Abstract
Falls are an important cause of morbidity in older adults and are an important source of health care spending. We hypothesize that falls are associated with systemic biomarkers of aging. The following functions, measured at the 1998–2000 and 2003–05 examinations of the Beaver Dam Eye study, were considered to be biomarkers of aging (frailties): poorer visual acuity, contrast sensitivity or discrepant vision between the eyes, inability to rise from a chair, slower gait time, poorer hand grip strength, and lower peak expiratory flow rate. We found that poorer values on biomarkers of aging (frailties) at the 1998–2000 examination were associated with 2 or more reported falls in the past year at the 2003–05 examination (p < 0.05 for all markers except peak expiratory flow rate). When the markers were combined as an index of biological aging (frailty), the index was significantly associated with falls after controlling for significant confounders (odds ratio per 1 step increase in the index: 1.33; 95% confidence interval = CI = 1.13–1.57) We conclude that biomarkers of aging, including any of three measures of visual function, are associated with falls. Improvement in these functional measures may lead to decreased risk of falls.
Keywords: falls risk, biomarkers of aging, visual function
1. Introduction
Falls are an important health problem for older adults with about 30% of persons 65 years of age or older sustaining at least one fall each year (O’Loughlin et al., 1993). Falls result in significant morbidity (Tinetti et al., 1988; Nevitt et al., 1991; Sattin, 1992) and mortality (CDCP, 2006). Costs for falls in the elderly in the United States are estimated at over $19 billion annually (Stevens et al., 2006). Falls and the resultant trauma are thought to be related to a variety of decreased physical functions that are sometimes grouped together as measures of biomarkers of aging or frailty. Frailty results from declines in multiple systems (Bortz 1993; Campbell and Buchner 1997; Hamerman 1999). It differs from disability or morbidity in that it is meant to describe a general decrease in functional status (Chin et al., 1999; Fried et al., 2001). Biomarkers of frailty include reduced lung function (Cook et al., 1991), decreased mobility, slower gait-time, decrease in muscle strength, and difficulty in rising from a chair (Klein et al., 2005). These signs may be accompanied by a reduced ability to rebound from illness or injuries, leading to increased mortality (Cook et al., 1991; Anstey et al., 2001; Fried et al., 2001; Klein et al., 2005) and morbidity (Tinetti et al., 1986; Nevitt et al., 1989; Tinetti et al., 1995; Klein et al., 2005). Because frailty involves several functional deficits, it would be expected that frail persons might be at risk of falls and other traumatic events. It is our purpose to investigate the relationship of individual biomarkers of aging, including three measures of visual function, to falls in older community residing adults. We examined these relationships both prospectively and cross-sectionally in these persons.
2. Subjects and methods
2.1. Population and setting
A private census in the town of Beaver Dam, Wisconsin, in 1987–88 enumerated 5924 persons between the ages of 43 to 84 years. A baseline examination was conducted in 1988–90 (N = 4926). All subjects who were eligible for participation in the baseline examination (Klein et al., 1991) were invited for three follow-up examinations (1993–95, 1998–2000–2003–05). Tenets of the Declaration of Helsinki were followed. Signed consent was obtained from each participant at each examination. Institutional review board approval was obtained yearly. The differences between participants and nonparticipants at each examination have been previously published (Klein et al., 1991, 1996, 2001, 2006). Those who were alive but not participating at later visits were older and had poorer visual acuity. For this report, we use measurements at the 1998–2000 and 2003–05 follow-up examinations of the cohort because all of the biomarker measurements were not taken at previous examinations. Only pertinent parts of the examination are described.
2.2. Falls assessment
Participants were asked, “During the past 12 months, have you fallen and landed on the floor or ground, or fallen and hit an object like a table or stair while not doing sports?” Persons that answered “yes” were further asked how many times they had fallen with categorical responses of 1, 2–3, 4–5, or 6 or more falls. For this report, we define a multiple faller as a person who had fallen 2 or more times in the previous 12 months as answered at the 2003–05 examination. We chose more than one fall as our definition because we assume that even the most robust individuals may trip on an unexpected obstacle or slip on the ice during a given year.
