Abstract
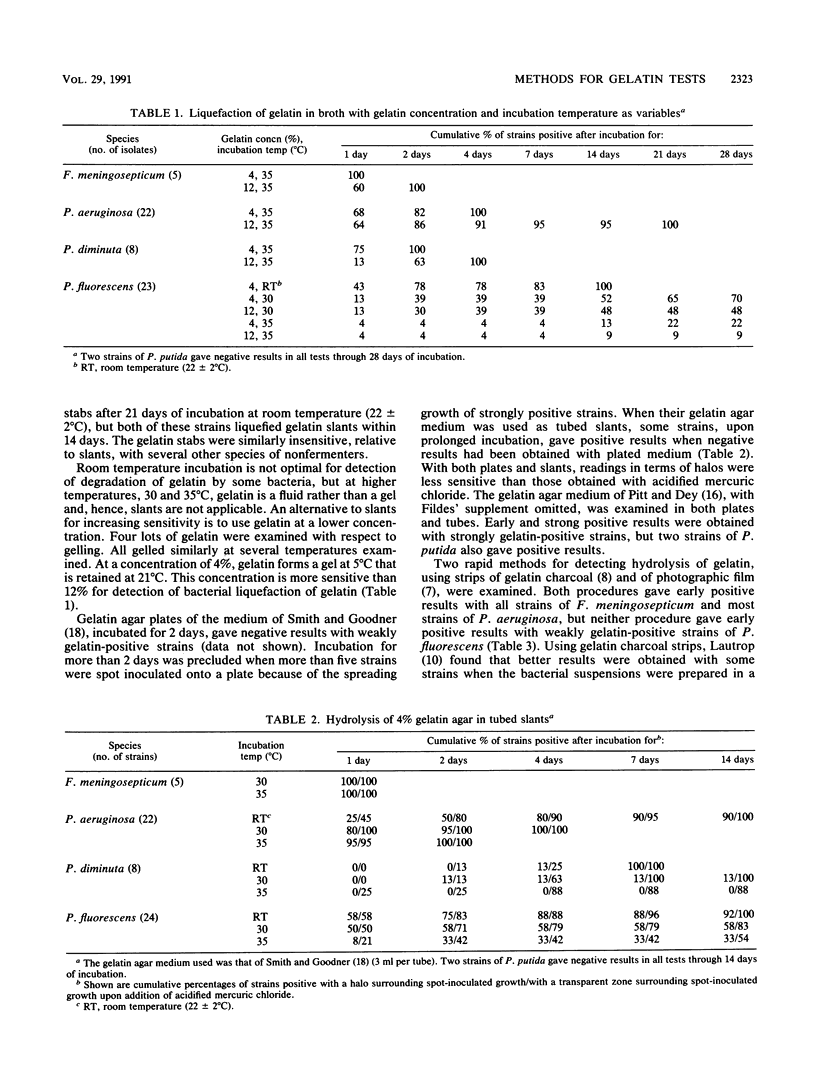
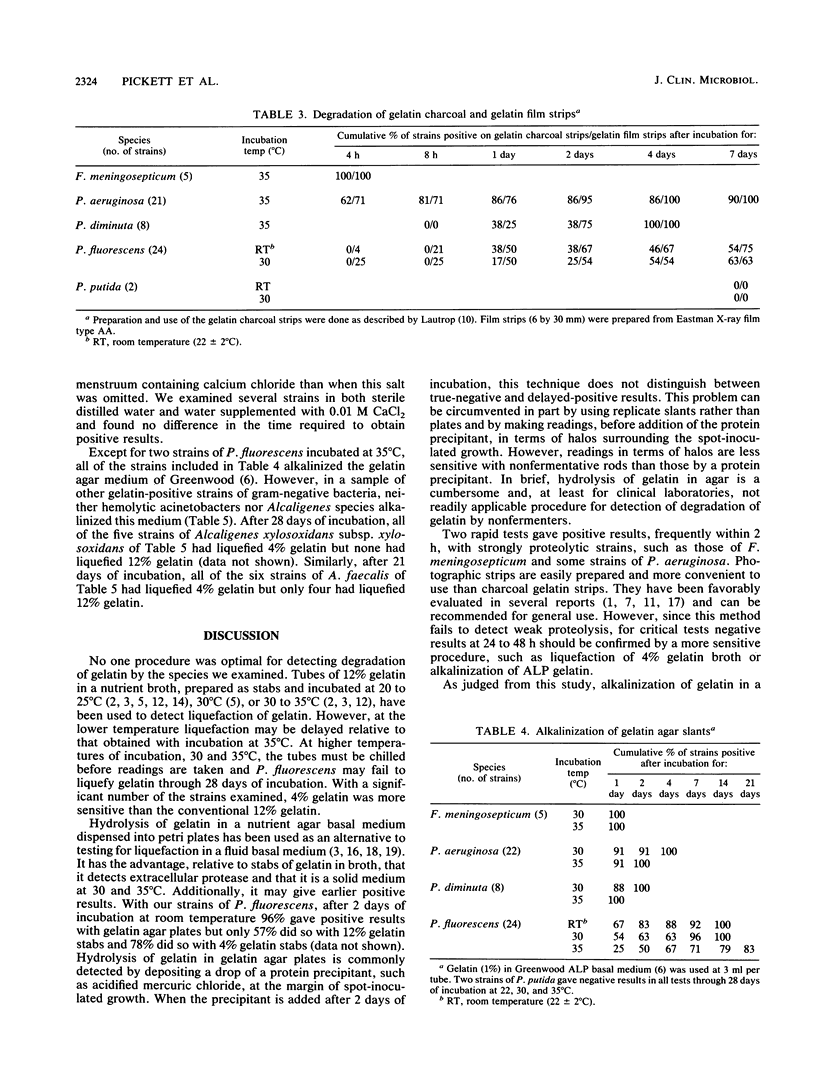
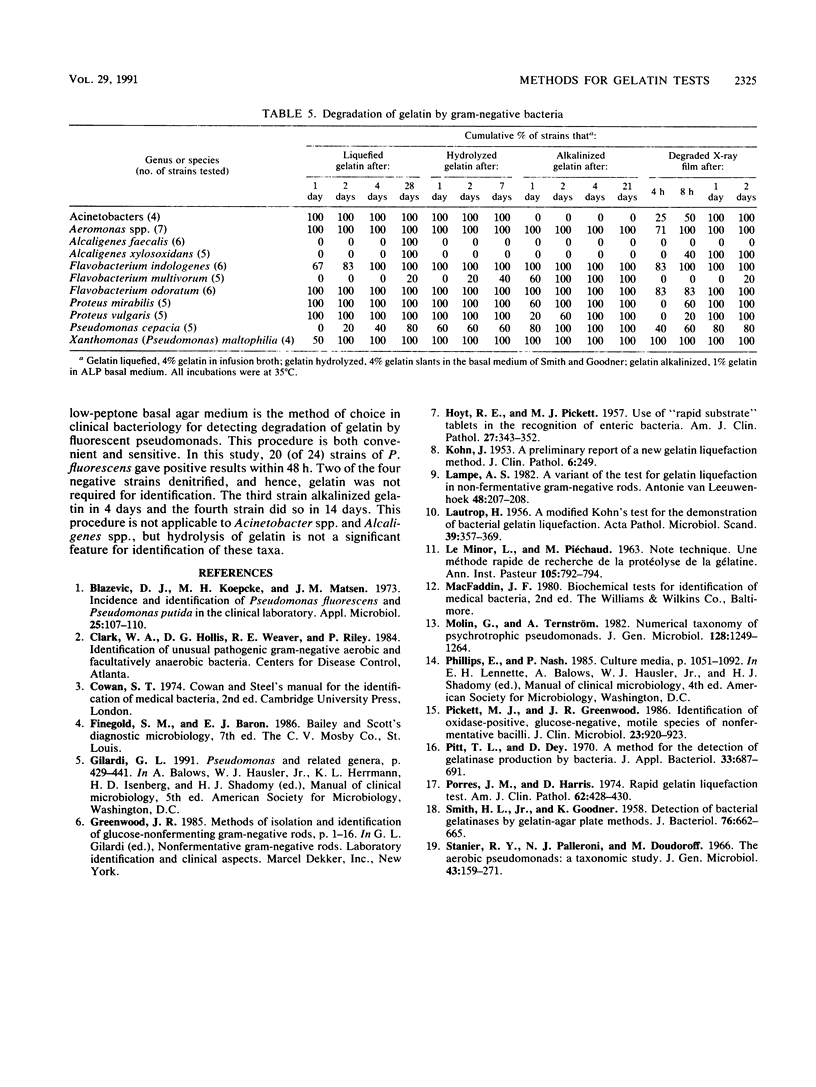
Five methods for detecting degradation of gelatin by bacteria were compared. These were liquefaction in nutrient broth, hydrolysis in nutrient agar, hydrolysis of charcoal gelatin strips, degradation of the gelatin on strips of photographic film, and alkalinization of gelatin agar. Degradation of photographic film is a rapid and convenient method but, like hydrolysis of gelatin in broth and in agar, may fail to detect weakly positive strains of bacteria. Alkalinization of gelatin in an agar medium is a convenient and sensitive method to detect degradation of gelatin, particularly by Pseudomonas fluorescens, but this method may not be applicable to some species.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blazevic D. J., Koepcke M. H., Matsen J. M. Incidence and identification of Pseudomonas fluorescens and Pseudomonas putida in the clinical laboratory. Appl Microbiol. 