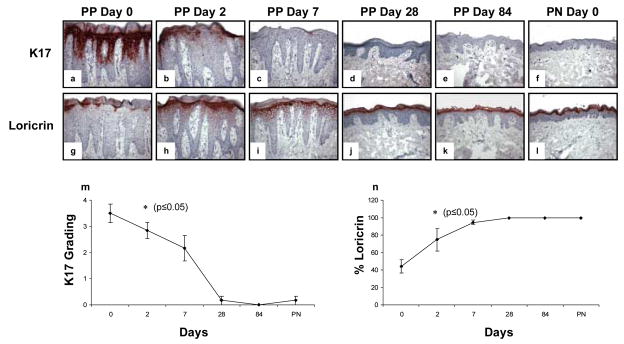
Untreated psoriatic plaques are characterized by strong and diffuse aberrant epidermal keratinocyte expression of keratin 17 (K17), accompanied by near complete loss of the terminal differentiation marker, loricrin (Figure 8a, g). As early as 2 days following initiation of adalimumab treatment, there was significant diminution in aberrant expression of K17 in six treated patients (Figure 8b), accompanied by enhanced upper level epidermal keratinocyte loricrin expression (Figure 8h). These molecular events in the epidermis continued to change over time and by day 28, minimal to absent K17 expression was present and normalization of loricrin expression was also observed in treated plaques, resembling PN skin (Figure 8c–f, i–l). Note the quantitative assessments for epidermal keratinocyte expression revealed statistically significant reductions in K17 as well as statistically significant increases in loricrin expression at day 2 (Figure 8m and n, respectively).
Figure 8. Expression and kinetic profile highlighting epidermal response in psoriatic patients before and after treatment with adalimumab focusing on aberrant keratin 17 and loricrin expression.
Untreated PP skin is characterized by strong and diffuse epidermal keratinocyte expression of the aberrant differentiation marker, keratin 17 (K17; a), accompanied by reduced expression of the terminal differentiation marker, loricrin (g). As early as 2 days following a single injection of adalimumab (n=6), there is significant reduction in K17 expression (b) with restoration of strong diffuse loricrin expression (h). By day 7, there is almost complete loss of K17 expression (c) with continued normalization of loricrin expression and orthokeratosis (h). Biopsies on day 28 and 84 revealed near complete healing of PP skin resembling PN skin as depicted (d–f, j–l).
Discussion
To investigate the mechanisms of action by which anti-TNF therapy improves psoriatic lesions, a sequential series of skin biopsies obtained from six different individuals were procured for immunohistochemical analysis including PN skin and PP skin, before and after 2, 7, 28, and 84 days of treatment [3,8–10]. Compared to PN skin, the pre-treatment PP skin biopsies contained significantly increased numbers of TNFα positive cells including CD11c+ DCs, as well as CD68+ and CD14+ macrophages. The PP skin was also characterized by aberrant differentiation program (loss of loricrin and appearance of keratin 17) in which terminal differentiation was incomplete and accompanied by confluent parakeratosis. The large number of TNFα+ mononuclear cells, including both CD68+ and CD14+ macrophages, as well as CD11c+ DCs, did not significantly change after 2 days of treatment, but by 7 days following a single injection of adalimumab, there was statistically significant reduction in the DC subset of mononuclear cells. By day 28, the number of TNFα, CD68+, and CD14+ mononuclear cells became significant and approached levels seen in PN skin, which became even closer to PN skin levels by day 84. The maturation markers for DCs (e.g. CD83 and CD86) became less apparent in the PP skin as early as day 2, and followed a downward trend parallel with the overall CD11c DC marker at subsequent time points. Similarly, the number of CD3+ T cells was reduced over the time course of study in PP skin by adalimumab; thereby resembling the tissue response to infliximab [34]. Taken together, these results clearly indicate that the elevated number of DCs, macrophages, and T cells in PP skin requires a continuous presence of TNFα, and once a TNFα inhibitor is administered, the PP skin becomes characterized by a rapid decrease in not only the total number of immunocytes, but also those immunocytes expressing TNFα.
