Abstract
Human umbilical cord blood as a source of stem cells has recently been reported in experimental treatment of cerebral disorders. However, little is known about the nature of cells and cellular mechanisms leading to neurofunctional improvement. Here we investigated the potential of separated CD34+ versus CD34- human umbilical cord blood cells (HUCBC) to promote functional recovery following stroke. The experiments were performed in spontaneously hypertensive (SH) rats, known for a risk profile comparable to stroke patients.
After three weeks of behavioral training in the RotaRod and Beamwalk test arrays, stroke was induced by permanent middle cerebral artery occlusion (MCAO). For cell therapy, 1x106 cryopreserved cells were administered systemically between 8 and 10 hours after MCAO. The behavioral tests were performed together with a neurological severity score (mNSS) until day 29 to assess neurofunctional disabilities. Nearly complete functional remission was observed with both subpopulations CD34+ as well as CD34- cells. To localize cells histologically, they were labeled with a fluorescence dye (CFSE) before injection. Again, after administration of CD34+ as well as CD34- cells, CFSE labelled cells were found that accumulated in the border zone between the central necrosis of the ischemic lesion and functional brain tissue, thus indicating active attraction towards the lesion for both cell populations. Immunohistology with anti-CD68 and antibodies to human neuronal markers (NF-L, chromogranin) indicated an accumulation of human and rat monocytes in the border zone of the lesion while neuronal cells of human origin could not be detected in host brains.
Keywords: behavioral test, cord blood, middle cerebral artery occlusion, stem cell, stroke, transplantation
Abstract
Obwohl humanes Nabelschnurblut als Quelle von Stammzellen für experimentelle Therapien von Erkrankungen des Zentralnervensystems derzeit intensiv untersucht wird, ist noch wenig über die zellulären Prozesse bekannt, die der funktionellen Verbesserung von Ausfallerscheinungen zugrunde liegen. In der vorliegenden Studie untersuchten wir das Potenzial humaner Nabelschnurblutzellen, funktionelle Verbesserungen nach einem experimentellen Schlaganfall zu unterstützen. Die Experimente wurden mit spontan hypertensiven (SH) Ratten durchgeführt, deren metabolische Grunderkrankungen dem Risikoprofil menschlicher Schlaganfallpatienten entsprechen.
Nach drei Wochen der Konditionierung für die Verhaltenstests RotaRod und Beamwalk wurde ein experimenteller Schlaganfall durch permanente Okklusion der mittleren Hirnarterie (middle cerebral artery occlusion, MCAO) ausgelöst. Im Zuge der Zelltherapie wurden 1x106 kryokonservierte CD34+- oder CD34--Zellen 8 bis 10 Stunden nach Verschluss der rechten mittleren Hirnarterie intravenös appliziert. Die Verhaltenstests wurden zusammen mit der Erhebung des modified neurological severity score (mNSS) bis 29 Tage nach Eintritt des experimentellen Schlaganfalls durchgeführt, um die Entwicklung neurofunktionaler Defizite im Verlauf beurteilen und quantifizieren zu können. Dabei wurde eine annähernd komplette Rückbildung von Ausfallerscheinungen bei Gabe beider Zellpopulationen beobachtet. Um transplantierte Zellen lokalisieren zu können, wurden diese mit dem Fluoreszenzfarbstoff CFSE unmittelbar vor der systemischen Zellgabe markiert. Sowohl nach der Gabe von CD34+- als auch CD34--Zellen konnten CFSE-markierte Zellen in der Grenzzone zwischen zentraler Kolliquationsnekrose und funktionellem Hirngewebe detektiert werden. Immunhistologische Untersuchungen mit anti-CD68 Antikörpern gegen neuronale Marker (NF-L, Chromogranin) zeigten eine verstärkte Ansammlung von Zellen monozytärer Natur, während neuronale Zellen humanen Ursprungs nicht identifiziert werden konnten.
Introduction
Cell transplantation after stroke has been used to either substitute stem cells for neuronal differentiation or to promote the endogenous regenerative potential and plasticity of the brain with the therapeutic goal being restoration of functional neuronal networks. However, the use of embryonic or fetal grafts in humans is restricted by ethical considerations, severe logistical problems, and because intracerebral administration of homologous embryonic stem cells may lead to teratocarcinomas as demonstrated in rodents [11]. Therefore, alternative stem cell sources for treatment of neurological disorders have been evaluated in rat models, among them human bone marrow [7] and human umbilical cord blood (HUCB, [23]).
HUCB mononuclear cells are easy to obtain and show extensive proliferative capacity in vitro [34]. Similar to the percentage observed in bone marrow, 1%-2% of mononuclear HUCB cells express CD34, the surface marker molecule of hemopoietic stem cells [2]. Sanchez-Ramos et al. [26] and Bicknese et al. [3] demonstrated, that HUCB-derived cells express neuronal markers when cultivated in media known to promote expansion and differentiation of neuronal precursors. Consequently, HUCB cells were used for treatment of experimental neurological diseases in animal models like traumatic brain injury [22], paraplegia caused by spinal cord injury [27], amyotrophic lateral sclerosis [14], and stroke [6]. The therapeutic benefit in stroke treatment was demonstrated by intravenous administration of HUCB cells enriched for CD34+ cells after middle cerebral artery occlusion (MCAO) in Wistar rats [6].
However, because of the heterogeneity of HUCB mononuclear cells, it is the question whether or not the classical hemopoietic stem cell characterized by the surface marker CD34 (as used in the study of Chen et al. [6]) is responsible for the therapeutic benefit. To evaluate whether CD34 is an appropriate marker to describe the cell population responsible for the therapeutic benefit upon experimental stroke in rats, we prepared by separation with magnetic beads highly enriched CD34+ cells and used them in rat MCAO in comparison to cell preparations which were depleted from CD34+ cells. RotaRod, Beamwalk, and mNSS, a modified neurological score system [6], were used to evaluate frequently the neuromotoric capability of the animals. In order to localize the cells in tissue sections the cells were labeled prior to injection intracellularly with the fluorescence dye carboxyfluorescein succinimidyl ester (CFSE) according to Parish [24].
