Abstract
Induction of c-fos in the spinal cord due to pain is well established. This study aims to look at the effects of acute swim stress on Fos-like immunoreactivity (FLI) induced by formalin and how it is modulated by ketamine and morphine. Acutely-stressed and non-stressed adult male Sprague Dawley rats were pretreated with intraperitoneal injection of ketamine 5 mg/kg (Ketava, Atlantic Lab), morphine 10 mg/kg (Rhotard, Custom Pharmaceutical), or saline, 5 minutes prior to experimentation. Rats were acutely stressed by swimming for 3 min in 20°C water. Dilute formalin (Formaldehyde, Merck) was injected to the hindpaw and the formalin score recorded. Rats were then sacrificed and spinal cords (L4-L5) removed for immunohistochemical analysis of FLI. Two-way ANOVA showed significant effects of stress, drug and stress-drug interactions in formalin test and FLI. Both morphine and ketamine produced analgesia in the formalin test. In the saline stressed group, FLI was suppressed on the ipsilateral side (p<0.01) but increased on the contralateral side (p<0.01) compared with non-stressed saline. In morphine and ketamine stressed groups, FLI was increased on both ipsilateral and contralateral sides for morphine (ipsilateral: p<0.05; contralateral: p<0.001) and ketamine (ipsilateral: p<0.05, contralateral: p<0.05) compared with their corresponding non-stressed groups. In conclusion, presence of stress may lead to discrepancy between behavioural manifestation of pain and c-fos induction in the spinal cord.
Keywords: Fos, stress, pain, formalin, morphine, ketamine
Abstract
Die Induktion von c-fos-Aktivität im Rückenmark unter Schmerz ist allgemein bekannt. Diese Studie hat zum Ziel, die Wirkung von Schwimmstress auf die durch Formalininjektion ausgelöste c-fos-ähnliche Immunreaktivität und wie diese unter Ketoamin- und Morphinbehandlung verändert wird zu untersuchen. Akut gestresste und nicht-gestresste, ausgewachsene, männliche Sprague-Dawley-Ratten wurden mit intraperitonealen Injektionen von Ketoamin (5 mg/kg) oder Morphin (10 mg/kg) oder durch Injektionen von physiologischer Kochsalzlösung 5 Minuten vor Beginn des Experimentes behandelt. Die akute Stresssituation wurde durch Schwimmen im Wasser bei 20°C über 3 Minuten erzeugt. Verdünntes Formalin (Formaldehyd, Merck) wurde in die hintere Pfote der Ratten injiziert und der Formalineffekt untersucht. Die Ratten wurden anschließend getötet, das Rückenmark (L4-L5) wurde für die immunhistologischen Analysen der c-fos-ähnlichen Immunreaktivität herauspräpariert. Die Auswertung in der zweifaktoriellen Varianzanalyse zeigte im Formalintest signifikante Effekte von Stress, von Pharmaka und von Stress-Pharmaka-Interaktionen auf die c-fos-ähnliche Immunreaktivität. Sowohl Morphin als auch Ketoamin erzeugten eine Analgesie im Formalintest. In der mit physiologischer Kochsalzlösung behandelten gestressten Gruppe war die c-fos-ähnliche Immunreaktivität verglichen mit den nicht-gestressten kochsalzbehandelten Tieren auf der ipsilateralen Seite unterdrückt (p<0,01), aber auf der contralateralen Seite (p<0,01) erhöht. Bei Morphin (ipsilateral p<0,05; contralateral p<0,001) und bei Ketoamin (ipsilateral p<0,05; contralateral p<0,05) war sie verglichen mit ihren korrespondierenden nicht gestressten Gruppen sowohl auf der ipsilateralen als auch auf der contralateralen Seite erhöht. Aus den Experimenten folgt, dass Stress zu diskrepanten Ergebnissen zwischen verhaltensbedingten Manifestationen von Schmerz und c-fos-Induktion im Rückenmark führen kann.
Introduction
The discovery of the c-fos proto-oncogene [1] led to extensive studies on pain-induced Fos-like immunoreactivity (FLI) expression in the central nervous system including the spinal cord [2], [3], [4], trigeminal brain stem neurones [5], supraoptic nucleus of the hypothalamus [6] and thalamus [7]. C-fos has been designated the marker for noxious stimulation and implicated in the development of central sensitization mediated by the N-Methyl-D-Aspartate (NMDA) receptor [8]. Endogenous opioids, on the other hand, are released under stressful conditions and produce analgesia by inhibiting the excitability of sensory nerves and/or the release of excitatory neurotransmitters that leads to modulation of pain and induction of analgesia [9]. These form the basis for utilizing morphine, an opiate, and/or ketamine, an NMDA receptor antagonist, in preemptive analgesia [10], [11].
