Abstract
From a prospective matched-pair study in 40 patients it can be concluded that the use of a new bipolar sealer system for electrocoagulation is effective in Total Knee Arthroplasty in reducing the visible total blood loss until drain removal on day two after surgery by more than 28% as compared to a conventional regimen. Together with shed blood autotransfusion, it reduced homologous blood transfusions by a factor of five. No complications occurred. The system can be recommended for use in knee surgery during, after opening, and without use of a tourniquet. It bears additional potential for further applications in orthopaedic surgery.
Keywords: blood transfusion, total knee replacement, surgical hemostasis, electrocoagulation
Abstract
In einer prospektiven Matched-Pair Studie an 40 Patienten wurde gezeigt, dass die Verwendung eines neuartigen elektrischen bipolaren Versiegelungssystems den sichtbaren Gesamtblutverlust bei Knieendoprothesenimplantation bis zur Drainentfernung an Tag 2 nach dem Eingriff um mehr als 28% im Vergleich mit herkömmlichen Verfahren reduzierte. Zusammen mit der Autotransfusion von Sekretblut verminderte sich damit die Fremdblutgabe um den Faktor 5. Es wurden keine Komplikationen beobachtet. Das System kann für den Einsatz in der Kniechirurgie mit und ohne Blutsperre empfohlen werden. Es bietet ein hohes Potenzial für weitere Anwendungen in der Orthopädischen Chirurgie.
Introduction
Reduced intra- and perioperative blood loss is an important quality factor in modern Total Knee Arthroplasty (TKA). Lesser bleeding results in faster recovery of the patient, better wound healing and lower complication rates. Vice versa, homologous blood transfusion has a high morbidity [1] and thus should be avoided. In a prospective matched pair study blood loss during and after TKA should be evaluated.
Materials and methods
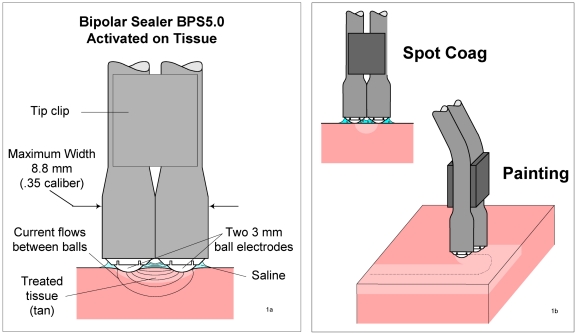
Firstly, the application of an innovative bipolar sealer system (BPS 5.0, TissueLink, Dover, NH, USA, Figure 1 (Fig. 1)) for excluding learning curve effects and unwanted side effects in combination with and without additional postoperative 40µ (filter pore size) filtrated shed blood retransfusion (SolcoTrans Plus, Davol, Cranston, RI, USA) was done with informed consent in five patients undergoing elective TKA. After that, a cohort of 20 consecutive patients with use of BPS during surgery according to the manufacturer's recommendations [11] was selected and visible blood loss was recorded intraoperatively, immediately postoperative, and at time of drain removal.
Figure 1. The Bipolar Sealer: principles of design (1a) and action (1b).
From a prior study in 80 patients it was obvious that blood loss after TKA is significantly related to age and gender: In a prospective design Sigg et al. [10] found in their raw data significantly higher total visible blood loss (TVBL) in men (p<0.003) and a positive correlation of visible immediate postoperative (but not total) blood loss with age (r=0.253, p<0.029). Thus, these two parameters were considered as criteria for a matched pair design. A linear stepwise regression analysis from their raw data also excluded preoperative Hb concentrations as a predictive factor for TVBL.
In an unpublished other data collection in 28 TKA patients the authors could exclude significant effects of Body Mass Index (BMI) upon TVBL (r=0.143, p<0.468). Thus BMI was recorded but not used for matching purposes.
The above mentioned cohort was matched in respect of age and gender with 20 patients from a pool of 40 other previous patients who did not obtain BPS but were otherwise operated the same way and had obtained the same vacuum drain management with drain removal two days after surgery. The latter control patient's data was blinded for blood loss and Hemoglobin (Hb) concentrations prior to matching.