When our experiments were started by establishing the MCAO model we found the dimension of functional deficits in Wistar rats not very suitable for the evaluation of subtle distinctions in the efficiency of treatment protocols. Therefore, further experiments were performed with spontaneously hypertensive (SH) rats. During maturation these animals develop an idiopathic hypertension, hyperinsulinemia, hypertriglyceridemia and hypercholesterinemia associated with reduced cerebral blood flow [28] very similar to the risk profile of stroke patients. Lesions are larger and more consistent as with normotensive rats [10], [31]. Permanent instead of transient surgical occlusion was performed in our MCAO model since the majority of stroke events in humans (85%) is caused by thrombembolism [8]. Another important parameter is the time window selected for therapy. We performed cell injection 8-10 hours after experimental MCAO which is earlier than in other studies [6], [33] in order to intervene in the early remodelling phase following the ischemic insult.
In this study, we could demonstrate a nearly complete recovery from functional deficits caused by permanent MCAO in spontaneously hypertensive rats by intravenously administered HUCB cells. Furthermore, human CD34- cells from HUCB were as sufficient for successful treatment of stroke in SH rats than CD34+ cells. Both cell populations were found accumulated in the border zone of the lesion. CD34- cells comprise more than 98% of HUCB cells.They are much easier to obtain than purified CD34+ cells and may provide an important source for treatment of stroke in future cell based therapies in humans.
Materials and methods
Human cord blood (HUCB) sources
Samples of HUCB were obtained from several hospitals in Leipzig, Germany. They were taken in accordance to ethical prescripts immediately after delivery. Mononuclear cells were isolated as described below and stored in the gaseous phase of liquid nitrogen. Cryopreserved samples of cord blood were obtained from the cord blood bank VITA34 Inc., and the cells were separated after thawing the frozen HUCB samples.
HUCB cell preparation
The mononuclear cell fraction of pooled cord blood samples was separated using Ficoll-Paque PLUS (Amersham Biosciences, Uppsala, Sweden) density gradient centrifugation according to the manufacturers protocol. Cells were stored by freezing after addition of 5.5% dimethyl sulfoxide (DMSO, Serumwerke Bernburg Inc., Bernburg, Germany) in small drops according to standard operation procedures using a computer-controlled freezing device (Sylab, Austria). Prior to transplantation, cryopreserved HUCB cell samples were thawed rapidly in a water bath at 37°C, transferred to a tube containing a mixture of 10 ml RPMI 1640 Medium (Biochrom Inc., Berlin, Germany), 2 ml DNAse I and 60 µl 0.5M MgCl2 (Sigma, Germany) at 37°C. Cells were centrifuged at 1250 rpm for 5 minutes at room temperature, the supernatant was removed and the pellet resuspended in 8 ml phosphate buffered saline (PBS, pH 7.4; Biochrom Inc.) and 2 ml DNAse I for a second washing step. Cell viability was then assessed by dye exclusion using a 0.4% Trypan Blue solution and a Neubauer counting chamber in order to adjust the cell number in the samples to viable cells. Only samples with a viability above 90% were used for further processing. Thereafter, cells were centrifuged and resuspended in a final volume of 300 µl PBS per 108 total cells.
Enrichment or depletion of CD34+ cells
The enrichment or depletion of CD34+ cells was performed using microbead-conjugated anti-CD34 antibodies (CD34 Progenitor Cell Isolation Kit), LS+/VS+ columns and a SuperMACS magnetic cell separator (all Miltenyi-Biotech Inc., Bergisch Gladbach, Germany) according to manufacturers instructions. Briefly, 100 µl of FcR blocking was added per 108 cells to inhibit unspecific Fc receptor-mediated binding. Samples were incubated with microbead-conjugated anti-CD34 antibody solution (clone QBEnd/10 against epitope II) for 30 minutes at 6°C. Cells were selected using a LS+/VS+ column and a SuperMACS magnetic cell separator. CD34- cells remaining in the column were washed out and collected in a separate tube. A representative fraction of separated cells was incubated with a conjugated non-competing anti-CD34 antibody (dilution 1:100, Immunotech, clone 581 against CD34 epitope III) for further FACS analysis.
Sample analyses
In order to characterize the cell samples, flow cytometric analyses were performed using a FacsCalibur™ Scanner equipped with the CellQuest™ software (both Becton-Dickinson, Franklin Lakes, USA). Integral cells were determined above the level of 200 arbitrary units on the sideward scatter scale (FSC-H). Events below this level were regarded as cell fragments according to control experiments.
Cell labeling with carboxy fluoresceindiacetate succinimidyl ester (CFSE)
For localization in histological sections, HUCB cells were labeled in vitro before injection with 0.1% CFSE (Molecular Probes Inc., Eugene, USA) for 10 minutes in RPMI 1640 medium at 37°C. Labeling efficiency was verified by cytofluorometric analysis and the samples were only taken for further experiments when at least 99% of the cells showed an increase in fluorescence intensity at least by a factor of 100. Cell viability was again calculated by the Trypan Blue method and a total amount of 1x106 viable CD34+ or CD34- cells was injected i.v. per animal. The distribution of CFSE-labeled cells was examined by UV microscopy in tissue sections as described below.
Animals
All experimental procedures were approved by the Experimental Animal Committee of the Regional Council of Leipzig. 48 male spontaneously hypertensive (SH) rats (Charles River Laboratories), weighting 220 g to 275 g were used in the experiments. Animals were maintained in individual home boxes at 12:12 hour light:dark cycle. Light was turned on at 6:00 a.m. Rats were allowed to have access to food and water ad libitum and were kept at room temperature (23°C). Conditioning of animals was started at least 7 days after animal delivery to allow for adaption to the new environment.