C-fos has been utilized as a marker to evaluate the efficacy of analgesic and anaesthetic drugs in preventing the “memory of pain” [12], [13]. However, the effects of ketamine on c-fos induction [14], [15] and its usage as preemptive analgesic agent produced conflicting results [16], [17], [18], [19]. Some reported beneficial effects [20] while others did not obtain supportive results [21]. While c-fos expression is clearly the result of noxious stimulation, the question arises as to whether its induction can be modified by other factors such as stress accompanying the pain stimulus.
Various stressors such as footshock [22], swimming [23] and restraint [24] are capable of producing analgesia in laboratory animals. This phenomenon is called stress-induced analgesia [25] that can be opioid and/or non-opioid mediated [26]. Studies have demonstrated that c-fos expression was induced by stress in certain areas of the brain [27] and in peripheral organs [28]. However, very few studies have looked at stress induced expression of c-fos in the spinal cord and how pain modulates its expression. The spinal cord plays a major role in the integration and modulation of nociceptive inputs prior to messages being sent to higher centres [29]. Harris et al. [30] demonstrated that rats preexposed to conditioned stimuli paired with an aversive event exhibited reduced pain behaviour in the formalin test that corresponded with reduction in Fos expression in the spinal cord. These conditioned hypoalgesic responses are mediated by descending pathways from the midbrain and brainstem that disrupt nociceptive processing in the spinal cord.
The aim of the present study was to determine the effects of acute swim stress on Fos-like immunoreactivity in the rat due to formalin injection and how ketamine and morphine modulate its expression.
Materials and methods
Animals
Experiments were performed on adult male Sprague-Dawley rats weighing between 230–350 g. The rats were maintained in a 12-h light dark cycle and allowed free access to food and water. They were housed in individual cages and allowed adaptation for at least four days in the physiology department laboratory. Each animal was used only once. Experiments were performed between 08:00 and 16:00 in the physiology department laboratory. Animals were obtained from Animal House, Health Campus, Universiti Sains Malaysia. This study was approved by the Animal Ethics Committee of Universiti Sains Malaysia.
Drugs used
All drugs and saline controls were administered as pretreatment i.e. before the swim stress and formalin injection procedures. Saline 0.9% was used as vehicle to dissolve the drugs. The drugs used were ketamine 5 mg/kg, morphine 10 mg/kg, and saline 0.9% as control. All drugs and saline were injected intraperitoneally at a volume of 1 ml/kg. As the ketamine dose used was low, the rats experienced minimal duration (less than five minutes) of loss of righting reflex and had recovered fully before undergoing swim stress. The morphine dose used was shown to produce analgesia in the rat formalin test [31].
Experimental groups
Rats were allocated to one of six experimental groups with eight animals in each group. The non-stressed groups were pretreated with ketamine, morphine, or saline, and formalin injection was administered 30 minutes after. The stressed groups received similar pretreatment as the non-stressed groups. However, 17 minutes after pretreatment, they were subjected to three minutes of swim stress in 20ºC water followed by formalin injection 10 minutes after cessation of swimming. Ten minutes was chosen as it corresponded to the time of peak antinociception following swim stress [32].
Acute swim stress procedure
A container measuring 92 cm x 46 cm x 46 cm high containing 20 cm of water at 20°C was used for this purpose. Rats were placed in the water individually and left to swim for three minutes before being removed [32], [33].
Formalin injection
Ten minutes after cessation of acute swim-stress, the rats were manually restrained by a second experimenter and 50 μl of dilute (1%) formalin [12] was injected to the plantar aspect of its right hindpaw. The rat was then placed in a perspex testing chamber measuring 26 cm x 20 cm x 20 cm. A mirror was placed below the floor of the chamber at 45° angle to allow an unobstructed view of the rat’s paws [31], [33], [34]. The amount of time spent in each of four behavioural categories, 0–3, was recorded with a videocam [35] starting from the time of injection until the end of one hour. The tape was later viewed by two observers blinded to the treatment of each rat and the formalin test score was tabulated every minute and averaged at 5-minute intervals [12]. The quantification was based on the total time spent in 4 behavioural categories [31]. The categories were:
0 = the injected paw is not favoured (i.e. foot flat on the floor with toes splayed) indicating insignificant or no pain felt
1 = the injected paw has little or no weight on it with no toe splaying indicating mild pain felt
2 = the injected paw is elevated and the heel is not in contact with any surface indicating moderate pain
3 = the injected paw is licked, bitten or shaken indicating severe pain
All rats were used only once and sacrificed after experiment.