Average age was 72 years, 7 patient pairs were male and 13 female. Patients with known coagulation and peripheral circulation disorders were generally excluded from participation. No preoperative autologous blood donation was utilized. A torniquet was used in both groups and opened prior to wound closure and high frequency coagulation. All patients obtained the same standardized recommended low molecular weight heparin prophylaxis to prevent deep vein thrombosis.
Results
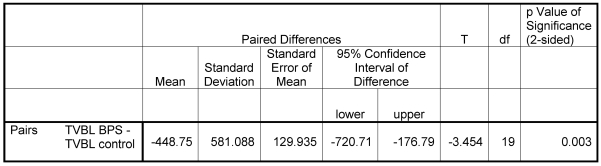
The results of this pilot study showed a significant reduction of 28.4% TVBL in the BPS group with a total average of 1130 vs. 1580 ml (p<0.003, Table 1 (Tab. 1)).
Table 1. Paired T-Test results for total visible blood loss (TVBL) with BPS vs. control group.
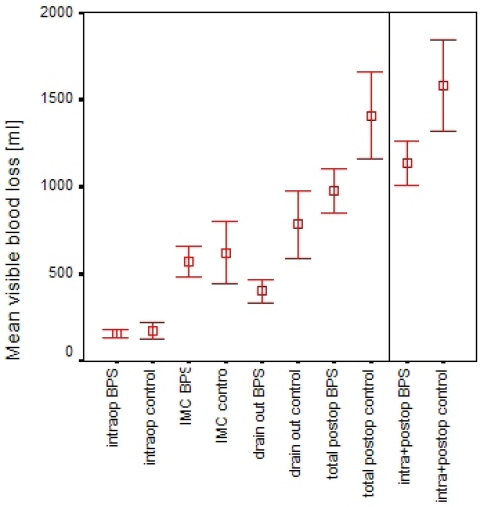
The intraoperative bleeding was less with BPS but the collected fluid amount did not differ significantly. This can be attributed to the fact that the time for bleeding and high frequency coagulation after opening of the torniquet was short in both cohorts. Compared with the postoperative blood loss, the intraoperative loss was negligible in the BPS group. The blood loss significantly started to differ among the groups after the patients stay in Intermediate Care (p<0.001, Figure 2 (Fig. 2)).
Figure 2. Mean visible blood loss in BPS vs. control group over time intraoperatively, on Intermediate Care Unit (IMC), on regular ward at time of drain removal (left from vertical line), and cumulated (TVBL, right from vertical line).
Group differences after IMC and cumulated differences as shown were highly significant. Bars represent 95% confidence intervals of mean values.
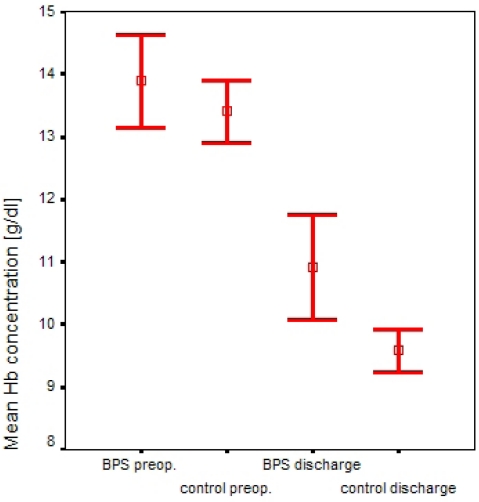
Both groups on average had the same normal preoperative Hb concentrations (13.6 g/dl) and the BPS group showed a smaller Hb reduction at discharge. Although additional postoperative autotransfusion of unwashed 40µ (filter pore size) filtered shed blood within 6 hours after surgery was added for the purpose of comprehensive blood salvage management in the BPS group, the average difference between the two groups at discharge was insignificant. This is explained by the fact that in the control group a total of 10 homologous erythrocyte concentrate packs were administered on the basis of clinical indications (BPS group: 2 packs). In the BPS group full suction power of 700 mbar (10.2 lbs/inch²) could be maintained in all patients whereas suction needed to be set to zero in 5 patients in the control group due to fast postoperative filling of the suction reservoir. In the BPS group, Hb concentration at discharge was 10.9 g/dl, in the control group 9.6 g/dl (Figure 3 (Fig. 3)). No re-operations became necessary in both groups and neither did any other complications occur.
