Abstract
Background: Common warts (verrucae vulgares) are human papilloma virus (HPV) infections with a high incidence and prevalence, most often affecting hands and feet, being able to impair quality of life. About 30 different therapeutic regimens described in literature reveal a lack of a single striking strategy. Recent publications showed positive results of photodynamic therapy (PDT) with 5-aminolevulinic acid (5-ALA) in the treatment of HPV-induced skin diseases, especially warts, using visible light (VIS) to stimulate an absorption band of endogenously formed protoporphyrin IX. Additional experiences adding waterfiltered infrared A (wIRA) during 5-ALA-PDT revealed positive effects.
Aim of the study: First prospective randomised controlled blind study including PDT and wIRA in the treatment of recalcitrant common hand and foot warts.
Comparison of "5-ALA cream (ALA) vs. placebo cream (PLC)" and "irradiation with visible light and wIRA (VIS+wIRA) vs. irradiation with visible light alone (VIS)".
Methods: Pre-treatment with keratolysis (salicylic acid) and curettage. PDT treatment: topical application of 5-ALA (Medac) in "unguentum emulsificans aquosum" vs. placebo; irradiation: combination of VIS and a large amount of wIRA (Hydrosun® radiator type 501, 4 mm water cuvette, waterfiltered spectrum 590-1400 nm, contact-free, typically painless) vs. VIS alone. Post-treatment with retinoic acid ointment. One to three therapy cycles every 3 weeks. Main variable of interest: "Percent change of total wart area of each patient over the time" (18 weeks). Global judgement by patient and by physician and subjective rating of feeling/pain (visual analogue scales).
80 patients with therapy-resistant common hand and foot warts were assigned randomly into one of the four therapy groups with comparable numbers of warts at comparable sites in all groups.
Results: The individual total wart area decreased during 18 weeks in group 1 (ALA+VIS+wIRA) and in group 2 (PLC+VIS+wIRA) significantly more than in both groups without wIRA (group 3 (ALA+VIS) and 4 (PLC+VIS)): medians and interquartile ranges:
-94% (-100%/-84%) vs. -99% (-100%/-71%) vs. -47% (-75%/0%) vs. -73% (-92%/-27%).
After 18 weeks the two groups with wIRA differed remarkably from the two groups without wIRA: 42% vs. 7% completely cured patients; 72% vs. 34% vanished warts.
Global judgement by patient and by physician and subjective rating of feeling was much better in the two groups with wIRA than in the two groups without wIRA.
Conclusions: The above described complete treatment scheme of hand and foot warts (keratolysis, curettage, PDT treatment, irradiation with VIS+wIRA, retinoic acid ointment; three therapy cycles every 3 weeks) proved to be effective. Within this treatment scheme wIRA as non-invasive and painless treatment modality revealed to be an important, effective factor, while photodynamic therapy with 5-ALA in the described form did not contribute recognisably - neither alone (without wIRA) nor in combination with wIRA - to a clinical improvement.
For future treatment of warts an even improved scheme is proposed: one treatment cycle (keratolysis, curettage, wIRA, without PDT) once a week for six to nine weeks.
Keywords: warts, waterfiltered infrared A (wIRA), photodynamic therapy (PDT), 5-aminolevulinic acid (5-ALA), human papilloma virus (HPV)
Abstract
Hintergrund: Vulgäre Warzen (Verrucae vulgares) sind humane Papillomvirus-Infektionen (HPV) mit einer hohen Inzidenz und Prävalenz, die am häufigsten Hände und Füße befallen und die in der Lage sind, die Lebensqualität zu beeinträchtigen. Etwa 30 in der Literatur beschriebene Therapieverfahren zeugen von einem Mangel an einer einzigen überzeugenden Strategie. Jüngste Veröffentlichungen zeigten positive Ergebnisse der Photodynamischen Therapie (PDT) mit 5-Aminolävulinsäure (5-ALA) in der Therapie von HPV-induzierten Hautkrankheiten, besonders Warzen, wobei sichtbares Licht (VIS) verwendet wird, um ein Absorptionsband des endogen aus 5-ALA gebildeten Protoporphyrin IX zu stimulieren. Weitere Erfahrungen, wassergefiltertes Infrarot A (wIRA) während der 5-ALA-PDT zusätzlich anzuwenden, offenbarten positive Wirkungen.
Ziel der Untersuchung: Erste prospektive randomisierte kontrollierte Blind-Studie, die PDT und wIRA in die Behandlung von therapierefraktären vulgären Hand- und Fußwarzen einbezieht.
Vergleich von "5-ALA-Salbe (ALA) vs. Placebo-Salbe (PLC)" und "Bestrahlung mit sichtbarem Licht und wIRA (VIS+wIRA) vs. Bestrahlung mit sichtbarem Licht allein (VIS)".
Methoden: Vorbehandlung mit Keratolyse (Salizylsäure) und Kürettage. Photodynamische Therapie (PDT): topische Applikation von 5-ALA (Medac) in "Unguentum emulsificans aquosum" vs. Placebo; Bestrahlung: Kombination von sichtbarem Licht (VIS) und einem hohen Maß an wassergefiltertem Infrarot A (wIRA) (Hydrosun®-Strahler Typ 501, 4 mm Wasserküvette, wassergefiltertes Spektrum 590-1400 nm, kontaktfrei, typischerweise schmerzlos) vs. sichtbares Licht (VIS) allein. Nachbehandlung mit Vitamin-A-Säure-Salbe. Ein bis drei Therapiezyklen im Abstand von 3 Wochen. Hauptzielvariable: "Prozentuale Änderung der Gesamtwarzenfläche jedes Patienten über die Zeit" (18 Wochen). Globales Urteil von Patient und von Arzt sowie subjektive Einschätzung von Empfindung/Schmerz (visuelle Analogskalen).
80 Patienten mit therapierefraktären vulgären Hand- und Fußwarzen wurden randomisiert einer der vier Behandlungsgruppen (mit vergleichbarer Anzahl an Warzen in vergleichbaren Lokalisationen in allen Gruppen) zugeteilt.
Ergebnisse: Die individuelle Gesamtwarzenfläche nahm während 18 Wochen in Gruppe 1 (ALA+VIS+wIRA) und in Gruppe 2 (PLC+VIS+wIRA) signifikant mehr als in den beiden Gruppen ohne wIRA (Gruppe 3 (ALA+VIS) und 4 (PLC+VIS)) ab: Mediane und Interquartil-Spannen:
-94% (-100%/-84%) vs. -99% (-100%/-71%) vs. -47% (-75%/0%) vs. -73% (-92%/-27%).
Nach 18 Wochen unterschieden sich die zwei Gruppen mit wIRA deutlich von den zwei Gruppen ohne wIRA: 42% vs. 7% komplett geheilte Patienten; 72% vs. 34% völlig verschwundene Warzen.
Das globale Urteil von Patient und von Arzt und die subjektive Einschätzung des Empfindens waren in den zwei Gruppen mit wIRA viel besser als in den zwei Gruppen ohne wIRA.
Folgerungen: Das oben beschriebene vollständige Therapieschema von Hand- und Fußwarzen (Keratolyse, Kürettage, Photodynamische Therapie, Bestrahlung mit VIS+wIRA, Vitamin-A-Säure-Salbe; drei Therapiezyklen im Abstand von 3 Wochen) erwies sich als effektiv. Innerhalb des Therapieschemas zeigte sich wIRA - als nicht-invasive und schmerzlose Therapiemodalität - als ein wichtiger, effektiver Faktor, während die Photodynamische Therapie mit 5-ALA in der beschriebenen Form nicht erkennbar - weder alleine (ohne wIRA) noch in Kombination mit wIRA - zu einer klinischen Verbesserung beitrug.
Für die zukünftige Behandlung von Warzen wird ein weiter verbessertes Schema vorgeschlagen: ein Therapiezyklus (Keratolyse, Kürettage, wIRA, ohne PDT) einmal pro Woche für sechs bis neun Wochen.