Sacrifice of animals and perfusion-fixation of spinal cord
Five rats from each group were selected for immunohistochemistry to detect Fos-like immunoreactivity (FLI). All rats were sacrificed 120 minutes after formalin injection to ensure that the pattern of Fos expression was contributed by the entire second phase of the formalin test [3].
Rats were anaesthetized deeply with an overdose of sodium pentobarbitone intraperitoneally [36]. Following the loss of pinch reflex, thoracotomy was done to expose the heart and an 18G branula was inserted into the apex of the left ventricle. An outlet for the perfusion solution to flow out was created by making a snip over the right atrium. Perfusion was then performed by gravity method using first phosphate-buffered saline (PBS) [37] until clear fluid was flushed out (approximately 120 ml). This was followed by 500 ml of cold 4% paraformaldehyde in phosphate buffer (PB) 0.1 mol litre-1 (pH=7.4) [34].
Dissection of spinal cord and cryostat sectioning
Rats were dissected by posterior approach and vertebrae were exposed. The sciatic nerve was identified and traced from the popliteal fossa to its origins at L4 and L5 segments of the spinal cord [38]. Laminectomy [37] was done to expose the spinal cord and the L4 and L5 segments were removed. Extracted spinal cords were labeled with a 1-mm lateral, longitudinal incision contralateral to the side of formalin injection [37]. The cut segments were then post-fixed in fresh perfusion solution (4% PFA in PB 0.1M) at 4°C for 4 hours, and cryoprotected overnight in 20% sucrose in phosphate buffer 0.1M at 4°C until the cord sank [39]. The segments were next embedded in tissue freezing medium (Jung) and sliced into 30 µm sections using a cryostat [37]. Sections were transferred using a paintbrush to a 24-well multiwell plate containing 500 µl of PBS in each well.
Immunohistochemistry
Sections were stained with a three-step peroxidase avidin-biotin complex (ABC) method (purified primary antibody, biotinylated secondary antibody, and ABC with DAB (diaminobenzidine)) for immunohistochemical localization of Fos protein [40]. After rinsing twice with Tris-buffered saline (TBS) at 5 minutes each, the sections were incubated with primary rabbit polyclonal anti-Fos antiserum directed against residues 4–17 of the N-terminal region of Fos peptide (Ab-5, Oncogene) diluted 1:20,000 with TBS containing 2% normal goat serum (NGS) and 0.2% Triton X-100 for 48 hours at 4°C with constant gentle agitation [12]. After rinsing with three changes of Tris-Triton [37] at 10 minutes each, the sections were incubated with biotinylated secondary antibody (goat anti-rabbit antiserum; Vectastain, Vector Laboratories) diluted 1:200 in TBS containing 2% normal goat serum (NGS) and 0.2% Triton X-100 for 1 hour at room temperature. Sections were then rinsed with three changes of Tris-Triton at 10 minutes each, and then reacted with an avidin-biotin complex (Elite ABC, Vectastain) diluted 1:50 in TBS for 1 hour at room temperature. They were then rinsed in Tris-Triton at three times 10 minutes and reacted with diaminobenzidine solution (0.5 mg per ml of TBS) for 10 minutes [2]. 0.2% hydrogen peroxide (H2O2) [2] was added to each well and a brown discolouration was observed after 15 minutes. The reaction was stopped by rinsing the sections with three changes of TBS at 10 minutes each. After a quick rinse in deionized water, all sections were then mounted on gelatin-subbed slides and air-dried overnight. Slides were then dehydrated with absolute ethanol for 15 minutes, mounted with Styrolyte Mounting Medium and protected with a coverslip [38].
To examine the specificity of the primary antibody, non-specific staining was tested by omitting one of each step simultaneously for the non-stressed saline control rats. Incubation without anti-c-fos antibody, secondary antibody, ABC and DAB was performed on different sections and dark staining of the nucleus was completely absent [41]. This approach ensured high quality of specificity for the antibody in use.