Compared to lack of consistent reductions in the number of TNF+, CD11c+, CD68+, and CD14+ mononuclear cells located predominantly in the dermis after 2 days of adalimumab therapy, the epidermal keratinocyte responses were surprisingly rapid and included significant reduction in keratin 17 expression in mid and upper epidermal layers, accompanied by prominent increases in loricrin expression in upper epidermal keratinocytes. Indeed, while untreated PP skin samples were characterized by absence of a granular cell layer and confluent parakeratosis, the day 2 post adalimumab biopsies were conspicuous for rapid re-appearance of a granular cell layer and orthokeratosis. Thus, it appeared the epidermal keratinocyte activation of a normal terminal differentiation program preceded actual reductions in the number of infiltrating mononuclear cells in the dermis.
As regards mechanistic implications, this study provides several insights into the dynamic role for TNFα in the maintenance of psoriatic lesions. First, the predominant source of TNFα is localized to DCs and macrophages. Second, upon administration of a biological agent capable of targeting and neutralizing TNFα, the large number of inflammatory mononuclear cells and T cells are rapidly reduced, indicating a key role for TNFα in the maintenance of high tissue infiltration by immunocytes. The self-reinforcing role for TNFα in sustaining TNFα expression by immunocytes was reflected by reduction in TNFα expressing DCs and macrophages. Third, the aberrant epidermal differentiation program was also found to be dependent on TNFα. Exactly how TNFα perturbs epidermal keratinocyte differentiation in this disease setting requires further study. Previously, we and others had noted barrier perturbation in normal skin itself triggers TNFα production [35,36], and the current results indicate dysregulated TNFα levels in PP skin can also contribute to altered terminal differentiation and barrier abnormalities. Fourth, the mechanism by which targeting TNFα produces reduction in the cell number of DCs, macrophages, and T cells in the dermis does not appear to involve a prominent induction of apoptosis. This lack of prominent apoptosis induction (only rare, scattered activated caspase 3+ cells on days 2, 7, 28, or 84) following TNF blockade was also previously observed by others, albeit at a single time point assessment (e.g. 48 hrs) in both skin and joints of psoriasis patients [13]. Another study found no induction of apoptosis in circulating monocytes after TNF blockade, but approximately 4–6% of dermal dendritic cells were activated caspase 3 positive after 1 month of treatment in responding patients [13]. It is possible that etanercept differentially influences skin cells compared to adalimumab.
Based on the current results using psoriatic skin samples examined at multiple time points, the neutralization of TNFα by adalimumab appears to reduce the number of immunocytes predominantly by a non-apoptotic mechanism. As trafficking of immunocytes in the skin is best regarded as a dynamic process including recruitment, retention, and return to recirculation [37], further studies are required to more precisely define the non-apoptotic mechanism underlying the rapid and dramatic reductions observed for DCs, macrophages, and T cells. As regards remodeling of the epidermal compartment following adalimumab treatment, the rapid induction of terminal differentiation (e.g. loricrin) and loss of aberrant keratinocyte maturation markers (e.g. K17) which is accompanied by a thinning of the epidermal layer, is reminiscent of the rapid non-apoptotic events observed when Myc induced changes are abruptly interrupted in a transgenic mouse model [38]. Given the apoptotic resistance of keratinocytes within psoriatic plaques [28,39], it is perhaps not too surprising that the normalization of epidermal thickness occurs in a non-apoptotic dependent fashion involving terminal differentiation and shedding. In the gut, mucosal barrier defects were also found to respond rapidly to anti-TNFα therapy [40].