Introduction
Common warts (verrucae vulgares) are human papilloma virus (HPV) infections with a high incidence and prevalence, most often affecting hands and feet, being able to impair quality of life of the patients by unsightly appearance, pain and contagion. About 30 different therapeutic regimens described in the literature [1], [2], [3] reveal a lack of a single striking strategy with high efficacy and low side-effects.
Photodynamic therapy (PDT) is one of the most accepted procedures with best published evidence of efficacy in the treatment of warts [4], [5].
At the starting point (June 2001) of an intensive ten month planning period for this first prospective randomised controlled blind study including PDT and waterfiltered infrared A (wIRA) in the treatment of common warts, the following knowledge was already available:
• Stender [6] had published in 2000 a very remarkable randomised controlled double-blind study showing very good results of PDT in recalcitrant warts with topical application of 5-aminolevulinic acid (5-ALA) compared to placebo treatment. She used visible light (VIS) to stimulate an absorption band of protoporphyrin IX, which is endogenously transformed out of 5-ALA.
• Some dermatologists, especially Strasser [7], reported - including computer based photodocumentation - very good results in large numbers of patients with hand and foot warts: Strasser combined a pre-treatment with keratolysis and curettage, a PDT treatment with topical application of 5-ALA and a combination of VIS and wIRA (Hydrosun® radiator, type 500), and a post-treatment with retinoic acid ointment.
PDT is based on the interaction of a photosensitiser with visible light (defined as within the range 380-780 nm) and reacting substances within the cells (e.g. lipids) either without oxygen (photooxidative reaction type I) or with oxygen (photooxidative reaction type II) [8]. Most widely used in dermatology is a topical application of 5-ALA, which is endogenously transformed in proliferating cells (e.g. keratinocytes) into the photosensitiser, protoporphyrin IX [5], [6], [8], [9], [10]. (Even combined irradiation with visible light and infrared in 5-ALA-PDT has been described [11].) This procedure is non-invasive, repeatable, and besides sunburn-like sensations (possible discomfort and itching during irradiation, possible erythema and photosensibilisation in case of sun exposure during approximately 1 day after application), free of known side-effects [12], [13], [14], [15]. Especially the discomfort during irradiation seems to depend upon the spectral property of the used radiator (e.g. unfiltered infrared parts of radiation and total thermal burden to the skin).
Waterfiltered infrared radiation, especially waterfiltered infrared A (wIRA, within 780-1400 nm), allows - compared to unfiltered heat radiation of conventional infrared bulbs with large amounts of infrared B (defined as 1400-3,000 nm) and C (defined as 3,000-1,000,000 nm) - a multiple energy transfer into tissue without irritating the skin. The filter effect of water decreases those parts of infrared radiation (absorption bands of water within infrared A and most parts of infrared B and C), which would bring - by reacting with water molecules in the skin - only a thermal burden to the surface of the skin. wIRA mainly consists of radiation with good penetration into tissue, similar to sun heat radiation in moderate climatic zones, which is filtered by water vapour in the atmosphere.
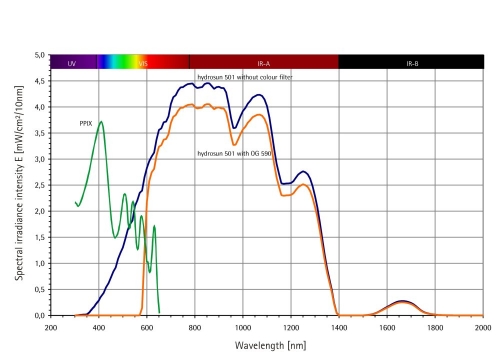
Technically wIRA is produced in special radiators, whose whole incoherent radiation of a 3,000 kelvin halogen bulb is passed through a cuvette, containing water (absorbing the described undesired wavelengths): the resulting spectrum with visible light (VIS) and wIRA is shown in Figure 1 (Fig. 1).
Figure 1. Spectra of Hydrosun® 501 and Protoporphyrin IX (PPIX).
Hydrosun®: Calculated for Hydrosun® 501 with 4 mm water cuvette and orange filter OG590 at 250 mW/cm² (= 2.5 x 103 W/m²) total irradiance intensity, from Measurement of University of Applied Sciences Munich, dated 30th June 1999
PPIX: Qualitative presentation (e.g. only relative scaling of ordinate scale) for comparison of absorption diameter of protoporphyrin IX (PPIX) monomeres in DMSO [25]
wIRA produces a therapeutically usable field of warmth in tissue by (i) good penetration of this radiation into the depth of tissue, by (ii) reaching capillaries near the surface and by transport of warmth by bloodflow into deeper layers, by (iii) increasing bloodflow near the surface and increasing by this the second mechanism, by (iv) conduction of warmth into the depth, and by (v) increased energy production (metabolism) of tissue caused by increased temperature. Beside these thermal effects infrared A - even with very small irradiance intensity and in the unfiltered form - is able to stimulate cells and evoke a cascade of reactions [16], [17], [18], [19], [20], [21].
In the planning phase of this study - taking all available information in published or unpublished form into account - the following two aims were achieved: first: to describe a practical optimised treatment procedure (including pre- and post-treatment) for recalcitrant HPV-induced hand and foot warts on the basis of PDT and waterfiltered infrared A. Second: to create a design for the study.
Aim of the study
Performing a prospective randomised controlled blind study including PDT and wIRA in the treatment of recalcitrant common hand and foot warts.
Comparison of "5-ALA cream (ALA) vs. placebo cream (PLC)" and "irradiation with visible light and wIRA (VIS+wIRA) vs. irradiation with visible light alone (VIS)".
Methods and design
Leading aspects of study design
• All aspects of therapy (pre-treatment, PDT treatment, and post-treatment) were given in the same optimised manner to all groups of patients with the exception of the two mentioned factors, resulting in 4 therapy groups:
Group 1: 20% 5-ALA cream + VIS+wIRA = ALA + VIS+wIRA (= PDT + wIRA)
Group 2: Placebo cream + VIS+wIRA = PLC + VIS+wIRA
Group 3: 20% 5-ALA cream + VIS = ALA + VIS (= "standard PDT")
Group 4: Placebo cream + VIS = PLC + VIS
This scheme gives the best possibility to examine the effects of 5-ALA-PDT, the effect of wIRA, and the interaction of both, when embedded in an optimal setting of pre-treatment and post-treatment. This gives the best chance to avoid overestimation of effects as all results can be evaluated relative to the minimally treated group 4 (getting keratolysis, curettage, placebo cream, visible light irradiation, and retinoic acid ointment), which can be expected to be better than the spontaneous course.
• In order to avoid severe biomathematical and interpretational problems, as seen in the already mentioned remarkable study of Stender [6], randomised assignment to therapeutical groups was not done for individual warts, but for patients (= all warts of a patient are assigned to the same treatment), as the bearer of characteristics is the patient and not the wart. This is the appropriate biomathematical way to exclude possible systemic interactions between different treatments of different warts within the same patient.
• 80 patients were randomised within 10 time blocks of 8 patients to the four therapy groups purely on the sequence of the patients being included in the study (= after checking all inclusion and exclusion criteria). This means, that there is no stratification with regard to the location of the warts. (Each time block consists of 2 patients belonging to each of the four therapeutic groups: smallest possible block size which allows no information about the allocation of another patient of the same time block to a therapy group, not even that he or she belongs to a different therapy group.)
Inclusion and exclusion criteria
• Inclusion criteria were (i) voluntary participation, (ii) age between 18 and 65 years, (iii) any sex, (iv) warts on hands and/or feet, clinically verified, resistant to at least one previous therapy, (v) preceding therapies of any type had to be unsuccessful for two months and they had to be discontinued at least 4 weeks before the start of the study, (vi) agreement not to use other therapies during the study, (vii) given written informed consent (with possibility to withdraw from the study at any time without a negative impact on their further treatment at the Department of Dermatology, Jena).
• Exclusion criteria were (i) immunosuppressive therapy, (ii) internal and/or dermatological diseases with suppressed immunity, (iii) pregnancy, lactation, and uncertain contraception, (iv) any local therapy of verrucae within the last 4 weeks before the start of the study, and (v) lack of cooperation.