2.3. Biomarkers of aging assessment
Participants able to walk were instructed to walk a measured 8 foot course at their usual pace. The time was recorded (gait-time) (Klein et al., 2003). The participant was then seated in a standard chair (seat 19.5 inches from the floor) which was against the wall. The participants who felt that it was safe for them to stand up without help were asked to do so without using the arms of the chair. If unable to do so, the participant was instructed to stand up using the arms of the chair. The method (with or without arms) was recorded. For purposes of this report, we defined inability to rise from the chair without arms in the first attempt. The peak expiratory flow rate was measured using the mini-Wright meter (Klein et al., 2003). While standing, the participant was asked to take as deep a breath as possible and to blow as hard and fast as possible into the meter as s/he was able. This was repeated two more times, and the highest value (greatest flow rate) was used in the analysis. Dynamometry was performed separately in each hand two times. The mean of two measures for the dominant hand was used in these analyses (Klein et al., 2003). Distance visual acuity was measured according to a modification of the Early Treatment Diabetic Retinopathy Study (ETDRS) protocol (Klein et al., 1996) for each eye and was denoted as best-corrected visual acuity. Results were given as the number of letters read correctly, as well as Snellen equivalents ranging from 20/10 to no light perception. Contrast sensitivity was measured using the Pelli-Robson chart in each eye (Pelli et al., 1988).
For purposes of analysis, we categorized the aging biomarkers as follows: best corrected visual acuity was categorized into 20/20 or better, 20/25–20/32, and 20/40 or poorer in better seeing eye; contrast sensitivity was categorized into ≥ 1.65, 1.50, and ≤ 1.35 log triplet score in the eye with the better contrast sensitivity (Pelli et al., 1988); discrepant vision was defined as a difference of 15 or more letters between eyes in the best-corrected visual acuity test; inability to rise from a chair without using arms; gait time, hand grip strength, and peak expiratory flow rate were categorized in sex-specific quartiles. The poorest category for each function was considered to indicate the presence of frailty for that function. The total of such frailties was summed into an index of biological aging (frailty) (Table 1) for each individual.
Table 1.
Biological aging (frailty) index definition
Add 1 to the index for any of the following:
|
2.4. Comorbidity assessment
Blood pressure, height, and body weight were measured according to study protocols (Klein and Klein, 1999) during the examination. Medications were brought to the examination and their names and frequency of use were recorded. Other lifestyle and medical history information was collected using a standard questionnaire. A comorbidity index was created by adding 1 to the index for presence of any of the following: hypertension (systolic ≥ 140 mmHg or diastolic ≥ 90 mmHg or use of anti-hypertensive medication), diabetes, history of cancer, arthritis, gout, Parkinson’s disease, Alzheimer’s disease, asthma, or cardiovascular disease (angina, myocardial infarction or stroke).
2.5. Data analysis
Analytic techniques included calculations of means and proportions and logistic regression for multivariate modeling of the probability of falling 2 or more times in the year prior to the 15-year follow-up examination. The biomarker measurements of interest were either collected 5 years prior or at the time the falling question was asked; we investigated biomarker measurements from both examinations in multivariate analyses. In some instances falls may cause poor function on one or more of the tests. Therefore, we present biomarker measurements taken at the 1998–2000 examination and reported falls at the 2003–05 examination as our primary analysis. However, measurements taken closer to the falling events may be more representative of the aging (frailty) statues at the time of the falls so we include biomarker measurements taken at the 2003–05 examination as a secondary analysis. A total of 2375 participated in the 15-year follow-up examination with 2106 (89%) having complete data for the primary analysis and 2256 (95%) having complete data for the secondary analysis.
3. Results
3.1. Population characteristics by falling history
A past history of falling 2 or more times in the previous year was reported in 7% of the population at the 2003–05 examination. Characteristics of the population at the 2003–05 examination by history of falling are shown in Table 2. Falling was related to age and after controlling for age was related to having history of diabetes, cancer, arthritis, Parkinson’s disease, Alzheimer’s disease, cardiovascular disease, being in a nursing home, and taking more medications. Sex, body mass index, waist-hip ratio, hypertension, smoking, taking vitamin D, vitamin B12, folic acid, statin drugs, or use of hormone replacement therapy were not related to falls in the previous year.
Table 2.