Counting of FLI-labeled neurons
Sections were examined using light microscopy attached to an image analyzer (Leica QWin) and the segmental level was determined according to the grey matter landmarks as described by Molander et al. [42]. Immunohistochemically detected nuclear-associated reaction product was referred to as Fos-like immunoreactivity (FLI) [43]. At least 8–12 of the L4/L5 sections were scanned for each rat, and six sections [36] with the greatest number of Fos positive cells, characterized by dark brown staining distinguishable from background [12], were selected. Images of the spinal cord sections were captured at X25 to determine the lamina distribution and at X50 to localize Fos positive cells. Saved images were then printed out in colour on glossy photo paper. The grey matter of each section was divided further into 4 regions according to Molander et al. [42]:
the superficial dorsal horn (laminae I–II)
the nucleus proprius (laminae III–IV)
the neck of the dorsal horn (laminae V–VI)
the ventral gray (laminae VII–X)
Fos positive cells were counted from the printed images with respect to each lamina by a blinded investigator and counterchecked with the image analyzer once to ensure that the correct dots were counted. The counts were then averaged for each rat. Counts that were questionable in either quantity or quality were excluded.
Statistical analysis
Pain behaviour scores by formalin test were analyzed using two-way analysis of variance (ANOVA) with repeated measures with one within subjects factor (time) and two between subjects factors (stress and drug). Two-way ANOVA was used to analyze the effects of phase 1 formalin test (mean score at 5 minutes) and phase 2 (mean of scores from 10 to 60 minutes) with stress (non stress vs. stress) and drug (saline vs. morphine vs. ketamine) as the main factors. Post hoc Tukey test was performed for drug and significance was accepted at p<0.05.
For each spinal cord section analyzed, the different laminae were divided into superficial lamina (laminae I–II), the nucleus proprius (laminae III–IV), the neck of the dorsal horn (laminae V–VI), and the ventral lamina (laminae VII–X). FLI expression was counted according to the lamina. Data were analyzed using two-way ANOVA by SPSS 11.0, with stress (non stress vs. stress) and drug (saline vs. morphine vs. ketamine) as the main factors. One-way ANOVA was used to compare the FLI between the ipsilateral (injected) and contralateral (non-injected) side of the spinal cord for each group and between the non-stressed and stressed groups for each treatment. Post hoc Tukey test was performed for the three drug groups. Data were expressed as mean ± SEM (standard error of mean) and statistical significance was taken when p<0.05.
Results
Formalin test
Analysis using two-way ANOVA with repeated measures revealed significant within subjects (time, time x stress, time x drug, time x stress x drug: all p<0.001) and between subjects effects of stress (F(1,42)=23.82, p<0.001), drug (F(2,42)=50.52, p<0.001) and stress-drug interaction (F(2,42)=7.31, p<0.05). Post hoc Tukey test showed significant difference between both morphine and ketamine groups compared to saline (morphine vs. saline: p<0.001; ketamine vs. saline: p<0.001). There was no significant difference between morphine and ketamine.
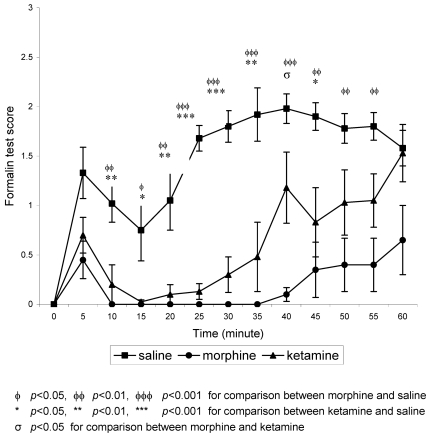
In the non-stressed groups, formalin produced the typical biphasic pain response in the saline group (Figure 1 (Fig. 1)). The first phase includes a burst of activity within 30 seconds of formalin injection. This phase lasted for about 5 minutes and was followed by a 5 to 10 minutes of reduced response i.e. the rats showed very little nociceptive behaviour, and then by a second phase of activity that lasts for at least 60 minutes after the formalin injection. For both morphine and ketamine groups, the biphasic response was markedly attenuated compared to the saline group signifying analgesia. This attenuation is marked at 10 minutes until 35 minutes post-formalin injection, after which the formalin scores for both treatment groups started to increase.
Figure 1. Mean formalin test scores in non-stressed groups against time.
n=8 for all groups. Values are means ± S.E.M.