Pre-treatment
• Keratolytic pre-treatment with Guttaplast® bandages (6 x 9 cm containing 1.39 g salicylic acid) [22], [23], being started by the patients 7 days in advance with foot warts and 3 days in advance with hand warts until the day of PDT treatment.
• Abrasion (curettage without bleeding) of well softened wart tissues by the treating physician in the morning of the PDT treatment day in order to optimise the penetration conditions for ALA cream.
PDT treatment
• 5-aminolevulinic acid (5-ALA) was chosen as photosensitiser with regard to the worldwide large experiences with 5-ALA (in a lyophilised, pyrogen-free, sterile form, produced by Medac GmbH, Wedel, Germany, according to the guidelines for "good manufacturing practice"). Especially the alternative methyl-ALA, which does not seem to bring better penetration, but has to be metabolised to 5-ALA by splitting of a potentially toxic methyl group, was not chosen.
• Emphasis was on using an optimised galenic preparation for 5-ALA in order to reach best penetration conditions:
As 5-ALA cream (abbreviated as ALA) was used: 20% 5-aminolevulinic acid (5-ALA) in "unguentum emulsificans aquosum" (water containing hydrophilic cream, German pharmacopoeia "DAB 2002"; consisting of: emulgating cetylstearylalcohol type A 9.0, viscous paraffin 10.5, white vaseline 10.5, water 70.0).
As placebo cream (abbreviated as PLC) "unguentum emulsificans aquosum" was used.
The two creams could not be distinguished by inspection nor by smell.
The creams were applied in a thin, yet occluding layer (0.2 g/cm²), covering the lesion and also the potentially subclinically affected areas, at least 5 mm around. A bandage (Tegaderm®) was applied over the test areas for 6 hours. After this period the bandage and residual cream was removed (before irradiation).
• In order to have good penetration conditions, the treated area (hand or foot) had to be kept warm (in passive manner) from before administering the cream until its removal, as a higher tissue temperature increases ALA penetration and production of protoporphyrin IX [24].
• For PDT irradiation two kinds of radiators, one with VIS+wIRA, one with VIS only (the latter serving as radiator for the control groups 3 and 4), were used, differing only concerning the missing of wIRA in the emitted spectrum of the latter, but both radiators emitting the same visible spectrum (for stimulating the photosensitiser protoporphyrin IX). By inspection, the two radiators could not be distinguished. (As the presence or absence of wIRA can be felt by the treating physician by comparing the radiation, the study was performed single instead of double blind.)
Used were Hydrosun® radiators (Hydrosun® Medizintechnik, Müllheim, Germany, radiator type 501, 4 mm water cuvette, orange filter OG590, waterfiltered spectrum: in case of VIS+wIRA: 590-1400 nm, in case of VIS 590-780 nm). The distance from the radiator to the treated uncovered skin area of the patient is standardized by a 25 cm distance rod. At that distance the irradiance intensity is nearly homogeneous with approximately 250 mW/cm² within a diameter of 10 cm (with the optical axis as center), consisting of approximately 60 mW/cm² in the visible range and approximately 190 mW/cm² wIRA, when using the radiator with VIS+wIRA. (When using the radiator with VIS only, irradiance intensity is approximately 60 mW/cm², being completely within the visible range.) In accordance with previous clinical experience in therapy of verrucae with 5-ALA-PDT [6] and the good acceptance of wIRA, we choose 30 minutes irradiation, resulting in a total radiation dose of 450 J/cm² with approximately 110 J/cm² in the visible range and approximately 340 J/cm² wIRA, when using the radiator with VIS+wIRA. (When using the radiator with VIS only, total radiation dose was approximately 110 J/cm², being completely within the visible range.) If the patient felt warmth to become uncomfortable during irradiation, this beginning discomfort could be stopped by increasing the distance of irradiation by 5 or 10 cm, keeping the duration of irradiation unchanged.
• The form eventually used with the orange filter OG590 (Schott, Mainz, Germany) (used in both radiators), excluding wavelengths below 590 nm, is in accordance with other common radiators for PDT with 5-ALA (including the one used by [6]). It uses only one (629 nm) absorption band of protoporphyrin IX and avoids the severe disadvantage of an undesired steeper gradient (from the surface to the depth of tissue) of the cumulated irradiance intensity of all absorption bands in case of not using the orange filter OG590, thus reaching the full spectrum of a white radiation with the stimulation of all five absorption bands (approximately 406 nm, 505 nm, 540 nm, 574 nm, 629 nm [25]) of protoporphyrin IX (as the four absorption bands towards blue show highest absorption and effectiveness, see Figure 1 (Fig. 1) and [25], but lower penetration into tissue than the single absorption band at approximately 629 nm [25]).
• In a limited number of patients of all groups, controls with Wood's light (approximately 406-407 nm as important wavelength to show up red (637 nm) fluorescence of protoporphyrin IX [26]) before and immediately after each PDT were planned to document the enrichment of protoporphyrin IX in wart tissue and to investigate a bleaching effect caused by irradiation. (Both recent publications about 5-ALA-PDT in warts [6], [10] found fluorescence of intralesionally formed protoporphyrin IX.)
Post-treatment
• Patients were warned not to expose the PDT-treated areas to direct sunlight for the first 2 days.
• After an interval of 2-3 days, the patients self applied a retinoic acid ointment (Balisa VAS-Creme®, containing 120 mg urea and 0.3 mg tretinoin (= retinoic acid) per g cream) twice daily.
Repetition of treatment cycle
• The entire procedure (pre-treatment, PDT treatment, and post-treatment) was repeated after 3 weeks and after 6 weeks, if warts had not cleared by then. Only areas (including a margin of approximately 5 mm) that differed in appearance from surrounding healthy skin were treated to avoid overtreatment.
The results were controlled after 3, 6, 9, 12 and 18 weeks.
Variables of interest, documentation, and statistical analysis
• The main variable of interest (to be tested concerning the whole time period in confirmatory statistics) was the outcome variable "percent change of total wart area of each patient over the time". The areas were measured planimetrically after drawing them onto transparent foils, scanning and analysing with free shareware software program (Scion Image for Windows, www.scioncorp.com) [27]. These measurements were made 0, 3, 6, 9, 12 and 18 weeks after the start of the therapy (t0 defined as morning of day of first PDT treatment before curettage). At the same time photodocumentation was taken with overview picture to localize each wart and detailed picture of each wart.
• Descriptive variables of interest
The aspect of subjective rating (feeling) was assessed before administering the cream, 6 hours later (= immediately before irradiation), and immediately after irradiation (at 0, 3, 6 weeks, and in addition once at 9 weeks) with the following question to be answered on a visual analogue scale (VAS): "How comfortable do you presently feel (with respect to pain, burn, itch etc.) about your wart covered region?" (0 = extremely uncomfortable, 100 mm = free of complaints).
To evaluate the over all judgement separately by the patient and the physician, the following question to be answered with VAS was used (after 3, 6 and 9 weeks): "How do you estimate the effect of the therapy?" (-50 mm = extreme deterioration, 0 = no effect, +50 mm = extreme improvement).
Visual analogue scales of 100 mm (well established to objectivise subjective ratings) have the advantage that the result can easily be transformed into one of 101 discrete steps, which brings a much higher differentiation than discrete verbal scales with e.g. a five point scale.
• In order to avoid all problems of parametric statistics (even descriptive parametric statistics) - e.g. as variables in clinical medicine often show an unsymmetrical distribution (this is especially true for the variable "percent change of total wart area of each patient") - only methods of non-parametric statistics (both descriptive and confirmatory) were used.
• Already in the planning phase it was decided to compare the four treatment groups concerning all clinically relevant time periods of all variables of interest descriptively (median, percentiles of 25 and 75, minimum, maximum) and concerning the only main variable of interest in addition the "percent change of total wart area of each patient between t0 and 18 weeks" confirmatorily with the non-parametric Mann-Whitney U test for unpaired samples. The total error probability was set to 0.05 (5%), applying Bonferroni alpha-error correction with alpha* = alpha/4, as the following four comparisons should be evaluated: group 1 vs. group 4, group 2 vs. group 4, group 3 vs. group 4, group 1 vs. group 2. Statistical analysis was performed with the Graph Pad Prism 4.0 for Windows package (San Diego, USA).