HUCB cell injection
8 to 10 hours after MCAO-induced brain ischaemia, the animals received either HUCB cells as single cell suspension or phospate buffered (pH 7.4) physiological saline (PBS) for control. Animals were shortly anaesthetized using a Plexiglas chamber flooded with CO2 and were rapidly transferred to an application desk on which the tail was fixed. A total amount of 1x106 viable CD34+ or CD34- cells in 1 ml total fluid volume (PBS) were injected into a tail vein.
Permanent middle cerebral artery occlusion (MCAO)
Rats were initially anaesthetized with ketamine chloride 100 mg/kg body weight (Merial Ltd., Hallbergmoos, Germany), 10 mg/kg xylazine (Bayer Inc., Leverkusen, Germany) and 0.1 mg/kg atropine (Ratiopharm Inc., Ulm, Germany), delivered intraperitoneally. Then, the animals were kept in their home cages until the corneal reflex could not be triggered anymore as a sign of deep anaesthesia. The head of the animal was then fixed on a tooth bar mounted in a flexible face mask allowing oxygen supply throughout the surgical procedure. The mask can be rotated up to 30 degrees around the longitudinal axis to turn the animal´s head to get better access to the middle cerebral artery (MCA). Animals were placed throughout the whole surgical procedure on a feedback controlled warming pad (Fine Science Tools Inc., Heidelberg, Germany) which included measurement of pulse rate and blood oxygenation status (Nonin Medical Inc., Plymouth, USA). Both eyes were protected against drying with sterile eye ointment.
The MCA was occluded according to a method reported by Tamura [30]. A L-shaped incision was performed in the scalp and the musculus temporalis between eye and ear on the right side. Then, the head of the animal was turned to the left for easy access to MCA proximally to its origin at the circulus arteriosus. The zygomatic arch was carefully removed using a bone forceps. A subtemporal craniotomy was carried out using a dental drill and the dura mater was locally incised with a fine needle. Then the MCA was carefully elevated from brain surface by a hook mounted on a stereotactic frame. For permanent vessel blocking an electrically heated wire loop was used to disrupt and immediately occlude the MCA. The incisions were sutured using absorbable 4-0 surgical threads and the wound surface was temporally anaesthetized by lidocaine gel (2%, Farcopharma Ltd, Cologne, Germany). Following surgery, animals were taken back to their home cages to recover. Postsurgical anaesthesia was provided by adding metamizole-Na (25 mg, Ratiopharm Inc., Ulm, Germany) and 5% glucose to the water storage can (40 ml) of each cage daily for one week.
Functional behavioral testing
All animals received a 3-week training on a set of behavioral tests before MCAO. The battery of functional tests comprised the computer controlled RotaRod system (TSE Systems Ltd., Bad Homburg, Germany), the beam walk test and the modified neurological severity score (mNSS, [6]).
The Rota Rod test was basically performed according to Dunham and Miya [9] and Jones and Roberts [17]. Rats were placed on a motor driven horizontal cylinder (the rod) in up to four individual chambers of the device. The time animals are able to remain on the accelerating cylinder was measured. The acceleration, final rotation speed and time of animal drop off were controlled and documented digitally. In our setting, rotation speed increased from 3 rpm to 60 rpm within 3 minutes. A trial was terminated if an animal fell or jumped off the rod or if the rat remained on the rod for 200 seconds. Animals were allowed to recover in their home cages for at least 3 minutes before a new trial was started. Mean duration (in seconds) was recorded from five individual trials.
In the beam walk test according to Hamm et al. [15], rats must move over a horizontally mounted bar (1 m in length, 14 mm in diameter) to enter their home boxes placed at the end of the beam. The time animals took to get over the bar was measured in five trials and the mean time was recorded. If the rat slipped of the beam and hung, a maximum time of 20 seconds was noted for this trial. If an animal fell off the bar, it received a 30-second malus.
Table 1 (Tab. 1) shows the items for the modified neurological severity score (mNSS) according to Chen et al. [6]. Neurological function including motoric and sensoric systems as well as reflexes and a balance test is graded on a numeric scale from 0 to 18 (0 for no disorders/normal function and a maximum lesion score of 18). If an animal was unable to perform a test or if the lack of a tested reflex was noticed, a score point was given. Thus, the higher the score the more severe the ischemic lesion caused by MCAO. 1-6 mNSS score points were considered as mild, 7-12 score points as medium and 13-18 score points as severe injury.
Table 1. Detailed description of the items forming the modified neurological severity score (mNSS) according to Chen et al. [6].
The beam walk is commonly used to evaluate sensomotoric deficits in rats following MCAO.
A baseline measurement was taken one day prior to surgery. From the day following the surgical procedure, RotaRod and beam walk testing were performed daily up to 7 days post operation. Thereafter, tests were carried out every second day until day 29. All animals underwent the mNSS test at day 1, 4, 7, 11, 15, 19, 23, and 27. Behavioral testing was conducted during the light phase and animals were not removed from their familiar environment. All experiments were done by one researcher blinded to the experimental groups, and the sequence of tests was randomised between the animals each day. Data are presented as absolute values in seconds (RotaRod and beam walk) or score points (mNSS).
Tissue preparation, immunohistochemistry and histological analysis
After termination of behavioral tests animals were deeply anaesthetized with CO2 and were perfused transcardially with physiological saline solution. The brains were removed immediately and evaluated for gross macroscopical pathology. 2 mm thick frontal slices of fresh rat brains were cut and then snap frozen on dry ice using precooled metal plates with polished surfaces. 10-15 µm thick serial sections throughout the whole brain were fixed in 8% ethanol 1/1 v:v for 15-30 minutes and then stained with either Hematoxylin/Eosin (HE) or Giemsa stain (Merck, Haar, Germany), dehydrated and mounted in Eukitt (Merck). Sections designated for fluorescence microscopy were mounted in non-fixed condition in aqueous medium (Aquamount; Polysciences, Warrington, USA). Microscopy was done using a Zeiss Axioplan 2 microscope with Neofluar objectives and the Zeiss Fluorescence filter sets 02, 05, 06, 09, 15 and 26.