Population characteristics by history of falling, Beaver Dam eye study 2003–05
| 0 or 1 fall past year (n=2100) | ≥ 2 falls past year (n=156) | |||
|---|---|---|---|---|
| Characteristic | Mean ± S.D., or % | Mean ± S.D., or % | p = | p-age adjusted |
| Age, mean years | 71.3 ± 8.7 | 76.0 ± 10.2 | < 0.001 | |
| Body mass index, mean kg/m2 | 30.4 ± 6.0 | 30.2 ± 6.7 | 0.63 | 0.50 |
| Waist-hip ratio, mean | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.36 | 0.37 |
| Female, % | 57.4 | 67.3 | 0.02 | 0.07 |
| Smoking history, % | ||||
| Never | 48.0 | 47.4 | 0.50 | 0.41 |
| Past | 42.9 | 46.2 | ||
| Current | 9.0 | 6.4 | ||
| Hypertension, % | 64.3 | 71.1 | 0.11 | 0.33 |
| Diabetes, % | 14.7 | 27.3 | < 0.001 | < 0.001 |
| Cancer, % | 24.6 | 35.3 | < 0.01 | 0.04 |
| Arthritis, % | 50.0 | 71.8 | < 0.001 | < 0.001 |
| Gout, % | 9.8 | 11.7 | 0.48 | 0.74 |
| Parkinson’s Disease, % | 0.7 | 5.1 | < 0.001 | < 0.001 |
| Alzheimer’s Disease, % | 0.7 | 6.5 | < 0.001 | < 0.001 |
| Asthma, % | 9.0 | 10.3 | 0.57 | 0.52 |
| CVD history, % | 16.3 | 36.8 | < 0.001 | < 0.001 |
| Nursing home, % | 3.0 | 16.0 | < 0.001 | < 0.001 |
| >2 comorbidities, % | 19.2 | 40.4 | < 0.001 | < 0.001 |
| Sedentary Lifestyle, % | 67.8 | 76.3 | 0.03 | 0.25 |
| Number of medications taken | 8.3 ± 4.4 | 11.2 ± 5.2 | < 0.001 | <0.001 |
Notes: BP = blood pressure; CVD = cardiovascular disease
3.2. Associations of biomarkers of aging with falling history
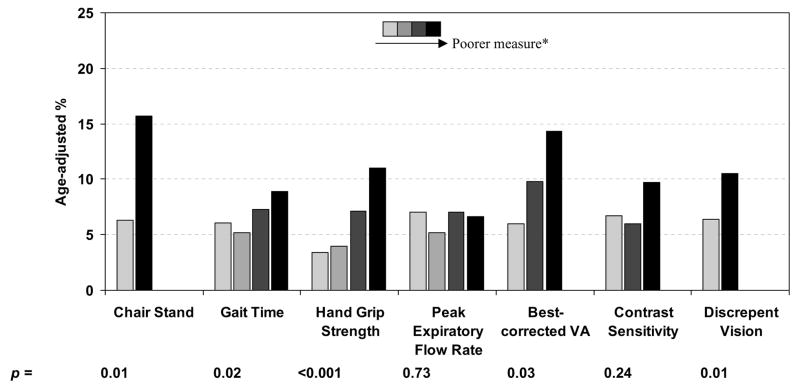
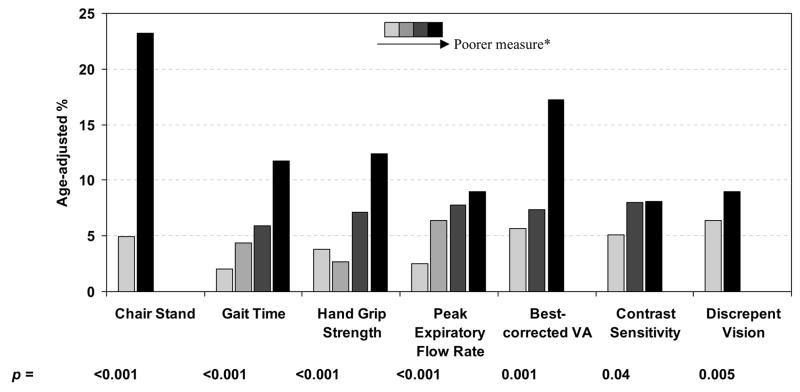
Age-adjusted risk for 2 or more falls in the previous year by the various biomarkers of aging measured in the 1998–2000 examination (Figure 1a) and the 2003–05 examination (Figure 1b) are shown in Figure 1. After controlling for age, poorer performance on all measured factors except peak expiratory flow rate at the 1998–2000 examination were associated with higher rates of multiple falls (p < 0.05 for all associations). We combined all measures into a biological aging (frailty) index (Table 1) and investigated the association of this index with falls (Table 3). After controlling for age, an increase in the index measured in the 1998–2000 examination was associated with an increase in falls risk (odds ratio = OR = per 1 step increase 1.38; 95% confidence interval = CI = 1.17–1.62). In a multivariate logistic regression analysis, sex, number of medications being taken, and number of comorbid conditions were the only additional factors significantly related to reported falls (data not shown). Controlling for these factors, the association between the index score and falls was slightly attenuated but still highly significant (OR per 1 step increase 1.33; 95% CI = 1.12–1.57).