Realisation
Prospective, randomised, controlled (2 factors [radiation, photosensitiser] controlled [VIS+wIRA vs. VIS, 5-ALA vs. Placebo], four armed) study in patients with therapy-resistant common warts on either hands and/or feet.
The protocol was approved by the Ethics Committee of the Friedrich Schiller University, Jena.
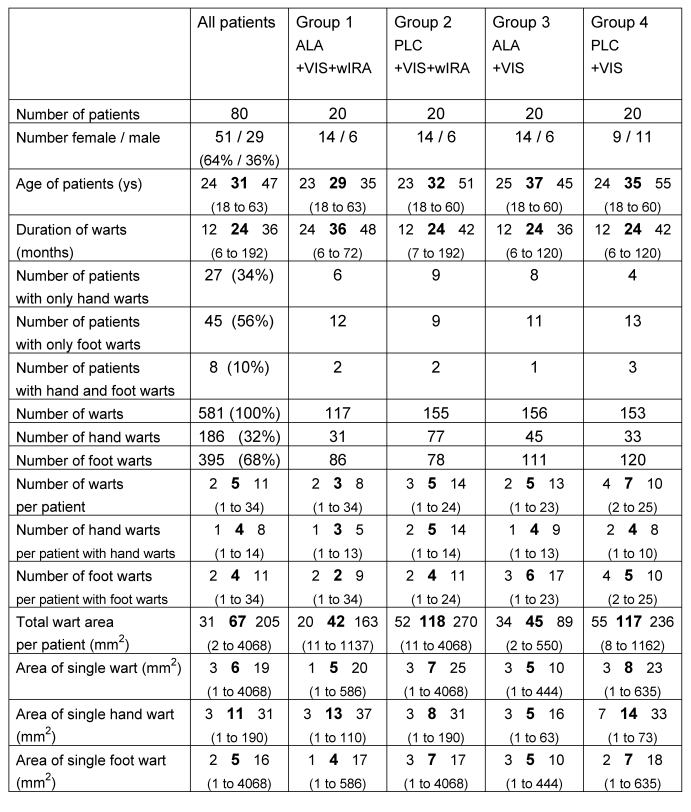
From May 2002 to October 2002, 80 patients (51 female, 29 male, median age 31 (range 18-63) years, see further characterization in Table 1 (Tab. 1)), fulfilling the inclusion criteria and none of the exclusion criteria, were assigned randomly into one of the four therapy groups (4 x 20) with comparable numbers of warts at comparable sites in all groups and treated in the described form. The 581 hand and foot warts were of different sizes and categories, including small to large solitary lesions and multiple lesions in clusters of mosaic warts, and the median duration of warts at entry was 24 (range 6-192) months.
Table 1. Characteristics of n=80 patients at time of entry.
(given as number and percentage or otherwise as percentile of 25, median, percentile of 75 and range (minimum to maximum))
Compliance with treatment was excellent. Especially no single patient complained pain after administering the cream nor during or after the irradiation.
8 patients did not continue to participate after one treatment, additional 3 after two treatments (in one patient caused by a change in living area), one patient did not show up for the controls after the third treatment: in total 12 patients did not continue till the end of the study. No systematic differences between the drop outs and their groups, to which they belonged, were seen. Perhaps the number of dropouts in the groups 3 and 4 was higher due to the smaller therapeutic effect.
So out of the 80 patients, 68 completed the entire study (43 female, 25 male) with a median age of 32 (range 18-63) years and a total of 500 hand and foot warts; the median duration of warts at entry was 24 (range 6-192) months. Within these 68 patients, 19 patients with 104 warts belonged to group 1 (ALA+VIS+wIRA), 19 patients with 141 warts to group 2 (PLC+VIS+wIRA), 14 patients with 125 warts to group 3 (ALA+VIS), and 16 patients with 130 warts to group 4 (PLC+VIS). All presented results refer to these 68 patients.
All 68 patients received 3 therapy cycles, as no patient was free of warts after three weeks and the first 6 patients being free of warts after 6 weeks showed a slight remaining hyperkeratosis and were therefore treated to be on the safe side.
Results
Embedded in the whole treatment scheme of hand and foot warts (keratolysis, curettage, PDT treatment, irradiation with VIS+wIRA, retinoic acid ointment; three therapy cycles every 3 weeks), wIRA alone as well as in combination with ALA-PDT reduced the "total wart area of each patient" significantly and increased the cure rate remarkably:
Results, when looking at the whole time period between beginning (t0) and week 18:
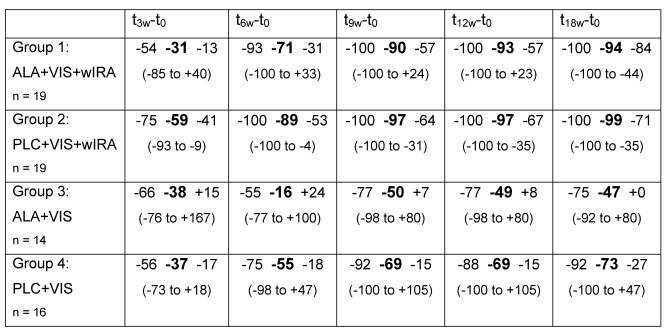
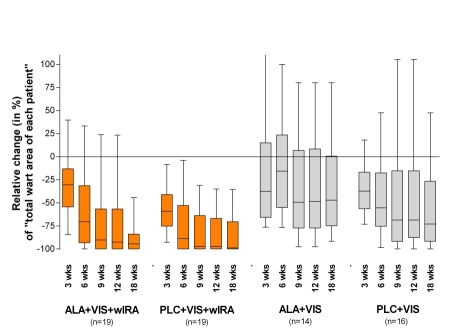
In group 1 (ALA+VIS+wIRA) and in group 2 (PLC+VIS+wIRA) the "total wart area of each patient" decreased significantly more than in both groups treated with visible light without wIRA (groups 3 and 4). The relative reduction (in percent) of "total wart area of each patient" within the 4 therapy groups is shown in Table 2 (Tab. 2) and Figure 2 (Fig. 2).
Table 2. Relative change (in %) of "total wart area of each patient".
between beginning of the treatment (t0 = morning of day of first PDT treatment before curettage) and examination day after 3 (t3w-t0), 6 (t6w-t0), 9 (t9w-t0), 12 (t12w-t0) and 18 (t18w-t0) weeks (given as percentile of 25, median, percentile of 75 and range (minimum to maximum))
Figure 2. Relative change (in %) of "total wart area of each patient".
between beginning of the treatment (t0 = morning of day of first PDT treatment before curettage) and examination day after 3, 6, 9, 12 and 18 weeks (given as minimum, percentiles of 25, median, percentiles of 75, and maximum (box and whiskers graph))
Group 1 (ALA+VIS+wIRA) had a significantly higher reduction of the "total wart area of each patient" (p = 0.0103) compared to group 4 (PLC+VIS).
In patients treated with PLC+VIS+wIRA (group 2) the "total wart area of each patient" decreased significantly more in comparison to patients treated with PLC+VIS (group 4) (p = 0.0107).
There was no significant difference between group 3 (ALA+VIS) and 4 (PLC+VIS) in reduction of the "total wart area of each patient". Also between group 1 (ALA+VIS+wIRA) and group 2 (PLC+VIS+wIRA) no significant difference occurred.