After morphologic differentiation between functional tissue versus colliquation necrosis or the border zone in HE-stained slices, borderlines between those areas were marked digitally. Then analysis of the following, corresponding fluorescence slices was performed after transfer of digital borderlines to the fluorescence image.
For further characterization, brain slices were incubated with mouse monoclonal antibodies against anti-human CD34, anti-human CD68, anti-human neurofilament-L fragment (NF-L, 70 kDa) antibodies, anti-human chromogranin (all Dako Cytomation, Glostrup, Denmark) or anti-rat CD68 (Dako, Glostrup, Denmark) according to manufacturers instructions.
Statistical analysis
All behavioral tests were evaluated for normality and equal variances. Analyses of significance were performed using the non-parametric Mann-Whitney test for comparison of the groups. We also tested the balance of the baseline variables in comparison of all groups to include any potential imbalances in the analysis of the treatment effect. Both, differences between treated groups and control animals as well as differences between CD34+ and CD34- treated subjects were evaluated for each behavioral test. P-values of 0.05 or less were considered statistically significant. All data are shown as mean ± standard deviation (SD).
Results
Characterization of separated HUCB cells
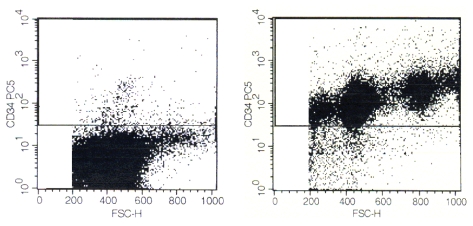
HUCB mononuclear cells were separated by anti-CD34 antibodies and magnetic beads as described above. Figure 1 (Fig. 1) shows characterization of the cell preparations used for treatment studies. The right panel (Fig. 1) shows the CD34+ cell enriched population containing a mean proportion of 98.9% CD34+ cells with a viability of >91% (5 independent experiments). The enrichment factor amounted to more than 50 (mean of 1.8% CD34+ cells in regular HUCB). The left panel (Fig. 1) shows the preparation depleted from CD34+ cells. The degree of depletion ranged from 3x to 6x i.e. from 0.1 to 0.5% CD34+ cells, as determined in five independent experiments. Viability was >92% in each experiment.
Figure 1. FACS analysis of HUCB cells used for treatment.
Left panel shows a CD34+ cell depleted sample stained with an anti-CD34 antibody (0.4% CD34+ cells) directed to a non-competing CD34 epitope as compared to the antibody used for cell separation. Right panel shows a CD34+ cell enriched fraction (98.9%).
Neurofunctional recovery after cell therapy
Animals were randomly assigned to one of three groups. Group 1 (n=18) received CD34+ cells while group 2 (n=12) was injected with CD34- cells. Group 3 (n=18) was taken as a control receiving a sham injection of PBS.
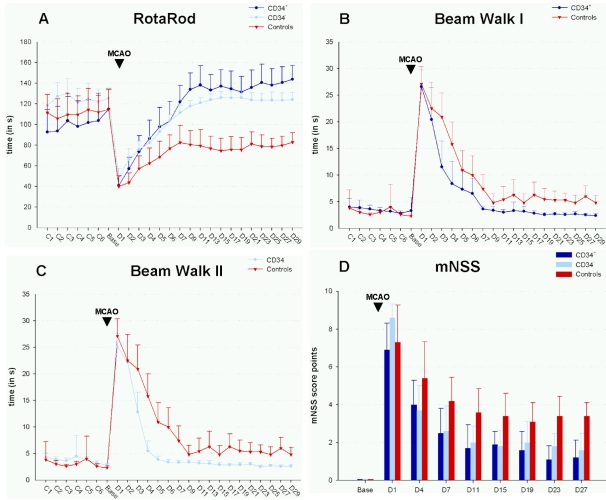
Rats receiving HUCB cells of both populations showed a remarkable reduction of functional and neurological deficits in each test with complete remission in the RotaRod and beam walk test as compared to baseline measurement (Figure 2A-D (Fig. 2)). Statistically significant differences (P<0.05) between animals treated with CD34+ cells and untreateted controls were first observed at day 2 in the RotaRod (P<0.05, Figure 2A (Fig. 2)), at day 3 in the beam walk array (P<0.01, Figure 2B and Figure 2C (Fig. 2)) and at day 4 in the mNSS (P<0.05, Figure 2D (Fig. 2)). CD34- HUCB cell administration became superior to saline injection at the third day in the RotaRod experiment (P<0.05) and the beam walk test (P<0.01) and at day 4 in the mNSS (P<0.05). With each functional test a high level of statistical significance (P<0.00001) was reached in the plateau phase of functional recovery (see Figure 2 (Fig. 2)). This level of highly significant difference was maintained at least from day 11 until the end of behavioural testing.
Figure 2. Results of behavioral tests before and after permanent MCAO (▼).
SH rats received 1x106 cells enriched or depleted from CD34+ cells by magnetic bead separation (n=18 for CD34+ cell treatment; n=12 for CD34- cell treatment) or 1 ml of PBS for control (n=18) 8 to 10 hours following ischemia. Behavioral tests (RotaRod, A; beam walk, B and C) and the modified neurological severity score (D) were performed during the phase of conditioning, before (Base line) and after MCAO throughout the evaluation phase. CD34+ and CD34- treated animals showed statistically significant improvement of neurological deficits in the plateau phase starting at day 11 (P<0.0001 in all test arrays). For statistical details in the early phase (D2-D9) see description in the text. Data are presented as absolute values ± SD.
