Abstract
Objective: Hypothermia has been shown to reduce neurologic deficits in patients after cardiopulmonary resuscitation (CPR). It was not clear if intravascular cooling is superior to standard external cooling in inducing hypothermia. Goal of this study was to compare intravascular cooling with an automated cooling device with external cooling in everyday practice on a cardiac-care ICU (intensive care unit).
Methods: Patients after successful CPR for unwitnessed cardiac arrest were subjected to cooling with an automated cooling system (CoolGard, Alsius) after initial hemodynamic stabilization. Goal was to achieve a core temperature of 33°C. Monitored were the time intervals from admission to begin of cooling and from begin of cooling to target temperature. Data were compared retrospectively with those from patients subjected to external cooling.
Results: 31 consecutive patients treated with intravascular cooling were analyzed. Cooling was initiated at a mean time of 58 min after admission, and the target temperature of 33°C was achieved after a mean of 3.48 hours after the begin of cooling. In contrast, 49 patients treated with external cooling achieved a minimum temperature of 34.8°C only 9.2 hours after admission.
Conclusion: In everyday practice, intravascular cooling using an automated cooling system is superior for a rapid induction of hypothermia after cardiac arrest.
Abstract
Hintergrund: Eine induzierte Hypothermie für mindestens 24 Stunden verbessert das neurologische Outcome von Patienten nach kardiopulmonaler Reanimation (CPR). Die optimale Methode zur Hypothermieinduktion ist unklar. Ziel dieser Studie war, den Effekt einer internen Kühlung mit einem automatisierten Kühlsystem mit dem einer externen Kühlung bei Patienten auf einer kardiologischen Intensivstation unter Alltagsbedingungen zu vergleichen.
Methoden: Untersucht wurden Patienten nach erfolgreicher CPR nach unbeobachtetem Herz-Kreislaufstillstand. Zur internen Kühlung wurden diese Patienten nach initialer hämodynamischer Stabilisierung baldmöglichst mit einem Kühlkathetersystem versorgt (CoolGard, Alsius) und auf eine Zieltemperatur von 33°C gekühlt. Ziel war das Erreichen der Zieltemperatur innerhalb von 4 Stunden nach Aufnahme. Falls notwendig, wurden zusätzlich externe Kühlkissen angewendet. Untersucht wurden die Zeitdauer von Aufnahme bis zum Beginn der Hypothermieinduktion und die Zeit bis zum Erreichen der Zieltemperatur. Diese Daten wurden verglichen mit Patienten, die nur extern (mit Kühldecken und -kissen) gekühlt wurden.
Ergebnisse: Bei 31 konsekutiven Patienten wurde eine Hypothermie mittels interner Kühlung induziert. Die Hypothermieinduktion begann im Durchschnitt 58 Minuten nach stationärer Aufnahme. Die Zieltemperatur von 33°C wurde im Durchschnitt nach 3,48 Stunden nach Beginn der Kühlungsmaßnahmen erreicht. Im Gegensatz dazu konnte bei 49 extern gekühlten Patienten nur eine minimale Temperatur von 34,8°C erreicht werden. Die Dauer bis zum Erreichen der Minimaltemperatur war mit 9,2 Stunden deutlich länger als in der intern gekühlten Patientengruppe.
Schlussfolgerung: Zur raschen Induktion einer Hypothermie nach CPR ist unter Alltagsbedingungen die interne Kühlung mit einem automatisierten Kühlsystem der externen Kühlung überlegen.
Background
Hypothermia has been shown by landmark studies to improve neurologic outcome in patients after cardiopulmonary resuscitation (CPR) [1], [2]. The detailed mechanisms by which hypothermia protects the brain are unresolved. It has, however, become clear that a rapid onset of hypothermia and a controlled, slow rewarming are critical to improve the outcome of those patients [3].
Despite this compelling evidence, hypothermia after cardiac arrest is underused [4]. This may be caused by the lack of standardized and user-friendly protocols for the cooling of patients. Initially, hypothermia has been introduced into clinical practice using conventional cooling methods. These methods consist mainly of the use of cooling blankets, cold fluid, and ice bags [1], [2]. This external cooling, however, does often not comply with other, invasive procedures required for the patients. Recently, devices for intravascular cooling have been introduced into the market [5] which seem to be much easier to handle. Data comparing intravascular with external, conventional cooling are, however limited to date.