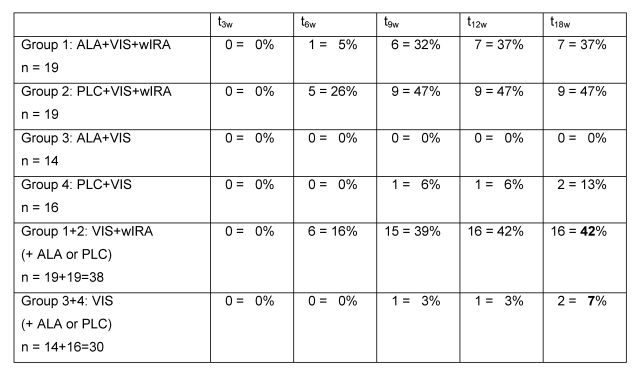
After 18 weeks in the two groups with wIRA (group 1 and group 2) 16 out of 38 patients (42%) were completely free of warts compared to only 2 out of 30 patients (7%) in the two groups without wIRA (group 3 and group 4) (see details in Table 3 (Tab. 3)). (All completely cured patients, who could be followed up one year later, remained free of warts.)
Table 3. Number and percentage of patients completely free of warts.
after 3 (t3w), 6 (t6w), 9 (t9w), 12 (t12w) and 18 (t18w) weeks
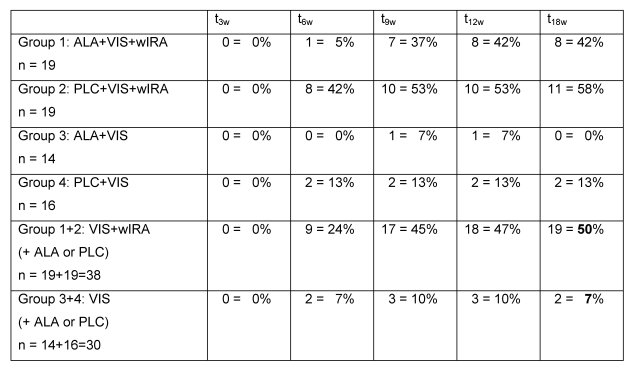
After 18 weeks in the two groups with wIRA (group 1 and group 2) 19 out of 38 patients (50%) were completely free of warts or with a remaining total wart area of less then 5% of the initial total wart area compared to only 2 out of 30 patients (7%) in the two groups without wIRA (group 3 and group 4) (see details in Table 4 (Tab. 4)).
Table 4. Number and percentage of patients completely free of warts or with a remaining total wart area of less then 5% of the initial total wart area .
after 3 (t3w), 6 (t6w), 9 (t9w), 12 (t12w) and 18 (t18w) weeks
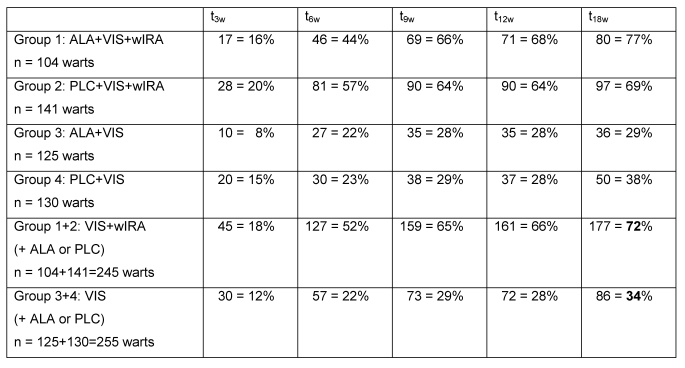
After 18 weeks in the two groups with wIRA (group 1 and group 2) 177 out of 245 warts (72%) had vanished completely (= median of 100% of reduction of "single wart area") compared to only 86 out of 255 warts (34%) in the two groups without wIRA (group 3 and group 4) (see details in Table 5 (Tab. 5)).
Table 5. Number and percentage of completely vanished warts.
after 3 (t3w), 6 (t6w), 9 (t9w), 12 (t12w) and 18 (t18w) weeks
The greatest part of these improvements was already achieved after 9 weeks (see details in Tables 3 (Tab. 3), 4 (Tab. 4), 5 (Tab. 5)).
Even the largest single wart (a plantar foot wart with 4068 mm2 in group 2) had been vanished already after 6 weeks.
The effect of therapy as estimated by patients and by physician separately in form of a rating given in a visual analogue scale (from -50 to +50) showed great improvement in the two groups with wIRA and much smaller effects in the two groups without wIRA: e.g. medians of estimation of effect of therapy by physician after 9 weeks: +41 (group 1), +41 (group 2); +0 (group 3), +0 (group 4) (see details in Figure 3 A+B (Fig. 3)).
Figure 3. A+B: Estimation of the effect of treatment by patients and by physician.
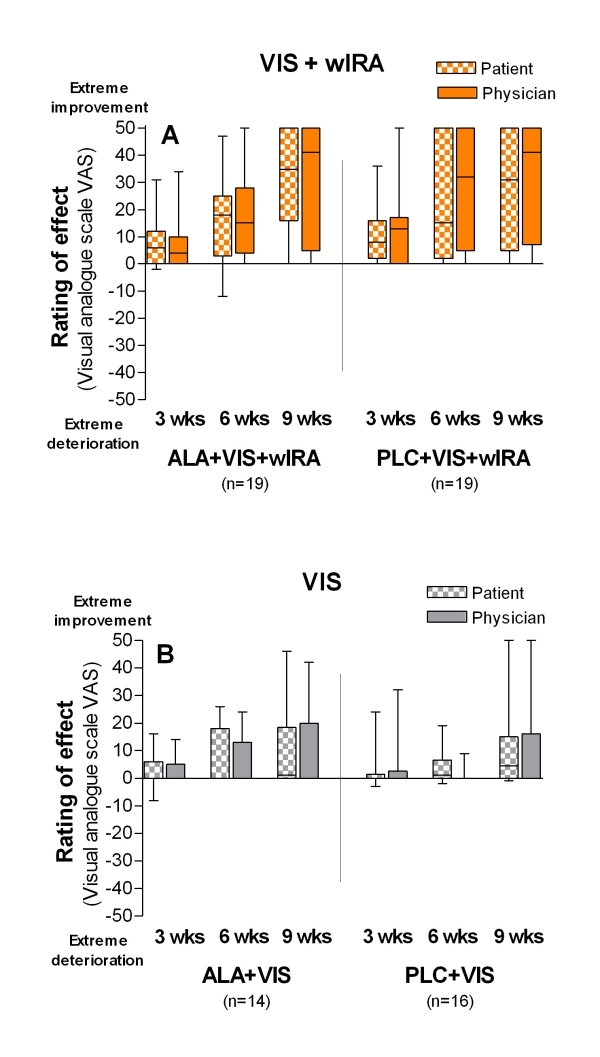
("How do you estimate the effect of therapy?") with visual analogue scale VAS (-50 mm = extreme deterioration, 0 = no effect, +50 mm = extreme improvement) after 3, 6 and 9 weeks (given as minimum, percentiles of 25, median, percentiles of 75, and maximum (box and whiskers graph)).
Medians from left to right:
A: VIS+wIRA treated groups (with and without ALA):
Group 1: ALA+VIS+wIRA: +6/ +4, +18/+15, +35/+41
Group 2: PLC+VIS+wIRA: +8/+13, +15/+32, +31/+41
B: VIS treated groups (with and without ALA):
Group 3: ALA+VIS: +0/+0, +0/+0, +1/+0
Group 4: PLC+VIS: +0/+0, +1/+0, +5/+0
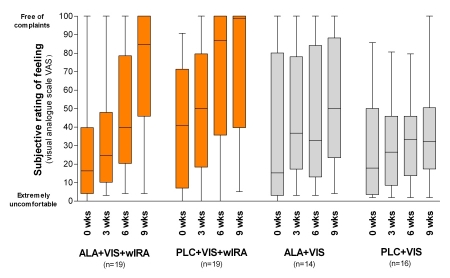
The complaints in the wart covered regions as estimated by the patients improved in the course of the study in all groups, but most in group 1 and 2: e.g. medians of subjective rating of feeling (visual analogue scale from 0 (= extremely uncomfortable) to 100 (= free of complaints)) after 9 weeks: 85 (group 1), 99 (group 2); 50 (group 3), 32 (group 4) (see details in Figure 4 (Fig. 4)). Patients with warts located on the soles of the feet and near nails often had a lot of pain. Only after one treatment with wIRA their complaints decreased within 3 weeks.