Comparing CD34+ HUCB cells with CD34- HUCB cells, no statistically significant difference (P<0.05) was observed with regard to the beneficial effect of both populations (Figure 2A-D (Fig. 2)). In the RotaRod test, marginal differences were only observed at day 9 (P=0.03), day 11 (P=0.04) and day 29 (P=0.04). Beam walk data were marginally different only at day 6 (P=0.01) while in the mNSS test a marginal divergence could be noticed at day 1 (P=0.02). In all cases, data obtained from animals that were administered CD34- HUCB cells tended to a slightly less effective treatment as compared to the use of CD34+ HUCB cells. However, the null hypothesis, i.e. no statistical difference between CD34+ versus CD34- HUCB cells, had to be accepted (P>0.4) in each case.
An example of the sensomotoric performance of experimental animals 14 days after MCAO with and without cell transplantation as well as before MCAO is given in the video file Attachment 1 attached. A cell-transplanted animal is able to pass the beam walk within 1.5 to 3.0 s like the healthy control animal without major stop overs or imbalances, while a non-transplanted MCAO subject clearly suffers from sensomotoric disabilities interrupting its walking frequently with the tendency to loose balance and to fall down.
Histological localization of HUCB cells in brain and other organs
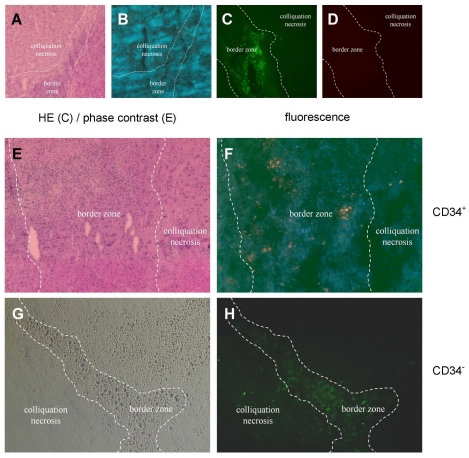
For histological studies the cells were labeled with CFSE prior to injection as described above and serial tissue sections were prepared after termination of the animal experiments at day 29 after MCAO for investigation by UV microscopy. Control experiments were performed without using HUCB cells as shown in Figure 3 (Fig. 3) for HE-staining (Figure 3A (Fig. 3)) and the green fluorescence channel (Figure 3B (Fig. 3)) displayed in parallel sections, while CFSE-labeled HUCB mononuclear cells were used in the experiment shown in Figure 3C (Fig. 3) in the green channel versus the red channel (Figure 3D (Fig. 3)) in order to demonstate exclusion of autofluorescence. CFSE-labeled cells could be detected clearly (Figure 3E-H (Fig. 3)) upon intravenous administration of both preparations, CD34+ and CD34- HUCB cells. They were found accumulating in the border zone (Figure 3F and H (Fig. 3)), while no fluorescent cells were observed at the contralateral side of lesion or in control animals (Figure 3B (Fig. 3)).
Figure 3. Tracking of CFSE-labelled CD34+ and CD34- HUCB cells in serial sections of SH in rat brains 29 days after MCAO.
A: control animal without cell therapy, HE staining; B: control animal, UV microscopy with green fluorescence; C: HUCB mononuclear cell treatment, green fluorescence, the borderline between the necrotic central area and the border zone to unaffected tissue is marked according to HE serial section; D: HUCB mononuclear cell treatment, red fluorescence; E: CD34+ HUCB cell treatment, HE staining; F: CD34+ HUCB cell treatment, green fluorescence (inverse colours); G: CD34- HUCB cell treatment, phase contrast microscopy; H: CD34- HUCB cell treatment, green fluorescence. 400fold magnification in all images.
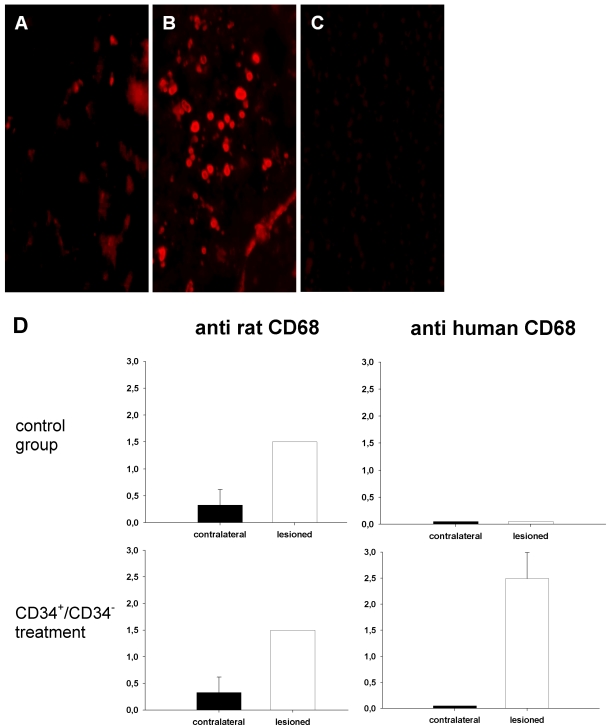
However, we were not able to identify neuronal cells of human origin by staining with antibodies against human NF-L or chromogranin as assessed four weeks after cell injection. Staining with anti-rat CD68 showed a moderate and diffuse activity of host microglial cells and monocytes in ischemic areas (Figure 4A and D (Fig. 4)). In both groups, animals transplanted with either human CD34+ or CD34- cells, an increase of human CD68+ cells could be found in the border zone of the lesion (Figure 4B and D (Fig. 4)), while human CD68 could not be found in the contralateral hemisphere or in animals that were not transplanted with human cells (Figure 4C and D (Fig. 4)). Staining with anti-human CD34 antibodies suffered from diffuse fluorescence activity in most endothelial cells. After HUCB cell administration, no tumor formation was found in any animal until termination of the experiment at day 29 post operation.