Goal of this work was therefore to retrospectively compare intravascular with conventional cooling in patients after successful CPR in everyday practice.
Methods
Clinical setting
Included in this analysis were patients after successful CPR treated at the cardiac-care ICU of one center. Since 2002, those patients are routinely treated with an intravascular cooling device (IC; CoolGard, Alsius). 31 consecutive patients treated with this device were compared with a cohort of 49 consecutive patients treated with conventional cooling only (CC, using the TheraCool device, KCI, San Antonio, USA; addtional use of cooling blankets and cold infusions was used as necessary) between 2000 and 2002. Treatment goal in all patients was to achieve a core temperature of 33°C as soon as possible after admission and to maintain this temperature for at least 24 hours.
Measurements
In all patients, the admission temperature, the minimum temperature, and the intervals from admission to begin of cooling and from begin of cooling to target temperature were evaluated.
Statistics
Mean and standard error were calculated. Comparisons were analyzed using the students T-test. P-values below 0.05 were considered significant.
Results
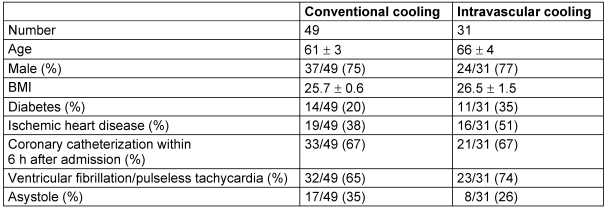
Patient's characteristics are shown in Table 1 (Tab. 1). There were no significant differences between the groups.
Table 1. Baseline characteristics.
The patients core temperature ad admission was 35.9°C in the patients subjected to intravascular cooling and 35.6°C in the patients with conventional cooling (p=n.s.). The time to the begin of cooling amounted to 81 min in IC and 60 min in CC.
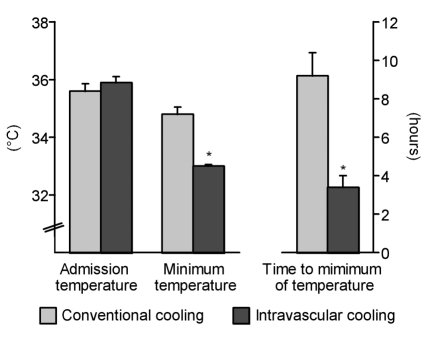
All patients in the IC group achieved the target temperature of 33°C. Patients were cooled to this temperature after 3.48±0.6 hours. Additional external cooling procedures were not performed in any patient in the IC group. In contrast, only 4 patients in the CC group (9%) reached this target temperature. In this group, a mean minimum temperature of 34.8°C was achieved 9.2±1.2 hours after the onset of cooling (Figure 1 (Fig. 1)).
Figure 1. Baseline and minimum temperatures and time to minimum temperature (mean ± SEM, *p<0.05).
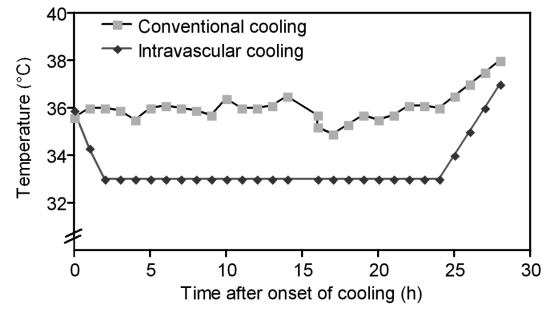
The time course of the core temperature in both groups is shown in Figure 2 (Fig. 2). IC proved suitable for a continuous and stable reduction of core temperature, which was due to the automated temperature control of the device. In contrast, temperature of the patients treated with CC was highly variable. Figure 2 (Fig. 2) illustrates that the IC method not only allows to achieve a stable target temperature but also allows controlled rewarming of patients.
Figure 2. Time course of core temperature in conventional and intravascular cooling (means).
This study was not powered to determine outcome or cost-effectiveness of the IC device. In-hospital-mortality and the length of hospital stay, however, was analyzed in both groups to exclude adverse effects of IC. This in-hospital mortality was 11/49 patients in the CC group (22%) and 8/31 patients in the IC group (26%, p=0.2). Patients were hospitalized for 16.5±1.6 days in CC and for 13.7±1.4 days in IC (p=0.17).