Figure 4. Subjective rating of feeling.
("How comfortable do you presently feel (with respect to pain, burn, itch etc.) about your wart covered region?") with visual analogue scale (VAS) (0 = extremely uncomfortable, 100 = free of complaints) before beginning of the treatment (morning of day of first PDT treatment before administering the cream) and after 3, 6 and 9 weeks (given as minimum, percentiles of 25, median, percentiles of 75, and maximum (box and whiskers graph)).
Medians from left to right:
VIS+wIRA treated groups:
Group 1: ALA+VIS+wIRA: 16 / 25 / 40 / 85
Group 2: PLC+VIS+wIRA: 41 / 50 / 87 / 99
VIS treated groups:
Group 3: ALA+VIS: 15 / 37 / 33 / 50
Group 4: PLC+VIS: 18 / 27 / 33 / 32
Within the treatment days the subjective rating of feeling did not change between "before administering the cream", "6 hours later (= immediately before irradiation)", and "immediately after irradiation" (therefore Figure 4 (Fig. 4) shows only 1 assessment time per day): Remarkably patients did not feel any pain during nor after treatment.
Figure 5 A-D (Fig. 5) shows pictures as an example of the course of treatment of hand warts: before starting with treatment (with PLC+VIS+wIRA), after 3, 6 and 18 weeks.
Figure 5. A-D: Example of hand warts: 18 year old boy with multiple periungual warts: before starting with treatment (with PLC+VIS+wIRA), after 3, 6 and 18 weeks.
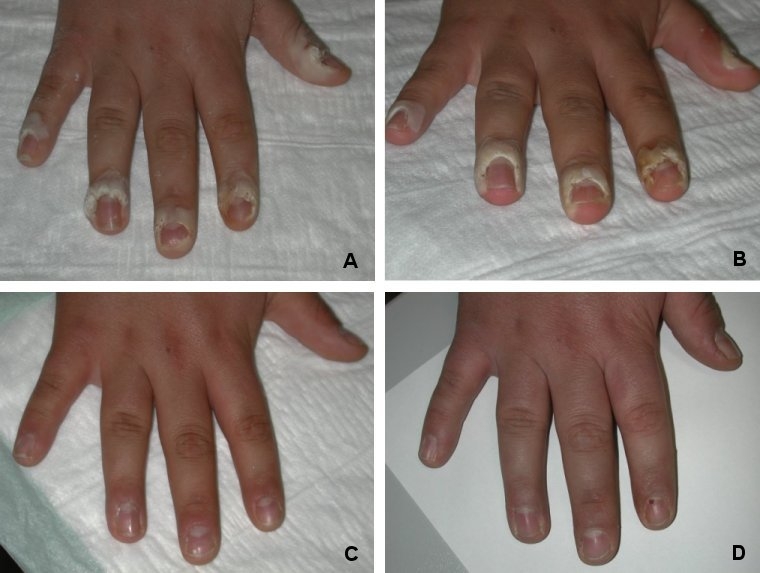
A: Before first treatment
B: Before second treatment
C: Before third treatment
D: 18 weeks after first treatment
Figure 6 A-D (Fig. 6) shows pictures as an example of the course of treatment of foot warts: before starting with treatment (with ALA+VIS+wIRA), after 3, 6 and 18 weeks.
Figure 6. A-D: Example of foot warts: 29 year old man with a solitary plantar foot wart: before starting with treatment (with ALA+VIS+wIRA), after 3, 6 and 18 weeks.
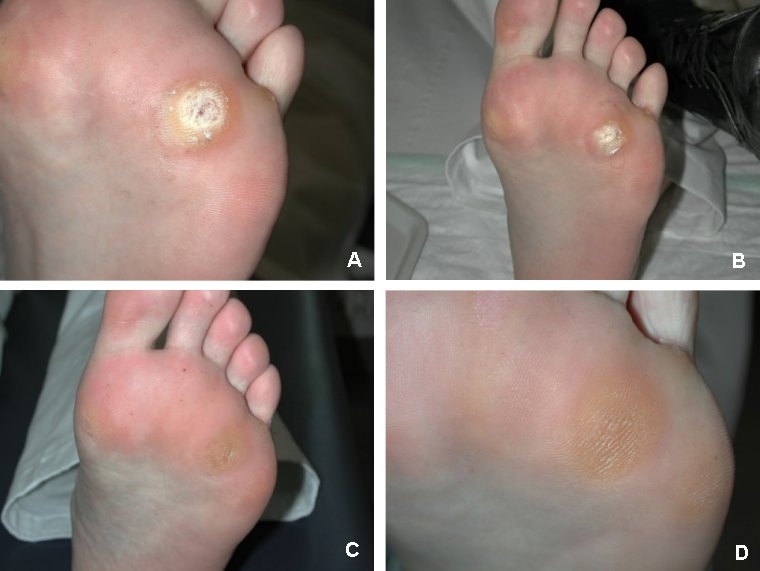
A: Before first treatment
B: Before second treatment
C: Before third treatment
D: 18 weeks after first treatment
Discussion
Warts impair quality of life of the patients due to their unsightly appearance, pain, and the concern that the warts might spread and be transferred to other people. The standard therapies are often invasive and/or painful and therefore especially in children traumatising for the patients. Thus we conducted the present study to enhance the reported effect of photodynamic therapy in warts combining it with wIRA. Generally irradiation with wIRA may be used with a preventive, regenerative and therapeutic intention in cases where a warming of deep tissue layers without skin contact and without overheating of the skin surface is desired. Thus, wIRA is already used in physiotherapy [28]; sports medicine [29]; internal medicine [30]; pediatrics [31]; and in dermatology for delayed wound healing [32], [33] (with cell physiological basics in [17]), sclerodermia, and penetration enhancement of topically applied substances [34], [35], or in combination with PDT [9], [36].
Photodynamic therapy (PDT) especially with topical application of 5-ALA is well established in dermatology [5], [8] with applications in the treatment of actinic keratosis and basal cell carcinomas. Recently, it has been reported to be efficient in warts [6], [10] and condylomata acuminata [37]. The combination of wIRA with 5-ALA based PDT has been reported for the treatment of actinic keratosis and basal cell carcinoma [9] and HPV-induced hand and foot warts [7]. The latter application, however, has not been validated by a randomised and controlled study. Despite a report of lack of efficacy of single-treatment 5-ALA-PDT in viral warts [6], [38], Stender [6] achieved in the mentioned remarkable randomised controlled study with three to six photodynamic treatment cycles with 5-ALA - embedded in a treatment with keratolysis and intensive paring in both groups - after 18 weeks a median of 100% (interquartile range 100%/57%) of reduction of "single wart area", compared to the control group without 5-ALA with 71% (100%/0%) (significant and relevant difference); 56% vs. 42% of all warts vanished completely, demonstrating superior efficacy of repetitive 5-ALA-PDT compared to placebo-PDT (based on a series of previously published experiences of this group, showing as well advantages compared to cryotherapy). As well, Fabbrocini showed - embedded in a treatment with keratolysis and curettage - a good effect of topical 5-ALA-PDT performed weekly for 3 weeks compared to placebo (75% vs. 23% vanished plantar warts) [10].
Compared to other radiation sources, there is much less sense of discomfort or burning during irradiation with the Hydrosun® radiator (in our study no complains occurred at all), most probably by excluding all kinds of unfiltered infrared.
This study shows that wIRA - with or without 5-ALA based PDT - significantly reduces "total wart area of each patient" of recalcitrant foot and hand warts and remarkably increases percentage of vanished warts and completely cured patients, if embedded in the described complete treatment scheme (including keratolysis and curettage as pre-treatment and retinoic acid ointment as post-treatment) of hand and foot warts. No scars or skin side effects were observed, and no disturbance of function was reported by patients after treatment.