Figure 1.
Age adjusted rates of 2 or more falls in the past year as reported at the 2003–05 examination between biomarker measurements at the 1998–2000 examination (a) and at the 2003–05 examination (b)
* Darker colored bars represent poorer measurements than lighter colored bars: Chair Stand: ability/inability to rise from chair without using arms in 1 attempt Gait Time, Hand Grip Strength, Peak Expiratory Flow Rate: defined in sex-specific quartiles (e.g., darkest bar represents highest quartile of gait-time).
Best corrected visual acuity categories: 20/20 or better; 20/25 or 20/32; 20/40 or poorer in better seeing eye
Contrast sensitivity categories: ≥ 1.65; 1.50; ≤ 1.35 log triplet score in better seeing eye
Discrepant vision: no/yes (best-corrected visual acuity difference between eyes ≥ 15 letters)
Table 3.
Biological aging (frailty) index and risk of 2 or more falls past year (reported during the 2003–05 examination of the Beaver Dam eye study)
| Age-adjusted |
Multivariate a adjusted |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Index Score | No. at risk | No. of multiple fallers | Crude % | Age- adjusted % | Odds Ratio | 95% CI | p = | Odds Ratio | 95% CI | p = |
| Index measured at the 1998–2003 examination | ||||||||||
| 0 | 1167 | 51 | 4.4 | 5.0 | 1.00 | -- | 1.00 | -- | ||
| 1 | 518 | 36 | 6.9 | 7.1 | 1.41 | 0.89–2.24 | 0.14 | 1.30 | 0.81–2.06 | 0.28 |
| 2 | 258 | 26 | 10.1 | 6.3 | 1.89 | 1.09–3.27 | 0.02 | 1.75 | 1.01–3.03 | 0.05 |
| 3 | 103 | 16 | 15.5 | 21.5 | 2.85 | 1.45–5.60 | 0.002 | 2.45 | 1.24–4.85 | 0.01 |
| 4 | 47 | 8 | 17.0 | 20.6 | 3.00 | 1.23–7.28 | 0.02 | 2.53 | 1.02–6.27 | 0.05 |
| 5 | 13 | 4 | 30.8 | 21.1 | 5.89 | 1.61–21.6 | 0.007 | 5.54 | 1.49–20.6 | 0.01 |
|
| ||||||||||
| Trend | 1.38 | 1.17–1.62 | < 0.001 | 1.33 | 1.13–1.57 | < 0.001 | ||||
|
| ||||||||||
| Index measured at the 2003–05 examination | ||||||||||
| 0 | 931 | 27 | 2.9 | 2.3 | 1.00 | -- | 1.00 | -- | ||
| 1 | 528 | 23 | 4.4 | 5.8 | 1.51 | 0.85–2.68 | 0.16 | 1.37 | 0.77–2.46 | 0.28 |
| 2 | 365 | 29 | 7.9 | 7.6 | 2.83 | 1.59–5.04 | < 0.001 | 2.38 | 1.32–4.28 | 0.004 |
| 3 | 234 | 39 | 16.7 | 11.1 | 6.49 | 3.57–11.8 | < 0.001 | 4.84 | 2.63–8.93 | < 0.001 |
| 4 | 159 | 27 | 17.0 | 29.9 | 6.61 | 3.41–12.8 | < 0.001 | 4.82 | 2.45–9.47 | < 0.001 |
| 5 | 39 | 11 | 28.2 | 24.3 | 12.69 | 5.34–30.2 | < 0.001 | 11.00 | 4.49–26.95 | < 0.001 |
|
| ||||||||||
| Trend | 1.67 | 1.46–1.91 | < 0.001 | 1.58 | 1.37–1.81 | < 0.001 | ||||
further adjusted for sex, total number of medications being taken and total number of comorbid conditions (hypertension, diabetes, cancer, arthritis, gout, Parkinson’s disease, Alzheimer’s disease, asthma, and cardiovascular disease).