Discussion
The data presented here show that in everyday practice of a single cardiac-care ICU, IC using an automated cooling device is superior to CC in achieving the recommended core temperature of patients after successful CPR.
Clearly, it has to be stated that the evidence grade of this study with a historical control group is only moderately high because no controlled randomized comparison of intravascular and conventional external cooling has been carried out but a comparison of 31 patients with intravascular cooling with a historical group of 49 patients with conventional external cooling. Goal of this analysis was only to test the feasibility and effectivity of various clinical methods in everyday practice.
The data obtained with CC in this study are in contrast to other data obtained with CC. The Hypothermia after Cardiac Arrest Study Group was, in 136 patients, able to reach a core temperature of 33°C with CC only; however, the time to reach this temperature was 12 hours [2]. Felberg and coworkers needed 301 min to achieve 33°C [6]. Bernard and coworkers were able to cool their patients to 33.3±0.98°C within 3 hours after admission with CC; the admission temperature of this cohort, however, was somewhat lower than of the patients examined here (35°C vs 35.6°C) [1]. Thus, in contrast to this study, other groups were able to induce hypothermia with CC only under the auspices of a controlled trial.
The main difference between the patients in those studies and the patients presented here is that in our study, two third of the patients underwent emergency coronary angiography (Table 1 (Tab. 1)). In previous studies, coronary angiography was performed in 4% [1], or patients with suspected myocardial ischemia were even precluded from the study [6]. Obviously, maintenance of CC measures is complicated during complex coronary procedures for technical reasons. This may explain why we were unable to reach the target temperature of 33°C in most patients treated with CC. In contrast, patients treated with IC in this study very predictably reached the target temperature despite of invasive procedures. Central to this favorable result was that the cooling catheters were rapidly placed during cardiac catheterization using the same femoral approach, thus avoiding to set up a separate sterile environment. Since most patients in this study had ischemic heart disease, and more than 70% of the patients subjected to coronary angiography underwent immediate coronary revascularization, the liberal access to coronary angiography used in this study seems justified.
A clear limitation of this study was that long-term outcomes of the patients were not monitored. The goal of this study was only to compare cooling methods in everyday practice. In-hospital mortality was not different between the groups. Despite of the (non-significant) reduction of hospital days in the IC group, we believe that the data presented here are not valid to perform cost estimates or further analyses on effectiveness of this treatment, which is due to the retrospective nature of this analysis and the sequential treatment of the groups.
Despite this limitation, the data presented here clearly favor intravascular cooling to induce hypothermia in patients after CPR, especially when emergency coronary revascularization is considered.
Notes
The authors Flemming and Simonis contributed equally to this work.
Conflicts of interest: none declared.
References
- 1.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 2.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. Erratum in: N Engl J Med. 2002;346(22):1756. [DOI] [PubMed] [Google Scholar]
- 3.Maramattom BV, Wijdicks EF. Postresuscitation encephalopathy. Current views, management, and prognostication. Neurologist. 2005;11(4):234–243. doi: 10.1097/01.nrl.0000159985.07242.22. [DOI] [PubMed] [Google Scholar]
- 4.Abella BS, Rhee JW, Huang KN, Vanden Hoek TL, Becker LB. Induced hypothermia is underused after resuscitation from cardiac arrest: a current practice survey. Resuscitation. 2005;64(2):181–186. doi: 10.1016/j.resuscitation.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Al-Senani FM, Graffagnino C, Grotta JC, Saiki R, Wood D, Chung W, Palmer G, Collins KA. A prospective, multicenter pilot study to evaluate the feasibility and safety of using the CoolGard System and Icy catheter following cardiac arrest. Resuscitation. 2004;62(2):143–150. doi: 10.1016/j.resuscitation.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Felberg RA, Krieger DW, Chuang R, Persse DE, Burgin WS, Hickenbottom SL, Morgenstern LB, Rosales O, Grotta JC. Hypothermia after cardiac arrest: feasibility and safety of an external cooling protocol. Circulation. 2001;104(15):1799–1804. doi: 10.1161/hc4001.097037. [DOI] [PubMed] [Google Scholar]