The effect of wIRA may be explained both as thermal and as non-thermal effect:
A thermal effect is the increase in tissue temperature (building up a well documented field of warmth as described in the introduction, e.g. up to approximately 4°C increase in 10 mm depth of tissue [28]), which might act directly towards the inactivation of thermolabilic human papilloma viruses and indirectly by increased tissue metabolism with following immunomodulatory effects including better local immune system competence of the patient. This explanation as thermal effect is supported by the old experience that heat (and changes between heat and cold) can be used in the treatment of warts and by published heat treatments of warts with either very short (one minute) [39] or medium (30-90 minutes) [40] or long (two hours) [41] procedures, the first probably more tending towards inactivation, the latter more towards immunomodulation. The explanation as thermal effect is as well in accordance with the fact that warts are most often located on hypothermic areas like hands and feet.
Beside all these aspects of thermal effects non-thermal effects of infrared as radiation directly effecting and stimulating cells seem to be a remarkable second major part of the explanation. Already Albrecht-Buehler [18] described cell reactions towards very weak (no thermal effect causing) infrared radiation sources, later Ehrlicher [42] reported similarly cell growth towards infrared sources (800 nm). Effects of special wavelengths within infrared A (e.g. 820 nm) on cellular substructures, like cytochrome c, have been described in detail [19], [20], [21]. Especially these non-thermal direct radiation effects would be able to explain different effects like immunomodulation or wound healing improvement even without large increase of tissue temperature.
We did not find a significantly different therapeutic response between wIRA with or without 5-ALA (group 1 with ALA+VIS+wIRA vs. group 2 with PLC+VIS+wIRA) and no significantly different therapeutic response between a standard-PDT (ALA+VIS) and a placebo-PDT (PLC+VIS): This could mean that ALA, in the present preparation (although using an optimised but difficult preparation with unguentum emulsificans aquosum), did not reach in a sufficient amount the infected tissue or that it was inactivated (e.g. pH dependent, if pH>5) or that it was not transformed in a sufficient amount into protoporphyrin IX or protoporphyrin IX was inactivated (e.g. by light ahead of the PDT irradiation). This is in accordance with the situation, that we did not observe any fluorescence visually of the treated verrucae vulgares during irradiation with Wood's light. Both recent publications about 5-ALA-PDT in common warts [6], [10] found fluorescence of intralesionally formed protoporphyrin IX, but using a fluorescence spectrometer. Visually observable fluorescence of common warts (even with an ideal stimulating Wood's light) during a well performing 5-ALA-PDT with good clinical results is described as very rare and weak even after intensive pre-treatment. This is in accordance with Ackermann [25], describing limitations of detectibility of fluorescence, e.g. the dependence from concentration ratio between affected tissue and surrounding tissue concerning protoporphyrin IX [25].
The "relative decrease of total wart area of each patient" in the two groups with only visible light without wIRA (47% and 73%) was - taking repeated pre-treatment with keratolysis and curettage and regular post-treatment with retinoic acid ointment into account ([6] reported as well under paring and keratolysis and placebo-PDT a median of 71% of reduction of single wart area) - within an expected range and with no necessity to explain a relevant part of the decrease by psychological or placebo effects or by spontaneous remission.
In conclusion, wIRA has been shown to be an effective, non-invasive treatment of recalcitrant common hand and foot warts when embedded in a complete procedure with pre-treatment (keratolysis, curettage) and post-treatment (retinoic acid ointment).
Especially as there are striking differences between the two groups treated with wIRA compared to the two other groups (42% vs. 7% completely cured patients; 72% vs. 34% vanished warts) with no additional benefit adding the time consuming ALA-PDT, the authors propose - including own recent experiences after having finished the presented study - a treatment of recalcitrant common hand and foot warts with wIRA without ALA-PDT, but embedded in a procedure with pre-treatment and possibly post-treatment (estimating keratolysis to be essential, curettage to bring additional benefit, retinoic acid ointment to be negligible), with an even improved result using one treatment cycle (keratolysis, curettage, wIRA) once a week for six to nine weeks.
Acknowledgements
We thank the Dr. med. h.c. Erwin Braun Foundation, Basel/Switzerland, for supporting the study by a research grant.
We thank Medac/Photonamics, Wedel/Germany, for supplying us with 5-ALA.
References
- 1.Sterling J, Kurtz JB. Viral infections. In: Champion RH, Burton JL, Burns DA, Breathnach SM, editors. Rook/Wilkinson/Ebling textbook of dermatology. 6th ed. Oxford: Blackwell Scientific; 1998. pp. 995–1095. [Google Scholar]
- 2.Combemale P, Delolme H, Dupin M. Traitment des verrues. Ann Dermatol Venereol. 1998;125(6-7):443–462. [PubMed] [Google Scholar]
- 3.Gibbs S, Harvey I, Sterling J, Stark R. Local treatments for cutaneous warts: systematic review. Br Med J. 2002;325(7362):461. [PMC free article] [PubMed] [Google Scholar]
- 4.Sterling JC, Handfield-Jones S, Hudson PM British Association of Dermatologists. Guidelines for the management of cutaneous warts. Br J Dermatol. 2001;144(1):4–11. doi: 10.1046/j.1365-2133.2001.04066.x. [DOI] [PubMed] [Google Scholar]
- 5.Kalka K, Merk H, Mukhtar H. Photodynamic therapy in dermatology. J Am Acad Dermatol. 2000;42(3):389–413. doi: 10.1016/s0190-9622(00)90209-3. quiz 414-6. [DOI] [PubMed] [Google Scholar]
- 6.Stender IM, Na R, Fogh H, Gluud C, Wulf HC. Photodynamic therapy with 5-aminolaevulinic acid or placebo for recalcitrant foot and hand warts: randomised double-blind trial. Lancet. 2000;355(9208):963–966. doi: 10.1016/S0140-6736(00)90013-8. [DOI] [PubMed] [Google Scholar]
- 7.Strasser W. Personal communication, not published. 2001.