We performed a parallel analysis investigating the relationship of the same biomarkers of aging at the 2003–05 examination to falls reported in the previous year at the 2003–05 examination. The association between the index and falls was similar to that found in the above analysis (Table 3). A one step increase in the index resulted in a 58% increased risk of falls (95% CI = 1.37–1.81).
4. Discussion
We have found that in adults living in a community setting, the biomarkers of aging that we studied are associated with risk of falls. In addition, the higher the index value the greater the risk. These data add to past reports concerning data from persons in selected communities (Ghodsi et al., 2003), from recently ill persons, or from persons in clinic or hospital settings (Kerse et al., 2004) of associations between biomarkers of aging and falls. Our population is younger than most and not selected based on disability, thus broadening the inferences of the findings of an association between the biomarkers and falls. This is notable since falls are an important cause of morbidity even in the population of persons who are still in an age range where some are working (Tinetti et al., 1988; Am. Geriatr. Soc. 2001).
Some (Gillespie et al., 2003; Cigolle et al., 2007), but not all, studies of biomarkers of aging include measures of visual function (Li et al., 2005; Sattin et al., 2005; Faber et al., 2006) and some use self-reported visual function (Cigolle et al., 2007). We describe falls with regard to three measures of visual function and included scores on any of the three measures as a marker of aging. We take this approach because we find that each of the three functions we include is associated with falls in our population. We include visual acuity because it is the most frequently measured function and is the basis for driver licensing in many places. It is, however, a high contrast acuity test and some have preferred testing of contrast sensitivity (Lord, 2006). In previous analyses in our data, contrast sensitivity and high contrast acuity are both correlated with risk of falls (Klein et al., 1998), but the same individuals are not necessarily deficient in both measures, so we use either of these measures to be more inclusive. We also include the third measure, discrepancy in visual acuity between the two eyes, because it is possible that this may be associated with diminished stereopsis and/or may cause visual confusion (Nevitt et al., 1989; Lord, 2006) leading to increased risk of falls.
We include more traditional biomarkers of aging in our panel, including ability to stand without using the arms of the chair, gait time, peak expiratory flow rate, and hand grip strength. The chair stand, gait time, and hand grip strength are likely to reflect muscle strength which is important in falls prevention. In addition, gait time and chair stand may reflect ability to balance. Peak expiratory flow rate is a general measure of robustness and is associated with risk of mortality (Cook et al., 1991). The measures of vision add information on sensory function to these measures of motor function and vitality, thus complementing each other and adding sensitivity to the combined biomarker of aging (frailty) index.
Province et al. (1995), Gillespie et al. (2001), Li et al. (2005), Faber et al. (2006) suggested that significant improvements in risk of falls can be achieved through exercise programs, although this finding is not universal (Cumming et al., 2007). However, there have been few studies aimed at decreasing the risk of falls by improving visual function (Day et al., 2002; Harwood et al., 2005; Foss et al., 2006). Some of these have been limited by the poor follow-up for treatment (Day et al., 2002) or dealt only with cataract surgery (Harwood et al., 2005; Foss et al., 2006). The randomized controlled trial by Cumming et al. (2007) took a more comprehensive approach to diagnosing and intervening upon vision abnormalities for the prevention of falls in older adults. The findings from that study were disappointing in that the intervention group fared no better than the control with respect to falls in the following year. It has been suggested that the changes in perception wrought by intervention may have been more difficult to adapt to than was the poor vision (personal communication of Dr. Robert G. Cumming). It is important to follow-up on this study and to also design trials to improve visual function as part of a multidisciplinary intervention program.