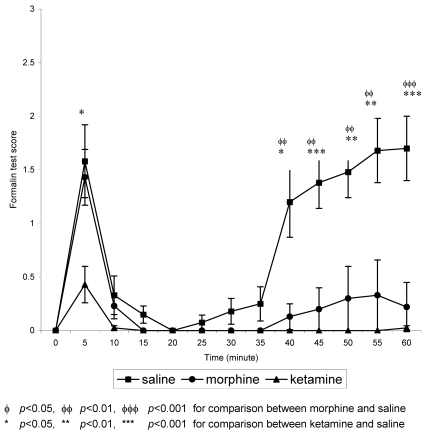
For the stressed groups, morphine and saline groups showed biphasic pattern but the second phase of the formalin test was depressed (Figure 2 (Fig. 2)). While for the ketamine group, the second phase was completely suppressed, obliterating the biphasic pattern. At 5 minutes post-formalin, which is equivalent to phase 1, ketamine demonstrated the lowest score which was significantly (p<0.05) lower than saline. After 5 minutes, however, all three groups showed marked attenuation of the formalin score. For saline this attenuation lasted until 35 minutes post-formalin when the score started to steadily increase again. Morphine showed slight increase in the score after 35 minutes but the scores were still significantly (p<0.01) lower than the saline group. Ketamine, however, demonstrated the greatest analgesia by complete attenuation of the formalin score until 60 minutes post-formalin.
Figure 2. Mean formalin test scores in stressed groups against time.
Values are means ± S.E.M.
Formalin test results during phase 1 and phase 2
Comparing both the non-stressed and the stressed groups during phase 1 (5 minutes post-formalin injection) by two-way ANOVA with stress and drug as main factors, there was significant effects of drug (F(2,42)=7.61, p<0.05) and stress-drug interaction (F(2,42)=3.78, p<0.05) on the formalin score. No significant effect of stress was seen during this phase. Post hoc Tukey test for drug showed significant reduction of formalin score for ketamine compared to saline (p<0.05).
During phase 2, significant effects of stress (F(1,42)=27.37, p<0.001), drug (F(2,42)=57.03, p<0.001) and stress-drug interaction (F(2,42)=5.22, p<0.05) on the formalin score were seen. Post hoc analysis showed significant reduction in the formalin test scores in the two treatment group morphine and ketamine compared to saline. There was no significant difference between morphine and ketamine.
Summary of formalin test results
These results show that in the non-stressed groups, both morphine 10 mg/kg and ketamine 5 mg/kg produced analgesia in the formalin test. All three stressed groups demonstrated stress-induced analgesia during phase 2. This analgesia was enhanced by prior treatment with morphine or ketamine.
FLI
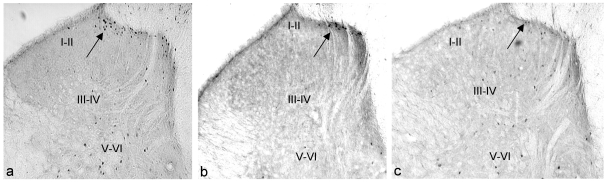
In the non-stressed animals given formalin, the highest density of FLI expression can be seen in the saline group, concentrated mainly on the ipsilateral side. In the morphine and ketamine groups, there were reductions in FLI expression (Figure 3 (Fig. 3)).
Figure 3. Photomicrographs showing FLI in spinal cord sections at level L4/L5 of non-stressed groups in different laminae (ipsilateral side).
(a) saline; (b) morphine; (c) ketamine. I–II: superficial lamina; III–IV: nucleus proprius; V–VI: neck of dorsal horn. Arrow indicates the dark staining Fos positive neurons (FLI) (magnification X 50).
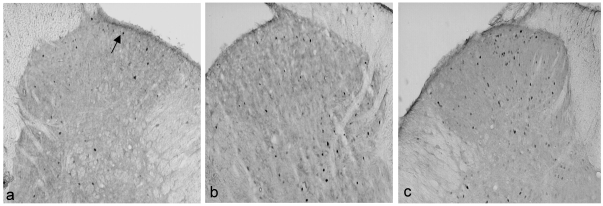
All stressed groups showed FLI expression on both the ipsilateral and contralateral sides. However, the distribution of the FLI in ipsilateral laminae I–II was more scattered compared with the non-stressed groups which concentrated mainly over the medial aspect of the laminae. Among the three groups, ketamine stressed group demonstrated the highest FLI expression on both the ipsilateral and contralateral sides of the spinal cord (Figure 4 (Fig. 4)).
Figure 4. Photomicrographs showing FLI in spinal cord sections at level L4/L5 of stressed groups (ipsilateral side).
(a) saline; (b) morphine; (c) ketamine. Arrow indicates the dark staining Fos positive neurons (FLI) (magnification X 50).