- 8.Wolf P. Photodynamische Therapie: Grundlagen und klinische Anwendung in der Dermatologie. Dtsch Ärztebl. 1999;96:1493–1498. [Google Scholar]
- 9.Foss P. Einsatz eines patentierten, wassergefilterten Infrarot-A-Strahlers (Hydrosun) zur photodynamischen Therapie aktinischer Dyskeratosen der Gesichts- und Kopfhaut. Z naturheilkundl Onkologie krit Komplementärmed. 2003;6(11):26–28. [Google Scholar]
- 10.Fabbrocini G, Di Costanzo MP, Riccardo AM, et al. Photodynamic therapy with topical delta-aminolaevulinic acid for the treatment of plantar warts. J Photochem Photobiol B. 2001;61(1-2):30–34. doi: 10.1016/s1011-1344(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 11.Harth Y, Hirshowitz B, Kaplan B. Modified topical photodynamic therapy of superficial skin tumors, utilizing aminolevulinic acid, penetration enhancers, red light, and hyperthermia. Dermatol Surg. 1998;24(7):723–726. doi: 10.1111/j.1524-4725.1998.tb04240.x. [DOI] [PubMed] [Google Scholar]
- 12.Fritsch C, Ruzicka T. Fluorescence diagnosis and photodynamic therapy of skin diseases. Wien: Springer; 2003. [Google Scholar]
- 13.Svanberg K, Andersson T, Killander D, et al. Photodynamic therapy of non-melanoma malignant tumours of the skin using topical delta-amino levulinic acid sensitization and laser irradiation. Br J Dermatol. 1994;130(6):743–751. doi: 10.1111/j.1365-2133.1994.tb03412.x. [DOI] [PubMed] [Google Scholar]
- 14.Szeimies RM, Calzavara-Pinton P, Karrer S, Ortel B, Landthaler M. Topical photodynamic therapy in dermatology. J Photochem Photobiol B. 1996;36(2):213–219. doi: 10.1016/s1011-1344(96)07375-7. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy JC, Pottier RH, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B. 1990;6(1-2):143–148. doi: 10.1016/1011-1344(90)85083-9. [DOI] [PubMed] [Google Scholar]
- 16.Menezes S, Coulomb B, Lebreton C, Dubertret L. Non-coherent near infrared radiation protects normal human dermal fibroblasts from solar ultraviolet toxicity. J Invest Dermatol. 1998;111(4):629–633. doi: 10.1046/j.1523-1747.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 17.Applegate LA, Scaletta C, Panizzon R, Frenk E, Hohlfeld P, Schwarzkopf S. Induction of the putative protective protein ferritin by infrared radiation: implications in skin repair. Int J Mol Med. 2000;5(3):247–251. doi: 10.3892/ijmm.5.3.247. [DOI] [PubMed] [Google Scholar]
- 18.Albrecht-Buehler G. Surface extensions of 3T3 cells towards distant infrared light sources. J Cell Biol. 1991;114(3):493–502. doi: 10.1083/jcb.114.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karu TI. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49(1):1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 20.Karu TI, Pyatibrat LV, Kalendo GS. Cell attachment to extracellular matrices is modulated by pulsed radiation at 820 nm and chemicals that modify the activity of enzymes in the plasma membrane. Lasers Surg Med. 2001;29(3):274–281. doi: 10.1002/lsm.1119. [DOI] [PubMed] [Google Scholar]
- 21.Karu TI. Low-power laser effects. In: Waynant RW, editor. Lasers in medicine. Boca Raton: CRC Press; 2002. pp. 171–209. [Google Scholar]
- 22.Gloor M, Fluhr J, Wasik B, Gehring W. Klinische Wirkung von Salicylsaure und hochdosiertem Harnstoff bei Applikation in standardisierten Magistralrezepturen aus dem Neuen Rezeptur Formularium (NRF) Pharmazie. 2001;56(10):810–814. [PubMed] [Google Scholar]
- 23.Thomsen JS, Simonsen L, Benfeldt E, Jensen SB, Serup J. The effect of topically applied salicylic compounds on serotonin-induced scratching behaviour in hairless rats. Exp Dermatol. 2002;11(4):370–375. doi: 10.1034/j.1600-0625.2002.110412.x. [DOI] [PubMed] [Google Scholar]
- 24.van den Akker JT, Boot K, Vernon DI, et al. Effect of elevating the skin temperature during topical ALA application on in vitro ALA penetration through mouse skin and in vivo PpIX production in human skin. Photochem Photobiol Sci. 2004;3(3):263–267. doi: 10.1039/b309284d. [DOI] [PubMed] [Google Scholar]
- 25.Ackermann G. Photophysikalische Grundlagen zur Fluoreszenzdiagnostik von Tumoren der Haut [Thesis] [Photophysical fundamentals of fluorescence diagnosis of skin tumors]. Regensburg: University Regensburg; 2001. (Ger). Available from: http://www.bibliothek.uni-regensburg.de/opus/volltexte/2001/27/ [Google Scholar]
- 26.Juzenas P, Sorensen R, Iani V, Moan J. Uptake of topically applied 5-aminolevulinic acid and production of protoporphyrin IX in normal mouse skin: dependence on skin temperature. Photochem Photobiol. 1999;69(4):478–481. [PubMed] [Google Scholar]
- 27.Fluhr JW, Pelosi A, Lazzerini S, Dikstein S, Berardesca E. Differences in corneocyte surface area in pre- and post-menopausal women. Assessment with the noninvasive videomicroscopic imaging of corneocytes method (VIC) under basal conditions. Skin Pharmacol Appl Skin Physiol. 2001;14 Suppl 1:10–16. doi: 10.1159/000056384. [DOI] [PubMed] [Google Scholar]
- 28.Vaupel P, Stofft E. Wassergefilterte Infrarot-A-Strahlung im Vergleich zu konventioneller Infrarotstrahlung oder Fango-Paraffin-Packungen: Temperaturprofile bei lokaler Wärmetherapie [Waterfiltered infrared A radiation compared with conventional infrared radiation or fango paraffine pack: temperature profiles in local thermal therapy] In: Vaupel P, Krüger W, editors. Wärmetherapie mit wassergefilterter Infrarot-A-Strahlung [Thermal therapy with waterfiltered infrared A radiation] 2nd ed. Stuttgart: Hippokrates; 1995. pp. 135–147. [Google Scholar]
- 29.Hoffmann G. Improvement of regeneration by local hyperthermia induced by waterfiltered infrared A (wIRA) Int J Sports Med. 2001;23 Suppl 2:S145. [Google Scholar]
- 30.Falkenbach A, Dorigoni H, Werny F, Gütl S. Wassergefilterte Infrarot-A-Bestrahlung bei Morbus Bechterew und degenerativen Wirbelsäulenveränderungen: Effekte auf Beweglichkeit und Druckschmerzhaftigkeit. Österr Z Physikal Med Rehab. 1996;6(3):96–102. [Google Scholar]
- 31.Singer D, Schröder M, Harms K. Vorteile der wassergefilterten gegenüber herkömmlicher Infrarot-Strahlung in der Neonatologie. Z Geburtsh Neonatol. 2000;204(3):85–92. doi: 10.1055/s-2000-10202. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann G. Improvement of wound healing in chronic ulcers by hyperbaric oxygenation and by waterfiltered ultrared A induced localized hyperthermia. Adv Exp Med Biol. 1994;345:181–188. doi: 10.1007/978-1-4615-2468-7_24. [DOI] [PubMed] [Google Scholar]
- 33.Biland L, Barras J. Die wassergefilterte Infrarot-A-Hyperthermie zur Behandlung venöser Ulcera. Hefte Wundbehand. 2001;5:41. [Google Scholar]
- 34.Haupenthal H. In vitro- und in vivo-Untersuchungen zur temperaturgesteuerten Arzneistoff-Liberation und Permeation [Thesis] [In vitro and in vivo investigations of temperature dependent drug liberation and permeation]. Mainz: Johannes Gutenberg-Universität; 1997. (Ger). [Google Scholar]
- 35.Bankova L, Heinemann C, Fluhr JW, Hoffmann G, Elsner P. Improvement of penetration of a topical corticoid by waterfiltered infrared A (wIRA). 1st Joint Meeting 14th International Congress for Bioengineering and the Skin & 8th Congress of the International Society for Skin Imaging; 2003 May 21-24; Hamburg. 2003. p. P96. [Google Scholar]
- 36.Kelleher DK, Bastian J, Thews O, Vaupel P. Enhanced effects of aminolaevulinic acid-based photodynamic therapy through local hyperthermia in rat tumours. Br J Cancer. 2003;89(2):405–411. doi: 10.1038/sj.bjc.6601036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fehr MK, Hornung R, Degen A, et al. Photodynamic therapy of vulvar and vaginal condyloma and intraepithelial neoplasia using topically applied 5-aminolevulinic acid. Lasers Surg Med. 2002;30(4):273–279. doi: 10.1002/lsm.10048. [DOI] [PubMed] [Google Scholar]
- 38.Ammann R, Hunziker T, Braathen LR. Topical photodynamic therapy in verrucae. A pilot study. Dermatology. 1995;191(4):346–347. doi: 10.1159/000246597. [DOI] [PubMed] [Google Scholar]
- 39.Stern P, Levine N. Controlled localized heat therapy in cutaneous warts. Arch Dermatol. 1992;128(7):945–948. [PubMed] [Google Scholar]
- 40.LoCricchio J, Haserick J. Hot-water treatment for warts. Cleve Clin Q. 1962;29:156–161. doi: 10.3949/ccjm.29.3.156. [DOI] [PubMed] [Google Scholar]
- 41.Dvoretzky I. Hyperthermia therapy for warts utilizing a self-administered exothermic patch. Review of two cases. Dermatol Surg. 1996;22(12):1035–1038. doi: 10.1111/j.1524-4725.1996.tb00657.x. discussion 1038-9. [DOI] [PubMed] [Google Scholar]
- 42.Ehrlicher A, Betz T, Stuhrmann B, et al. Guiding neuronal growth with light. Proc Natl Acad Sci USA. 2002;99(25):16024–16028. doi: 10.1073/pnas.252631899. [DOI] [PMC free article] [PubMed] [Google Scholar]