In conclusion, this study demonstrates for the first time a series of rapidly occurring molecular and cellular events by which adalimumab can reverse pathological events in both the epidermis and dermis of psoriatic plaques. The mechanism of action for adalimumab in psoriatic skin involves reduction in the inflammatory cell infiltration by TNFα positive DCs and macrophages, followed by T cells predominantly involving a non-apoptotic sequence of events. Similarly in the epidermis, adalimumab rapidly restores epidermal homeostasis without a significantly detectible apoptotic series of events. Targeting TNFα clearly impacts a multitude of cellular responses at the morphological and cellular level; and these results clearly highlight the dynamic and reversible interplay between immunocytes and keratinocytes that contribute to the maintenance of psoriatic plaques [41].
Acknowledgments
The authors thank Ms. Lynn Walter and Mrs. Lori Kmet for manuscript preparation. This study was supported by a grant from the National Institutes of Health (AR40065), and Abbott Laboratory, Abbott Park, IL, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. J Am Acad Dermatol. 2002;46:1–23. doi: 10.1067/mjd.2002.120568. [DOI] [PubMed] [Google Scholar]
- 2.McKay IA, Leigh IM. Altered keratinocyte growth and differentiation in psoriasis. Clin Dermatol. 1995;13:105–114. doi: 10.1016/0738-081x(95)93817-8. [DOI] [PubMed] [Google Scholar]
- 3.Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest. 2004;113:1664–1675. doi: 10.1172/JCI22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752–759. doi: 10.1046/j.1523-1747.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- 5.Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and “Type 1” inflammatory gene expression. Trends Immunol. 2004;25:295–305. doi: 10.1016/j.it.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Uyemura K, Yamamura M, Fivenson DF, Modlin RL, Nickoloff BJ. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701–705. doi: 10.1111/1523-1747.ep12371679. [DOI] [PubMed] [Google Scholar]
- 7.Nickoloff BJ, Karabin GD, Barker JN, Griffiths CE, Sarma V, Mitra RS, Elder JT, Kunkel SL, Dixit VM. Cellular localization of interleukin-8 and its inducer, tumor necrosis factor-alpha in psoriasis. Am J Pathol. 1991;138:129–140. [PMC free article] [PubMed] [Google Scholar]
- 8.Gisondi P, Gubinelli E, Cocuroccia B, Girolomoni G. Targeting tumor necrosis factor-alpha in the therapy of psoriasis. Curr Drug Targets Inflamm Allergy. 2004;3:175–183. doi: 10.2174/1568010043343903. [DOI] [PubMed] [Google Scholar]
- 9.Krueger G, Callis K. Potential of tumor necrosis factor inhibitors in psoriasis and psoriatic arthritis. Arch Dermatol. 2004;140:218–225. doi: 10.1001/archderm.140.2.218. [DOI] [PubMed] [Google Scholar]
- 10.Saripalli YV, Gaspari AA. Focus on: biologics that affect therapeutic agents in dermatology. J Drugs Dermatol. 2005;4:233–245. [PubMed] [Google Scholar]
- 11.Gottlieb AB, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA, Chen F, Magliocco M, Krueger JG. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol. 2005;175:2721–2729. doi: 10.4049/jimmunol.175.4.2721. [DOI] [PubMed] [Google Scholar]