Figure 3. Hemoglobin concentrations for the two groups preoperatively and at discharge, bars depict 95% confidence intervals of mean values.
Discussion
The BPS system can effectively reduce the postoperative blood loss after TKA. Together with postoperative shed blood autotransfusion the device reduces the probability for allogeneic transfusion by a factor of 5 and for overall transfusion of stored blood from 39% [1] to 5%. There are no contraindications for its combination with postoperative autologous retransfusion but it also works well without it. It is easy to handle and deserves consideration particularly in a comprehensive fast-track recovery regimen after arthroplasty. BPS can reduce TVBL to the same order of magnitude as the use of Aprotinin [10], yet without its possible drug related side effects such as anaphylaxis after repetitive use, and without conflicts from lacking FDA approval for use in alloarthroplasty.
There may be other strategies as well to reduce visible blood loss by reducing or regulating suction or, reducing the number of drains or, by avoiding drains at all [4]. Pfeiffer et al. in a prospective study of blood loss after TKA [7] found an average visible blood loss of only 500 ml (150-1280) with a closed system, equipped with regulated suction from 0 mbar until day 1 and 900 mbar until drain removal on day 2. The losses during surgery were not measured in that study. Since the surgical technique was not much different in this study, it may well be speculated that the use of BPS may further reduce that loss in a favourable combination with regulated suction.
Parker et al. [6] in the biggest meta analysis of drain issues in endoprosthetics ever conducted found that patients with drained wounds generally more often obtained postoperative transfusions. However, the transfusion regimen may have varied considerably among the included studies from different centres. Namely one very subjective factor that makes the surgeon opt for transfusion seems to be rapid and high volume effusion into the drains at the time he looks for the patient after surgery, thus possibly reducing transfusions in patients without drains.
It is obvious from the literature that the relation between visible and calculable hidden blood loss can not necessarily be the same in all patients with and without drains, and a postoperative hidden internal or external ("non-collectible") loss may increase to the same or even higher extent than reduction of visible loss without drains. Mengal et al. [5] calculated even higher total blood loss in non-drained vs. drained TKA. This is also supported by the analysis of Parker et al. [6] who showed that "reinforcement of wound dressings was required more frequently in the group managed without drains", the latter indicating protracted secretion from the stitched-up tissue.
Sehat and Evans [9] calculated visible and hidden blood loss in patients undergoing drained TKA and found the proportion of hidden blood loss to be 50% of the total blood loss. Thus, visible blood loss can be interpreted as being equal to the hidden loss. Other investigators have found similar results using mathematical models that calculate blood loss based on the relationship between hematocrit (Hct) and estimated blood volume [2], [3], [12], [13]. On the basis of this considerations the present study can yield also valid estimates of the total and hidden blood loss. TVBL is the one criterion which is immediately accessible to the bare eye, does not rely on lab tests, is apparently most influential for conventional decision making towards transfusion, and thus was chosen as key parameter in our study.
Alas, it seems to be very difficult to standardize parameters such as "swelling" and "hematoma" as indicators for hidden blood loss. It must be borne in mind that the above mentioned 50/50 ratio can only be assumed if drain pipes are not prematurely clotted, which they are inclined to be when they work by gravity only. A beneficial "self-tamponade" effect of an hemarthros is not likely to be proven from clinical experience.
Retransfusion is mostly considered useful, when great blood losses are expected. With the known risks of every transfusion, avoidance of bleeding must be the primary goal. Also remarkable is that autotransfusion systems obviously increase total blood loss which makes the blood saving characteristics of the BPS as compared to the control group even more noteworthy. Martin et al. [4] found significantly highest total blood loss in case of autotransfusion. Rosolski, in a meta-analysis, did not find significant blood saving effects of autotransfusion [8]. From a German university hospital we were also confidentially informed that in a yet unpublished prospective study autotransfusion of filtered non-centrifuged shed blood in TKA more than doubled the visible as well as the total (hidden plus visible) blood loss, as compared to a control group. The underlying mechanisms, alas, are still unclear but it must be noted that after autotransfusion bleeding appears to be less reduced than without it.
Conclusions
On the basis of our data and evidence from the literature our future strategy for primary cemented TKA will be as follows:
Use of BPS, two regulated suction drains, no shed blood autotransfusion.