Figure 4. Detection of cells positive for rat or human CD68 in control animals and subjects that received CD34+/CD34- cell transplantation.
Moderate and diffuse fluorescence signals from cells positive for rat CD68 occurs in the lesioned hemisphere of transplanted and control animals (A). A strong increase of fluorescence caused by cells positive for human CD68 cells could be detected in animals transplanted with both, CD34+ and CD34- cells indicating a "monocyte fate" of administered cells (B). Cells positive for human CD68 could not be detected in control rats (C) or in the contralateral hemisphere. D shows the level of fluorescence according to a score point system (0=none, 1=low, 2=moderate, 3=high fluorescence activity).
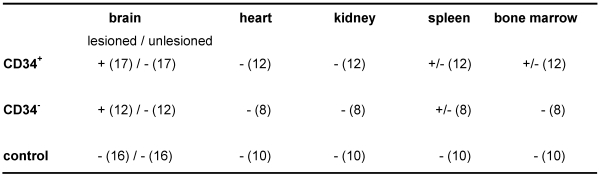
Tissue samples from heart, kidney, spleen and bone marrow of experimental animals were examined for CFSE-positive cells. Only few CFSE-positive cells were found in spleen and bone marrow of animals transplanted with CD34+ cells (Table 2 (Tab. 2)).
Table 2. Detection of CFSE-labeled cells in rat organs 29 days upon transplantation.
Number of evaluated animals is given in brackets.
+ = high density of CFSE-positive cells
+/- = only few CFSE-positive cells were detected
- = no CFSE-positive cells were detected in slices of harvested organs
Discussion
The experimental model
In order to verify the benefit of new therapeutical procedures for future use in humans, it is important to design experimental animal models in close relation to clinical requirements. In many human patients suffering from stroke, the capillary system is damaged by long-term hypertension, hypertriglyceridemia and hypercholesterinemia. Therefore, we performed the experiments in SH rats instead of strains like Wistar or Sprague Dawley which were used in previous studies [6], [33]. According to Traystman [31], SH rats suffer from larger and more consistent ischemic lesions and showed less variable functional deficits within the experimental groups which could be confirmed by own experiences (data not shown).
Close relation to clinical reality for potential treatment protocols was also the reason to adopt the permanent artery occlusion instead of transient blood vessel blockage by a surgical filament [20]. The time frame for cell therapy (8-10 hours after MCAO) would be feasible in the clincal practice of a stroke unit considering even the most unfortunate delay until the patients had arrived and passed all diagnostic procedures.
In principle, the use of xenogeneic (human) cells might induce a local immune response in the (rat) host which could hypothetically interfere with treatment e.g. by releasing neurotropic cytokines. However, Chen et al. [6] could demonstrate functional recovery by using HUCB cells in rat MCAO without immunosuppression. Their experimental design and results were comparable to the study of Willing et al. [33] using cyclosporine A. Since neuroprotective effects have been described recently by immunosuppressive drugs [18] we decided to omit their use. Moreover, recent control experiments (data not shown) with an irradiated human T cell clone with irrelevant specifity instead of HUCB cells showed no therapeutic effect at all. Because of irradiation at 5 Gy, T cells were not able to divide but remained viable in parallel in vitro culture for 29 days and were still able to produce TNF-α. By this experiment, we wanted to show that a xenogeneic immune reaction of the host towards cells of human origin was not responsible for the success of treatment.
Functional improvement in behavioral tests by CD34- cells
In this study, cell therapy with HUCB-derived cells demonstrated a significant improvement of neuromotoric function in SH rats but CD34- cells were nearly as effective as CD34+ cells. The number of remaining CD34+ cells in the depleted preparation was below 0.5% i.e. less than 5000 cells, while the enriched preparation contained nearly 1 million viable CD34+ cells. If CD34+ cells would be the cellular entity responsible for the therapeutic benefit there should be a clear distinction between both preparations in their functional potential.
Very recently, Taguchi et al. [29] have reported that HUCB-derived CD34- cells in contrast to CD34+ cells were not able to promote angiogenesis in SCID mice upon experimental focal cerebral ischemia. However, their cells were administered much later i.e. after 24 hours and persistent focal motor deficits were not observed in their model. Our results indicate that other cells than CD34+ cells seem to be involved. Interestingly, a cell population isolated from adult human brain and characterized as CD133+, CD34- and CD45- could expand and differentiate effectively within the brain of neonatally immunodeficient mice [32]. Despite that the cells in the study of Uchida et al. [32] were described as central nervous system stem cells, it is a possibility that more immature stem cells, described by another denominator, such as CD133, were responsible for the therapeutic benefit. Gallacher et al. [13] demonstrated that CD133+, CD34- cord blood cells are capable of forming heamtopoietic cells in vitro as well as in NOD/SCID mice and these immature cells may be responsible for the therapeutic effect seen in our CD34- cells.
The number of 106 cells injected systemically is relatively small as compared to the mass of ischemic tissue. Significant functional recovery could be detected at least at day 4 in the mNSS test, while the maximum improvement of neuromotoric functions was reached at day 11 and was maintained until the end of study. It seems to be unlikely that such a small number of cells integrates, develops appropriate neuronal connections, restores functional cerebral networks and leads to a conspicuous behavioural improvement within such a relatively short period of time. However, these cells may initiate or accelerate endogenous regeneration processes.
In general, an ischemic cerebral lesion consists of a central colliquation necrosis surrounded by the penumbra, an area of incomplete i.e. reversible cell damage [21]. The penumbra, as the “tissue at risk”, is a border zone dividing the regions of total ischemic cell destruction from those containing unaffected and functional tissue. This area is the main therapeutic target for treating stroke by regeneration and cellular restoration [12].