- 12.Malaviya R, Sun Y, Tan JK, Wang A, Magliocco M, Yao M, Krueger JG, Gottlieb AB. Etanercept induces apoptosis of dermal dendritic cells in psoriatic plaques of responding patients. J Am Acad Dermatol. 2006;55:590–597. doi: 10.1016/j.jaad.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Goedkoop AY, Kraan MC, Teunissen MB, Picavet DI, de Rie MA, Bos JD, Tak PP. Early effects of tumour necrosis factor alpha blockade on skin and synovial tissue in patients with active psoriasis and psoriatic arthritis. Ann Rheum Dis. 2004;63:769–773. doi: 10.1136/ard.2003.018085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansbridge JN, Knapp AM, Strefling AM. Evidence for an alternative pathway of keratinocyte maturation in psoriasis from an antigen found in psoriatic but not normal epidermis. J Invest Dermatol. 1984;83:296–301. doi: 10.1111/1523-1747.ep12340429. [DOI] [PubMed] [Google Scholar]
- 15.Bockelmann R, Horn T, Gollnick H, Bonnekoh B. Interferon-gamma-dependent in vitro model for the putative keratin 17 autoimmune loop in psoriasis: exploration of pharmaco- and gene-therapeutic effects. Skin Pharmacol Physiol. 2005;18:42–54. doi: 10.1159/000081685. [DOI] [PubMed] [Google Scholar]
- 16.Eckert RL, Crish JF, Efimova T, Dashti SR, Deucher A, Bone F, Adhikary G, Huang G, Gopalakrishnan R, Balasubramanian S. Regulation of involucrin gene expression. J Invest Dermatol. 2004;123:13–22. doi: 10.1111/j.0022-202X.2004.22723.x. [DOI] [PubMed] [Google Scholar]
- 17.Tomic-Canic M, Komine M, Freedberg IM, Blumenberg M. Epidermal signal transduction and transcription factor activation in activated keratinocytes. J Dermatol Sci. 1998;17:167–181. doi: 10.1016/s0923-1811(98)00016-4. [DOI] [PubMed] [Google Scholar]
- 18.Iizuka H, Takahashi H, Honma M, Ishida-Yamamoto A. Unique keratinization process in psoriasis: late differentiation markers are abolished because of the premature cell death. J Dermatol. 2004;31:271–276. doi: 10.1111/j.1346-8138.2004.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 19.Ishida-Yamamoto A, Iizuka H. Differences in involucrin immunolabeling within cornified cell envelopes in normal and psoriatic epidermis. J Invest Dermatol. 1995;104:391–395. doi: 10.1111/1523-1747.ep12665870. [DOI] [PubMed] [Google Scholar]
- 20.Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10:119–126. [PubMed] [Google Scholar]
- 21.Milstone LM. Epidermal desquamation. J Dermatol Sci. 2004;36:131–140. doi: 10.1016/j.jdermsci.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Pittelkow MR. Psoriasis: more than skin deep. Nat Med. 2005;11:17–18. doi: 10.1038/nm0105-17. [DOI] [PubMed] [Google Scholar]
- 23.Banno T, Gazel A, Blumenberg M. Effects of tumor necrosis factor-alpha (TNF alpha) in epidermal keratinocytes revealed using global transcriptional profiling. J Biol Chem. 2004;279:32633–32642. doi: 10.1074/jbc.M400642200. [DOI] [PubMed] [Google Scholar]
- 24.Gordon KB, Bonish BK, Patel T, Leonardi CL, Nickoloff BJ. The tumour necrosis factor-alpha inhibitor adalimumab rapidly reverses the decrease in epidermal Langerhans cell density in psoriatic plaques. Br J Dermatol. 2005;153:945–953. doi: 10.1111/j.1365-2133.2005.06816.x. [DOI] [PubMed] [Google Scholar]
- 25.Baima B, Sticherling M. How specific is the TUNEL reaction? An account of a histochemical study on human skin. Am J Dermatopathol. 2002;24:130–134. doi: 10.1097/00000372-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995;21:1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez-Steil C, Wrone-Smith T, Sun X, Krueger JG, Coven T, Nickoloff BJ. Sunlight-induced basal cell carcinoma tumor cells and ultraviolet-B-irradiated psoriatic plaques express Fas ligand (CD95L) J Clin Invest. 1998;101:33–39. doi: 10.1172/JCI1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wrone-Smith T, Mitra RS, Thompson CB, Jasty R, Castle VP, Nickoloff BJ. Keratinocytes derived from psoriatic plaques are resistant to apoptosis compared with normal skin. Am J Pathol. 1997;151:1321–1329. [PMC free article] [PubMed] [Google Scholar]
