Abstract
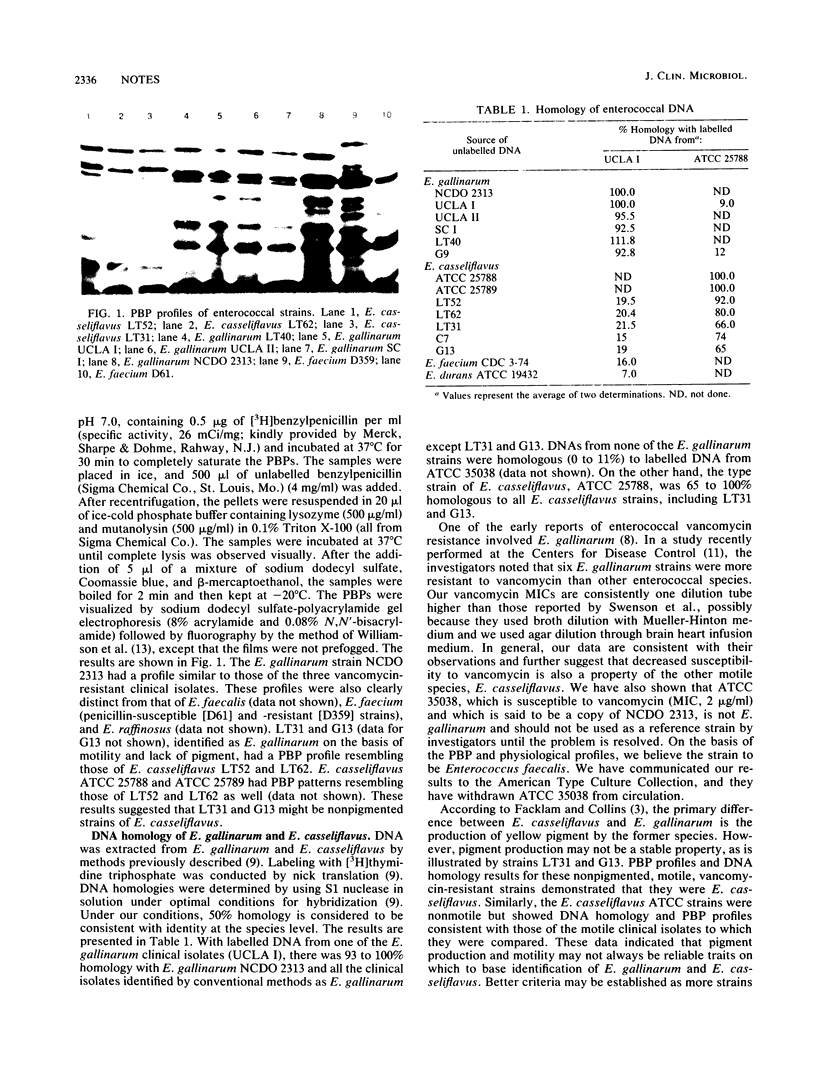
Thirty-seven clinical isolates of Enterococcus gallinarum and Enterococcus casseliflavus and three type or reference strains of the species were studied with respect to vancomycin susceptibility and key identification characteristics. With the exception of one clinical isolate of E. casseliflavus (MIC, 4 micrograms/ml), MICs of vancomycin were 8 to 32 micrograms/ml. The type strain of E. gallinarum, NCDO 2313, and five of the clinical isolates had similar penicillin-binding protein profiles and shared 90 to 100% DNA homology. Two isolates, identified as E. gallinarum by conventional tests, were shown to be non-pigment-producing E. casseliflavus on the basis of penicillin-binding protein profile and DNA homology. The type and reference strains of E. casseliflavus, ATCC 25788 and ATCC 25789, were nonmotile in our experiments. However, both shared 65 to 100% DNA homology with each other and with five clinical isolates of E. casseliflavus. These data suggest that the MICs of vancomycin observed for strains of E. gallinarum and E. casseliflavus are higher than those usually associated with other enterococci and may be a common property of these species. Additionally, pigment production and motility may occasionally be misleading criteria for definitive identification of these organisms.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Facklam R. R., Collins M. D. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989 Apr;27(4):731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow J. A., Jones D., Phillips B. A., Collins M. D. Taxonomic studies on some group D streptococci. J Gen Microbiol. 1983 May;129(5):1423–1432. doi: 10.1099/00221287-129-5-1423. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Douglas G. J., Hobbs S. J. Self-transferable plasmids determining the hemolysin and bacteriocin of Streptococcus faecalis var. zymogenes. J Bacteriol. 1975 Mar;121(3):863–872. doi: 10.1128/jb.121.3.863-872.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. P., Uttley A. H., Woodford N., George R. C. Resistance to vancomycin and teicoplanin: an emerging clinical problem. Clin Microbiol Rev. 1990 Jul;3(3):280–291. doi: 10.1128/cmr.3.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Gilligan P. H., Facklam R. R. Recovery of resistant enterococci during vancomycin prophylaxis. J Clin Microbiol. 1988 Jun;26(6):1216–1218. doi: 10.1128/jcm.26.6.1216-1218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Hill B. C., Thornsberry C. Problems with the disk diffusion test for detection of vancomycin resistance in enterococci. J Clin Microbiol. 1989 Sep;27(9):2140–2142. doi: 10.1128/jcm.27.9.2140-2142.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Gutmann L., Horaud T., Delbos F., Acar J. F. Use of penicillin-binding proteins for the identification of enterococci. J Gen Microbiol. 1986 Jul;132(7):1929–1937. doi: 10.1099/00221287-132-7-1929. [DOI] [PubMed] [Google Scholar]