Keeping additional options such as cell saver and aprotinin available for cardiopulmonary and vascular high risk patients.
Exclusion of surgeon-related "subjective" trigger parameters of decision making for self- and foreign donated blood transfusion.
It may be assumed that there are other applications in orthopaedic surgery for the system (e.g. sealing defects after large bone graft harvesting, total hip and knee revisions, tumour surgery) which should be evaluated on a larger scale in the future. The same holds true for use in patients with elevated bleeding risks.
References
- 1.Callaghan J. An analysis of current transfusion practice in patients undergoing total hip and knee replacement. In: Brown A, editor. Analyst interviews & surgeon interviews, re: cost of blood. 1999. [Google Scholar]
- 2.Hay SN, Monk TG, Brecher ME. Intraoperative blood salvage: a mathematical perspective. Transfusion. 2002;42(4):451–455. doi: 10.1046/j.1525-1438.2002.00085.x. [DOI] [PubMed] [Google Scholar]
- 3.Levy O, Martinowitz U, Oran A, Tauber C, Horoszowski H. The use of fibrin tissue adhesive to reduce blood loss and the need for blood transfusion after total knee arthroplasty. A prospective, randomized, multicenter study. J Bone Joint Surg Am. 1999;81(11):1580–1588. doi: 10.2106/00004623-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Martin A, Prenn M, Spiegel T, Sukopp C, von Strempel A. Die Bedeutung der Wunddrainage in der Knieendoprothetik - eine prospektive Vergleichsstudie [Relevance of wound drainage in total knee arthroplasty--a prospective comparative study] Z Orthop Ihre Grenzgeb. 2004;142(1):46–50. doi: 10.1055/s-2004-817656. [DOI] [PubMed] [Google Scholar]
- 5.Mengal B, Aebi J, Rodriguez A, Lemaire R. Drainage ou non-drainage postopératoire dans les arthroplasties totales primaires de hanche et de genou : étude prospective randomisée [A prospective randomized study of wound drainage versus non-drainage in primary total hip or knee arthroplasty] Rev Chir Orthop Reparatrice Appar Mot. 2001;87(1):29–39. [PubMed] [Google Scholar]
- 6.Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86-A(6):1146–1152. doi: 10.2106/00004623-200406000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Pfeiffer M, Schuler P, Wirth T, Griss P. Sekretverluste bei orthopadisch-chirurgischen Standardeingriffen unter Anwendung einer neuartigen geschlossenen Vakuumdrainage. Ein Erfahrungsbericht [Wound secretions in standard orthopedic surgery interventions using a new kind of closed vacuum drainage. A report of experiences] Unfallchirurg. 1991;94(4):194–197. [PubMed] [Google Scholar]
- 8.Rosolski T. Autologe Direkt-Retransfusion (ADR)- Contra Diskussion [Autologous transfusion -- from euphoria to reason: clinical practice based on scientific knowledge (Part II). Autologous direct re-transfusion -- contra] Anasthesiol Intensivmed Notfallmed Schmerzther. 2004;39(11):697–700. doi: 10.1055/s-2004-825910. [DOI] [PubMed] [Google Scholar]
- 9.Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty?. Correct blood loss management should take hidden loss into account. Knee. 2000;7(3):151–155. doi: 10.1016/s0968-0160(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 10.Sigg A, Grosse C, Henche HR. Fremdblutsparende Maßnahme bei knieendoprothetischen Eingriffen durch Aprotinin [Blood saving in Total Knee Arthroplasty by Aprotinin] Orthopädische Praxis. 2003;39(8):471–473. [Google Scholar]
- 11.TissueLink Medical's HemoSealing technology - Procedure guide for Total Knee Arthroplasty. Dover, NH: TissueLink Medical Inc.; 2003. [Google Scholar]
- 12.Waters JH, Lee JS, Karafa MT. A mathematical model of cell salvage efficiency. Anesth Analg. 2002;95(5):1312–1317. doi: 10.1097/00000539-200211000-00040. [DOI] [PubMed] [Google Scholar]
- 13.Weiskopf RB. Efficacy of acute normovolemic hemodilution assessed as a function of fraction of blood volume lost. Anesthesiology. 2001;94(3):439–446. doi: 10.1097/00000542-200103000-00013. [DOI] [PubMed] [Google Scholar]