Ipsilateral side
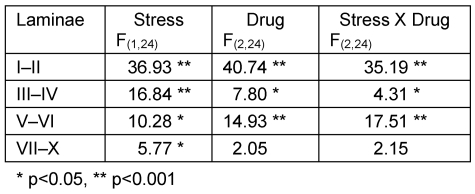
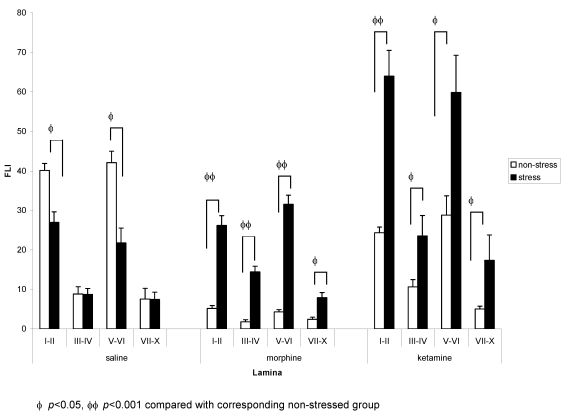
On the ipsilateral side, analysis by two-way ANOVA revealed significant effects of stress in all laminae. There were also significant effects of drug and stress-drug interaction in laminae I–II, III–IV and V–VI (Table 1 (Tab. 1)). One-way ANOVA with post hoc Tukey test for the drug groups showed that in the non stressed groups, both morphine and ketamine had significantly lower density of FLI on the ipsilateral side (morphine: laminae I–II and laminae V–VI, p<0.001; ketamine: laminae I–II, p<0.001 and laminae V–VI, p<0.05) compared to saline. Presence of stress caused a significant reduction in FLI expression in the saline group (laminae I–II and laminae V–VI, p<0.01) compared with saline non-stressed. In morphine and ketamine groups, however, stress caused increases in FLI expression compared with their respective non stressed groups (morphine: laminae I–II, III–IV and V–VI: p<0.001; laminae VII–X: p<0.01 compared with morphine non stressed; ketamine: laminae I–II, p<0.001; laminae III–IV, p<0.05; laminae V–VI, p<0.05 compared with ketamine non stressed). Comparing all stressed groups, ketamine stressed group showed a significantly higher FLI expression compared to the saline stressed group (laminae I–II, p<0.001; laminae III–IV, p<0.05; laminae V–VI, p<0.01) and morphine stressed group (laminae I–II, p<0.001; laminae V–VI, p<0.05) (Figure 5 (Fig. 5)).
Table 1. F values obtained with two-way ANOVA on FLI for stress and non-stress groups and the three drug treatment (ipsilateral side).
Figure 5. Comparison of FLI in (a) saline, (b) morphine, and (c) ketamine stressed and non-stressed groups (ipsilateral side).
For comparison between drug groups, please refer to text. n=5 for both goups. Values are means ± S.E.M.
Contralateral side
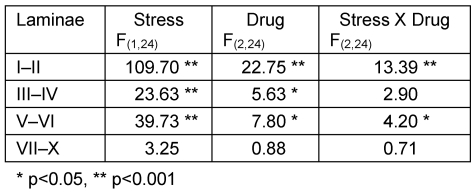
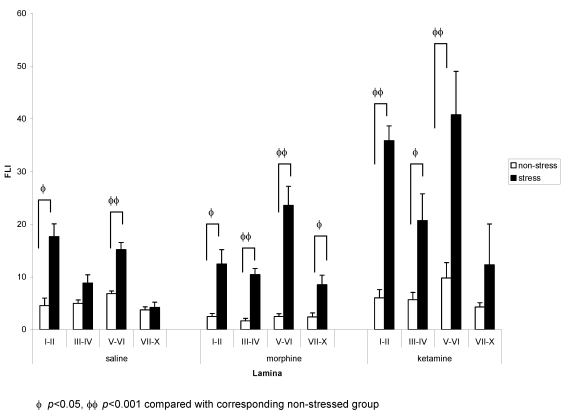
On the contralateral side, analysis by two-way ANOVA revealed significant effects of both stress and drug factors in laminae I–II, III–IV and V–VI, and stress-drug interaction in laminae I–II and V–VI (Table 2 (Tab. 2)). One-way ANOVA with post hoc Tukey test showed that in the non-stressed groups, no significant differences were seen among the three groups. Saline stressed group had a significantly higher FLI expression in laminae I–II (p<0.01) and laminae V–VI (p<0.001) compared to saline non-stressed group. Morphine stressed group demonstrated significantly higher FLI expression in all laminae but is most marked in laminae III–IV and laminae V–VI (p<0.001) compared to morphine non-stressed group. Ketamine stressed group also showed a significantly higher FLI expression in laminae I–II (p<0.001), laminae III–IV (p<0.05) and laminae V–VI (p<0.01) compared to ketamine non-stressed group. Comparing all stressed groups, the FLI expression was significantly higher in the ketamine stressed group compared to saline stressed group (laminae I–II, p<0.01; laminae V–VI, p<0.05) and morphine stressed group (laminae I–II, p<0.001) (Figure 6 (Fig. 6)).