- 29.Qin JZ, Chaturvedi V, Denning MF, Bacon P, Panella J, Choubey D, Nickoloff BJ. Regulation of apoptosis by p53 in UV-irradiated human epidermis, psoriatic plaques and senescent keratinocytes. Oncogene. 2002;21:2991–3002. doi: 10.1038/sj.onc.1205404. [DOI] [PubMed] [Google Scholar]
- 30.Ettehadi P, Greaves MW, Wallach D, Aderka D, Camp RD. Elevated tumour necrosis factor-alpha (TNF-alpha) biological activity in psoriatic skin lesions. Clin Exp Immunol. 1994;96:146–151. doi: 10.1111/j.1365-2249.1994.tb06244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansen C, Funding AT, Otkjaer K, Kragballe K, Jensen UB, Madsen M, Binderup L, Skak-Nielsen T, Fjording MS, Iversen L. Protein expression of TNF-alpha in psoriatic skin is regulated at a posttranscriptional level by MAPK-activated protein kinase 2. J Immunol. 2006;176:1431–1438. doi: 10.4049/jimmunol.176.3.1431. [DOI] [PubMed] [Google Scholar]
- 32.Lowes MA, Chamian F, Abello MV, Fuentes-Duculan J, Lin SL, Nussbaum R, Novitskaya I, Carbonaro H, Cardinale I, Kikuchi T, Gilleaudeau P, Sullivan-Whalen M, Wittkowski KM, Papp K, Garovoy M, Dummer W, Steinman RM, Krueger JG. Increase in TNF-{alpha} and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a) Proc Natl Acad Sci U S A. 2005;102:19057–19062. doi: 10.1073/pnas.0509736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abrams JR, Kelley SL, Hayes E, Kikuchi T, Brown MJ, Kang S, Lebwohl MG, Guzzo CA, Jegasothy BV, Linsley PS, Krueger JG. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells. J Exp Med. 2000;192:681–694. doi: 10.1084/jem.192.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, Bala M, Marano CW, Menter A. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51:534–542. doi: 10.1016/j.jaad.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Tsai JC, Feingold KR, Crumrine D, Wood LC, Grunfeld C, Elias PM. Permeability barrier disruption alters the localization and expression of TNF alpha/protein in the epidermis. Arch Dermatol Res. 1994;286:242–248. doi: 10.1007/BF00387595. [DOI] [PubMed] [Google Scholar]
- 36.Nickoloff BJ, Naidu Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J Am Acad Dermatol. 1994;30:535–546. doi: 10.1016/s0190-9622(94)70059-1. [DOI] [PubMed] [Google Scholar]
- 37.Nickoloff BJ. The cytokine network in psoriasis. Arch Dermatol. 1991;127:871–884. [PubMed] [Google Scholar]
- 38.Flores I, Murphy DJ, Swigart LB, Knies U, Evan GI. Defining the temporal requirements for Myc in the progression and maintenance of skin neoplasia. Oncogene. 2004;23:5923–5930. doi: 10.1038/sj.onc.1207796. [DOI] [PubMed] [Google Scholar]
- 39.Wrone-Smith T, Johnson T, Nelson B, Boise LH, Thompson CB, Nunez G, Nickoloff BJ. Discordant expression of Bcl-x and Bcl-2 by keratinocytes in vitro and psoriatic keratinocytes in vivo. Am J Pathol. 1995;146:1079–1088. [PMC free article] [PubMed] [Google Scholar]
- 40.Suenaert P, Bulteel V, Vermeire S, Noman M, Van Assche G, Rutgeerts P. Hyperresponsiveness of the mucosal barrier in Crohn’s disease is not tumor necrosis factor-dependent. Inflamm Bowel Dis. 2005;11:667–673. doi: 10.1097/01.mib.0000168371.87283.4b. [DOI] [PubMed] [Google Scholar]
- 41.Nickoloff BJ, Bonish BK, Marble DJ, Schriedel KA, DiPietro LA, Gordon KB, Lingen MW. Lessons learned from psoriatic plaques concerning mechanisms of tissue repair, remodeling, and inflammation. J Investig Dermatol Symp Proc. 2006;11:16–29. doi: 10.1038/sj.jidsymp.5650010. [DOI] [PubMed] [Google Scholar]