Upon intravenous administration, CFSE-labeled cells accumulated in a border zone comprising the brain area remaining from the penumbra after 4 weeks. Moreover, we found HUCB cells in the ipsilateral hemisphere of lesion and only very few CFSE-labeled HUCB in the contralateral ones as shown first by Chen et al. [6]. We were not able to detect cells of human origin expressing neuronal markers indicating that neurofunctional restoration is not related to neurogenesis from the transplanted cells. The detection of cells being positive for human CD68, a cellular marker for monocytes and macrophages, demonstrated that human cells take part in local processes of organisation of the lesion and the surrounding tissue. Findings of Borlongan et al. [5] indicate that trophic and/or neuroprotective factors produced by transplanted cells are mere important for neurofunctional restoration than the generation of new neurons.
Here, we could demonstrate that this accumulation was not selective for CD34+. The accumulation indicates chemotactic attraction of transplanted cells towards the lesion or topic expression of adhesion molecules with the same result: focal accumulation of migrating cells close to the ischemic lesion. Ischemic brain tissue expresses chemotactic proteins like MCP-1 [19] and microvascular endothelial cells significantly upregulate the expression of ICAM 1, vascular adhesion molecule-1 (VCAM) and E-selectin following acute ischemic cerebral injury as reported by Zhang et al. [35] and Blann et al. [4]. It is important to note that the blood brain barrier becomes leaky from 3 hours to 4 days after stroke in rats [16], [1], [25] which may allow for unselected entry of migrating cells. However, more work has to be done to investigate the mechanisms of cell migration and recruitment of regenerative potential in detail.
In conclusion, we could demonstrate that CD34+depleted HUCB cells accumulate in the border zone of the ischemic lesion and are highly sufficient for successful treatment of an ischemic insult after permanent occlusion of the middle cerebral artery in SH rats showing a risk profile. This population of cells is much easier to obtain than purified CD34+ cells which are a rare fraction in HUCB. Because HUCB cells are widely available, easy to store and to handle, they may provide an important source for treatment of stroke in future cell based therapies.
Acknoledgements
The authors wish to thank Manuela Ackermann, Mandy Glona, Silke Lehnert, and Christine Schulz for technical support as well as Sigrid Weisheit and Petra Madaj-Sterba for their valuable help considering animal care. This work was financially supported by a grant given by the state of Saxony (SAB) and by the Interdiszipinäres Zentrum für Klinische Forschung (IZKF) project A 00 supported by the BMBF (Bundesministerium für Bildung und Forschung).
Abbreviations
APC - allophycocyanine
bFGF - basic fibroblast growth factor
CD - cluster of differentiation
CFSE - carboxy fluoresceindiacetate succinimidyl ester
CNS - central nervous system
CSA - cyclosporine A
DMSO - dimethyl sulfoxide
FITC - fluorescein isothiocyanate
FSC-H - foreward scatter scale
HE - hematoxylin/eosin
HFS - high foreward scatter
HUCB - human umbilical cord blood
ICAM - intercellular adhesion molecule
LFS - low foreward scatter
LSS - low sideward scatter
MCA(O) - middle cerebral artery (occlusion)
MCP-1 - monocyte chemoattractant protein-1
mNSS - modified neurological severity score
NF-L - neurofilament L fragment
NOD/SCID - nonobese diabetic/severe combined immunodeficiency
PBS - phosphate buffered saline
PC5 - phycoerythrin-cyanin 5
PE - phycoerythrin
SH - spontaneously hypertensive
VCAM - vascular adhesion molecule
VEGF- vascular endothelial growth factor
Supplementary Material
A cell-transplanted animal is able to pass the beam walk within 1.5 to 3.0 s like the healthy control animal without major stop overs or imbalances, while a non-transplanted MCAO subject clearly suffers from sensomotoric disabilities interrupting its walking frequently with the tendency to lose balance and to fall down.
References
- 1.Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739(1-2):88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- 2.Bender JG, Unverzagt KL, Walker DE, Lee W, Van Epps DE, Smith DH, Stewart CC, To LB. Identification and comparison of CD34-positive cells and their subpopulations from normal peripheral blood and bone marrow using multicolor flow cytometry. Blood. 1991;77(12):2591–2596. [PubMed] [Google Scholar]
- 3.Bicknese AR, Goodwin HS, Quinn CO, Henderson VC, Chien SN, Wall DA. Human umbilical cord blood cells can be induced to express markers for neurons and glia. Cell Transplant. 2002;11(3):261–264. [PubMed] [Google Scholar]
- 4.Blann A, Kumar P, Krupinski J, McCollum C, Beevers DG, Lip GY. Soluble intercelluar adhesion molecule-1, E-selectin, vascular cell adhesion molecule-1 and von Willebrand factor in stroke. Blood Coagul Fibrinolysis. 1999;10(5):277–284. doi: 10.1097/00001721-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35(10):2385–2389. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32(11):2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 7.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1(2):92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 8.Delcker A, Diener HC, Timmann D, Faustmann P. The role of vertebral and internal carotid artery disease in the pathogenesis of vertebrobasilar transient ischemic attacks. Eur Arch Psychiatry Clin Neurosci. 1993;242(4):179–183. doi: 10.1007/BF02189960. [DOI] [PubMed] [Google Scholar]