Table 2. F values obtained with two-way ANOVA on FLI for stress and non-stress groups and the three drug treatment (contralateral side).
Figure 6. Comparison of FLI in (a) saline, (b) morphine, and (c) ketamine stressed and non-stressed groups (contralateral side).
For comparison between drug groups, please refer to text. n=5 for both goups. Values are means ± S.E.M.
From these results, it can be concluded that the significant interactions between stress and drug indicate that there is significant effect of stress but it is not the same in each drug. It also indicates that the effects of drugs are significant, but the drug effects are different between those with stress and those without stress.
Discussion
The biphasic response after formalin injection is due to direct chemical stimulation of nociceptors for phase 1 [31], and local inflammatory processes [44] as well as processes in the spinal cord [45] for phase 2. The finding that ketamine suppressed phase 2 but not phase 1 of the formalin test in this study is consistent with previous studies that used even higher doses of ketamine [46]. Lee and Lee [47] demonstrated suppression of both phases but the quantification of pain behaviour was only by counting the incidence of flinching. This study shows that a ketamine dose as low as 5 mg/kg is antinociceptive in the rat formalin test as shown in previous studies [37], [47]. Studies done with other NMDA antagonists such as dextromethorphan and memantin [46] and MK-801 [48] also showed similar pattern of phase 2 suppression. The fact that ketamine produced preemptive analgesia by preventing central sensitization during phase 1 as shown by Gilron et al. (37) is supported by clinical data suggesting preemptive analgesia with ketamine [20], [49] and by electrophysiological study demonstrating inhibition of dorsal horn neuronal firing by ketamine after noxious stimulation [50].
Following systemic administration of ketamine, several mechanisms have been proposed to be involved in producing the analgesia. The first one reflects actions of ketamine on mechanisms within the spinal cord involving central sensitization [51]. Other mechanisms include supraspinal actions, either by inhibiting NMDA receptors at, for example, thalamic sites, or activation of descending pain inhibitory mechanisms involving biogenic amines [52]. Active metabolites such as norketamine also contribute to systemic actions of ketamine [53]. It has also been shown that antagonists of NMDA receptors modulate elevated discharge of spinal nociceptive dorsal horn neurons that manifests as suppression of phase 2 of the formalin test [54]. Benrath et al. [55], in an in vivo experiment, demonstrated that low-dose S(+)-ketamine does not affect C-fibre-evoked potentials alone but blocks long term potentiation induction in pain pathways.
Swim stress, as expected, reduced formalin nociceptive response during the second phase. Previous studies using similar swim stress paradigm also produced similar result [56]. The analgesia produced by this swim stress paradigm has been shown to be mediated by δ-opioid receptor [56]. However another study by Vaccarino et al. [33] showed that subjecting mice to the same swim-stress paradigm produced a non-opioid analgesia in the formalin test. This and another study [23] demonstrated that another NMDA antagonist, MK-801 (dizocilpine maleate), blocked the analgesia produced by swim stress. This is in contrast with this study which showed enhancement of stress-induced analgesia by ketamine. However, Vaccarino et al. [33] only measured formalin-induced nociceptive response during the initial 10 minutes following formalin injection i.e. equivalent to phase 1. Therefore, the NMDA mediation of the swim stress may be involved only during phase 1. However, in this study, ketamine inhibited the first phase after swim stress i.e. producing analgesia instead of blocking it so some other explanation may be likely for this discrepancy [56]. The reason may be due to diminished NMDA-mediated neural transmission by swim stress [57]. This study also demonstrated the additive effects of swim stress and the two treatment groups morphine and ketamine during phase 2. Post hoc analysis did not show significant difference between ketamine and morphine.
Formalin injection induced FLI expression on the ipsilateral side of the spinal cord in the unstressed rats. Intraperitoneal administration of morphine and ketamine significantly reduced FLI expression on the ipsilateral side. These findings are consistent with earlier studies demonstrating that morphine [58], [59] and ketamine [40], [60] reduce FLI expression on the ipsilateral side of the spinal cord. This is consistent with the behavioural analgesia observed in the formalin test.