While there are many strengths to our study, there are also limitations. For cross-sectional findings, falls data reflect responses to a question about the year prior to measurements of the biomarkers. However, the results are essentially unchanged when evaluating the history of falls about five years after measuring the biomarkers. In addition, although we control for many relevant confounders in our analyses, we do not have a measure of cognitive ability. While age and other co-morbidities are likely to capture much of the effect of diminished cognition, we cannot be sure that this is the case. Further study of this is planned. In addition, persons with fatal or severely disabling falls did not participate fully in all procedures at the relevant examinations. This, however, is likely to lead to an underestimate of the relationship between the biomarkers and falls. Lastly, we defined history of falls as more than one in the past year. This perhaps conservative definition may have decreased our sensitivity and thus may have caused us to underestimate the relationship as well.
5. Conclusion
In summary, persons with inability to rise from a chair without using the arms of the chair, slower gait time, weaker hand grip strength, poorer visual function, and a higher score on the index of biological aging (frailty) had significantly increased history of falling. This was independent of other risk factors. The biological aging index may be a good measure to evaluate persons at risk of falling.
Acknowledgments
Role of funding source: The National Eye Institute provided funding for entire study including collection and analyses and of data (Grant No. EY06594; R Klein, BEK Klein); Research to Prevent Blindness provided further additional support for data analyses (R. Klein, BEK Klein, Senior Scientific Investigator Awards, New York, NY). There was no other involvement by the sponsors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Am. Geriatr. Soc., British Geriatr. Soc., Am. Acad. Orthop. Surg. Panel on falls prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc. 2001;49:664–672. [PubMed] [Google Scholar]
- Anstey KJ, Luszcz MA, Giles LC, Andrews GR. Demographic, health, cognitive, and sensory variables as predictors of mortality in very old adults. Psychol Aging. 2001;16:3–11. doi: 10.1037/0882-7974.16.1.3. [DOI] [PubMed] [Google Scholar]
- Bortz WM. The physics of frailty. J Am Geriatr Soc. 1993;41:1004–1008. [PubMed] [Google Scholar]
- Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age Ageing. 1997;26:315–318. doi: 10.1093/ageing/26.4.315. [DOI] [PubMed] [Google Scholar]
- CDCP (Centers for Disease Control Prevention) Fatalities and injuries from falls among older adults - United States, 1993–2003 and 2001–2005. Morb Mortal Wkly Rep. 2006;55:1221–1224. [PubMed] [Google Scholar]
- Chin MH, Jin L, Karrison TG, Mulliken R, Hayley DC, Walter J, Miller A, Friedmann PD. Older patients’ health-related quality of life around an episode of emergency illness. Ann Emerg Med. 1999;34:595–603. doi: 10.1016/s0196-0644(99)70161-7. [DOI] [PubMed] [Google Scholar]
- Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007;147:156–164. doi: 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- Cook NR, Evans DA, Scherr PA, Speizer FE, Taylor JO, Hennekens CH. Peak expiratory flow rate and 5-year mortality in an elderly population. Am J Epidemiol. 1991;133:784–794. doi: 10.1093/oxfordjournals.aje.a115957. [DOI] [PubMed] [Google Scholar]
- Cumming RG, Ivers R, Clemson L, Cullen J, Hayes MF, Tanzer M, Mitchell P. Improving vision to prevent falls in frail older people: a randomized trial. J Am Geriatr Soc. 2007;55:175–181. doi: 10.1111/j.1532-5415.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- Day L, Fildes B, Gordon I, Fitzharris M, Flamer H, Lord S. Randomised factorial trial of falls prevention among older people living in their own homes. Br Med J. 2002;325:128. doi: 10.1136/bmj.325.7356.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber MJ, Bosscher RJ, Chin APM, Van Wieringen PC. Effects of exercise programs on falls and mobility in frail and pre-frail older adults: A multicenter randomized controlled trial. Arch Phys Med Rehabil. 2006;87:885–896. doi: 10.1016/j.apmr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Foss AJ, Harwood RH, Osborn F, Gregson RM, Zaman A, Masud T. Falls and health status in elderly women following second eye cataract surgery: a randomised controlled trial. Age Ageing. 2006;35:66–71. doi: 10.1093/ageing/afj005. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, Mcburnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A: Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Ghodsi SM, Roudsari BS, Abdollahi M, Shadman M. Fall-related injuries in the elderly in Tehran. Injury. 2003;34:809–814. doi: 10.1016/s0020-1383(02)00376-5. [DOI] [PubMed] [Google Scholar]
- Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev. 2001:CD000340. doi: 10.1002/14651858.CD000340. [DOI] [PubMed] [Google Scholar]
- Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev. 2003:CD000340. doi: 10.1002/14651858.CD000340. [DOI] [PubMed] [Google Scholar]
- Hamerman D. Toward an understanding of frailty. Ann Intern Med. 1999;130:945–950. doi: 10.7326/0003-4819-130-11-199906010-00022. [DOI] [PubMed] [Google Scholar]
- Harwood RH, Foss AJ, Osborn F, Gregson RM, Zaman A, Masud T. Falls and health status in elderly women following first eye cataract surgery: a randomised controlled trial. Br J Ophthalmol. 2005;89:53–59. doi: 10.1136/bjo.2004.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerse N, Butler M, Robinson E, Todd M. Fall prevention in residential care: a cluster, randomized, controlled trial. J Am Geriatr Soc. 2004;52:524–531. doi: 10.1111/j.1532-5415.2004.52157.x. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Lee KE, Cruickshanks KJ. Performance-based and self-assessed measures of visual function as related to history of falls, hip fractures, and measured gait time. The Beaver Dam Eye Study. Ophthalmology. 1998;105:160–164. doi: 10.1016/s0161-6420(98)91911-x. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Knudtson MD, Lee KE. Relationship of measures of frailty to visual function: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2003;101:191–196. [PMC free article] [PubMed] [Google Scholar]
- Klein BE, Klein R, Knudtson MD, Lee KE. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;41:141–149. doi: 10.1016/j.archger.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE. US Department of Commerce. Springfield; VA: 1999. The Beaver Dam Eye Study III. Manual of Operations. NTIS Accession No. PB99–137861. [Google Scholar]
- Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98:1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103:1169–1178. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Lee KE, Cruickshanks KJ, Chappell RJ. Changes in visual acuity in a population over a 10-year period: The Beaver Dam Eye Study. Ophthalmology. 2001;108:1757–1766. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Lee KE, Cruickshanks KJ, Gangnon RE. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142:539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Li F, Harmer P, Fisher KJ, McAuley E, Chaumeton N, Eckstrom E, Wilson NL. Tai Chi and fall reductions in older adults: a randomized controlled trial. J Gerontol A: Biol Sci Med Sci. 2005;60:187–194. doi: 10.1093/gerona/60.2.187. [DOI] [PubMed] [Google Scholar]
- Lord SR. Visual risk factors for falls in older people. Age Ageing. 2006;35(Suppl 2):ii42–ii45. doi: 10.1093/ageing/afl085. [DOI] [PubMed] [Google Scholar]
- Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. J Am Med Assoc. 1989;261:2663–2668. [PubMed] [Google Scholar]
- Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: a prospective study. J Gerontol. 1991;46:M164–M170. doi: 10.1093/geronj/46.5.m164. [DOI] [PubMed] [Google Scholar]
- O’Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137:342–354. doi: 10.1093/oxfordjournals.aje.a116681. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci. 1988;2:187–199. [Google Scholar]
- Province MA, Hadley EC, Hornbrook MC, Lipsitz LA, Miller JP, Mulrow CD, Ory MG, Sattin RW, Tinetti ME, Wolf SL. The effects of exercise on falls in elderly patients. A preplanned meta-analysis of the FICSIT Trials Frailty and Injuries: Cooperative Studies of Intervention Techniques. J Am Med Assoc. 1995;273:1341–1347. [PubMed] [Google Scholar]
- Sattin RW. Falls among older persons: a public health perspective. Annu Rev Public Health. 1992;13:489–508. doi: 10.1146/annurev.pu.13.050192.002421. [DOI] [PubMed] [Google Scholar]
- Sattin RW, Easley KA, Wolf SL, Chen Y, Kutner MH. Reduction in fear of falling through intense tai chi exercise training in older, transitionally frail adults. J Am Geriatr Soc. 2005;53:1168–1178. doi: 10.1111/j.1532-5415.2005.53375.x. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj Prev. 2006;12:290–295. doi: 10.1136/ip.2005.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80:429–434. doi: 10.1016/0002-9343(86)90717-5. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Doucette J, Claus E, Marottoli R. Risk factors for serious injury during falls by older persons in the community. J Am Geriatr Soc. 1995;43:1214–1221. doi: 10.1111/j.1532-5415.1995.tb07396.x. [DOI] [PubMed] [Google Scholar]