1973 Jan;25(1):107–110. doi: 10.1128/am.25.1.107-110.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYT R. E., PICKETT M. J. Use of rapid substrate tablets in the recognition of enteric bacteria. Am J Clin Pathol. 1957 Mar;27(3):343–352. doi: 10.1093/ajcp/27.3_ts.343. [DOI] [PubMed] [Google Scholar]
- KOHN J. A preliminary report of a new gelatin liquefaction method. J Clin Pathol. 1953 Aug;6(3):249–249. doi: 10.1136/jcp.6.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAUTROP H. A modified Kohn's test for the demonstration of bacterial gelatin liquefaction. Acta Pathol Microbiol Scand. 1956;39(5):357–369. doi: 10.1111/j.1699-0463.1956.tb03413.x. [DOI] [PubMed] [Google Scholar]
- LEMINOR L., PIECHAUD M. NOTE TECHNIQUE. UNE M'ETHODE RAPIDE DE RECHERCHE DE LA PROT'EOLYSE DE LA G'ELATINE. Ann Inst Pasteur (Paris) 1963 Oct;105:792–794. [PubMed] [Google Scholar]
- Molin G., Ternström A. Numerical taxonomy of psychrotrophic pseudomonads. J Gen Microbiol. 1982 Jun;128(6):1249–1264. doi: 10.1099/00221287-128-6-1249. [DOI] [PubMed] [Google Scholar]
- Pickett M. J., Greenwood J. R. Identification of oxidase-positive, glucose-negative, motile species of nonfermentative bacilli. J Clin Microbiol. 1986 May;23(5):920–923. doi: 10.1128/jcm.23.5.920-923.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt T. L., Dey D. A method for the detection of gelatinase production by bacteria. J Appl Bacteriol. 1970 Dec;33(4):687–691. doi: 10.1111/j.1365-2672.1970.tb02251.x. [DOI] [PubMed] [Google Scholar]
- Porres J. M., Harris D. Rapid gelatin liquefaction test. Am J Clin Pathol. 1974 Sep;62(3):428–430. doi: 10.1093/ajcp/62.3.428. [DOI] [PubMed] [Google Scholar]
- Risse S. C., Lampe T. H., Cubberley L. Very low-dose neuroleptic treatment in two patients with agitation associated with Alzheimer's disease. J Clin Psychiatry. 1987 May;48(5):207–208. [PubMed] [Google Scholar]
- SMITH H. L., Jr, GOODNER K. Detection of bacterial gelatinases by gelatin-agar plate methods. J Bacteriol. 1958 Dec;76(6):662–665. doi: 10.1128/jb.76.6.662-665.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]