- 9.Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharmacol Assoc. 1957;46(3):208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- 10.Duverger D, MacKenzie ET. The quantification of cerebral infarction following focal ischemia in the rat: influence of strain, arterial pressure, blood glucose concentration, and age. J Cereb Blood Flow Metab. 1988;8(4):449–461. doi: 10.1038/jcbfm.1988.86. [DOI] [PubMed] [Google Scholar]
- 11.Erdo F, Buhrle C, Blunk J, Hoehn M, Xia Y, Fleischmann B, Focking M, Kustermann E, Kolossov E, Hescheler J, Hossmann KA, Trapp T. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab. 2003;23(7):780–785. doi: 10.1097/01.WCB.0000071886.63724.FB. [DOI] [PubMed] [Google Scholar]
- 12.Fisher M, Ratan R. New perspectives on developing acute stroke therapy. Ann Neurol. 2003;53(1):10–20. doi: 10.1002/ana.10407. [DOI] [PubMed] [Google Scholar]
- 13.Gallacher L, Murdoch B, Wu DM, Karanu FN, Keeney M, Bhatia M. Isolation and characterization of human CD34(-)Lin(-) and CD34(+)Lin(-) hematopoietic stem cells using cell surface markers AC133 and CD7. Blood. 2000;95(9):2813–2820. [PubMed] [Google Scholar]
- 14.Garbuzova-Davis S, Willing AE, Zigova T, Saporta S, Justen EB, Lane JC, Hudson JE, Chen N, Davis CD, Sanberg PR. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: distribution, migration, and differentiation. J Hematother Stem Cell Res. 2003;12(3):255–270. doi: 10.1089/152581603322022990. [DOI] [PubMed] [Google Scholar]
- 15.Hamm RJ, Pike BR, O'Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11(2):187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 16.Hatashita S, Hoff JT. Brain edema and cerebrovascular permeability during cerebral ischemia in rats. Stroke. 1990;21(4):582–588. doi: 10.1161/01.str.21.4.582. [DOI] [PubMed] [Google Scholar]
- 17.Jones BJ, Roberts DJ. A rotarod suitable for quantitative measurements of motor incoordination in naive mice. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;259(2):211. doi: 10.1007/BF00537801. [DOI] [PubMed] [Google Scholar]
- 18.Kaminska B, Gaweda-Walerych K, Zawadzka M. Molecular mechanisms of neuroprotective action of immunosuppressants--facts and hypotheses. J Cell Mol Med. 2004;8(1):45–58. doi: 10.1111/j.1582-4934.2004.tb00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JS. Cytokines and adhesion molecules in stroke and related diseases. J Neurol Sci. 1996;137(2):69–78. doi: 10.1016/0022-510x(95)00338-3. [DOI] [PubMed] [Google Scholar]
- 20.Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema, I: a new experimental model of cerebral embolism in rats in recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- 21.Lassen NA, Vorstrup S. Ischemic penumbra results in incomplete infarction: is the sleeping beauty dead? Stroke. 1984;15(4):755–758. doi: 10.1161/01.str.15.4.755. [DOI] [PubMed] [Google Scholar]
- 22.Lu D, Sanberg PR, Mahmood A, Li Y, Wang L, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002;11(3):275–281. [PubMed] [Google Scholar]
- 23.Newman MB, Davis CD, Kuzmin-Nichols N, Sanberg PR. Human umbilical cord blood (HUCB) cells for central nervous system repair. Neurotox Res. 2003;5(5):355–368. doi: 10.1007/BF03033155. [DOI] [PubMed] [Google Scholar]
- 24.Parish CR. Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol Cell Biol. 1999;77(6):499–508. doi: 10.1046/j.1440-1711.1999.00877.x. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29(10):2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Ramos JR, Song S, Kamath SG, Zigova T, Willing A, Cardozo-Pelaez F, Stedeford T, Chopp M, Sanberg PR. Expression of neural markers in human umbilical cord blood. Exp Neurol. 2001;171(1):109–115. doi: 10.1006/exnr.2001.7748. [DOI] [PubMed] [Google Scholar]
- 27.Saporta S, Borlongan CV, Sanberg PR. Neural transplantation of human neuroteratocarcinoma (hNT) neurons into ischemic rats. A quantitative dose-response analysis of cell survival and behavioral recovery. Neuroscience. 1999;91(2):519–525. doi: 10.1016/s0306-4522(98)00610-1. [DOI] [PubMed] [Google Scholar]
- 28.Swislocki A, Tsuzuki A. Insulin resistance and hypertension: glucose intolerance, hyperinsulinemia, and elevated free fatty acids in the lean spontaneously hypertensive rat. Am J Med Sci. 1993;306(5):282–286. doi: 10.1097/00000441-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114(3):330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1(1):53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- 31.Traystman RJ. Animal models of focal and global cerebral ischemia. ILAR J. 2003;44(2):85–95. doi: 10.1093/ilar.44.2.85. [DOI] [PubMed] [Google Scholar]
- 32.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97(26):14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willing AE, Lixian J, Milliken M, Poulos, Zigova T, Song S, Hart C, Sanchez-Ramos J, Sanberg PR. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003;73(3):296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi M, Hirayama F, Murahashi H, Azuma H, Sato N, Miyazaki H, Fukazawa K, Sawada K, Koike T, Kuwabara M, Ikeda H, Ikebuchi K. Ex vivo expansion of human UC blood primitive hematopoietic progenitors and transplantable stem cells using human primary BM stromal cells and human AB serum. Cytotherapy. 2002;4(2):109–118. doi: 10.1080/146532402317381811. [DOI] [PubMed] [Google Scholar]
- 35.Zhang RL, Chopp M, Zaloga C, Zhang ZG, Jiang N, Gautam SC, Tang WX, Tsang W, Anderson DC, Manning AM. The temporal profiles of ICAM-1 protein and mRNA expression after transient MCA occlusion in the rat. Brain Res. 1995;682(1-2):182–188. doi: 10.1016/0006-8993(95)00346-r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A cell-transplanted animal is able to pass the beam walk within 1.5 to 3.0 s like the healthy control animal without major stop overs or imbalances, while a non-transplanted MCAO subject clearly suffers from sensomotoric disabilities interrupting its walking frequently with the tendency to lose balance and to fall down.