Following acute swim stress, however, marked difference was observed in the effects of formalin injection on the FLI expression in the three groups. FLI expression was markedly reduced on the ipsilateral side of the formalin injection in the saline stressed group possibly through descending pain inhibitory system [61], and segmental pain modulation in the spinal cord [62], [63]. On the contralateral side, however, FLI expression was increased. This indicates that for FLI to be induced on the contralateral side, both pain stimulus and stress have to be present. Bruijnzeel et al. [64] and de Lange et al. [65] demonstrated how a single stressful experience caused altered brain and brain stem responsivity as evidenced by increased c-fos expression although behavioural sensitization was not expressed. Crane et al. [66] showed that restraint stress induced c-fos expression in the sympathetic preganglionic neurons of the thoracic spinal cord. All these findings suggest alterations in the neural pathways involved in the processing of stress and pain.
For the experimental groups, both morphine and ketamine increased FLI expression on both sides of the spinal cord (laminae I–II and laminae V–VI). This again shows that in the presence of stress, the analgesia seen by behavioural assessment is not mirrored by the FLI expression which is indicative of neuroplastic changes in the pain pathway. While the behavioural component of pain was suppressed, the c-fos proto-oncogene continued to be expressed. Moreover, stress and morphine or ketamine increased the induction of FLI expression, suggesting synergistic action of stress and either of the two drugs on FLI induction. This indicates that in the presence of acute swim stress, both morphine and ketamine fail to prevent spinal sensitization despite inhibition of behavioural manifestation of pain. The discrepancy between behavioural responses and FLI could be contributed by the potential analgesic effects of ketamine unrelated to NMDA receptor antagonism [67], or induction of FLI by ketamine via the σ receptors [43]. This discordance between pain behaviour and spinal FLI was also shown by Gilron et al. [37]. The increase in FLI expression in the swim stress groups could also be due to neuronal activation of both excitatory and inhibitory neurons activated by noxious stimulation and stress-induced modulatory pathways respectively [68]. However, in this study, receptor-labelling was not done to determine the neurons that expressed FLI. Of more concern is whether the neuronal activation can lead to long-term detrimental changes in the central nervous system. This study used only one indicative factor (FLI) and cannot be used to conclude for overall neuronal activity since each visualization parameter such as FosB, FRA, or Jun proteins has its own characteristics of expression [69]. Also, most neuronal activity might not be linked with induction of transcription factors. Alternative approaches to monitor neuronal activity should be employed such as examination of signal transduction intermediates, transcriptional/translational intermediates, and receptor translocation [70].
In clinical situations, the fact that ketamine pretreatment produces conflicting results suggests the presence of confounding effects of stress either in the form of operative, psychological, or other forms of stress. However, further research is required before this parallelism between clinical and animal studies can be made. At this point, it can be suggested that pain assessment based on what is felt by the patient, such as the Visual Analogue Scale (VAS), does not necessarily mirror the plasticity changes taking place in the spinal cord. Similarly, this study shows that although c-fos is often considered as a marker for neuroplasticity changes in the spinal cord, it may not necessarily reflect the only changes in neuroplasticity that occur in relation to pre-emptive analgesia. Stress is a major factor that must be considered in acute pain management in order to prevent c-fos expression and neuroplastic modification of the pain pathway [71]. Findings from the present study suggest that stress together with pain can enhance neuroplasticity changes.
In conclusion, these investigators report significant increase in FLI expression in the spinal cord in association with stress and pretreatment with ketamine/morphine in the formalin pain model. This study suggests that equal emphasis has to be placed on the management of both pain and stress as neuroplasticity may lead to long-term changes in the central nervous system.
Notes
Conflicts of interest
None declared.
Acknowledgement
This study was made possible by the USM short term grant.
References
- 1.Curran T, Teich NM. Identification of a 39,000-dalton protein in cells transformed by the FBJ murine osteosarcoma virus. Virology. 1982;116(1):221–235. doi: 10.1016/0042-6822(82)90415-9. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328(6131):632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 3.Abbadie C, Taylor BK, Peterson MA, Basbaum AI. Differential contribution of the two phases of the formalin test to the pattern of c-fos expression in the rat spinal cord: studies with remifentanil and lidocaine. Pain. 1997;69(1-2):101–110. doi: 10.1016/s0304-3959(96)03285-x. [DOI] [PubMed] [Google Scholar]
- 4.Catheline G, Le Guen S, Besson JM. Effects of opioid receptor antagonists on the effects of i.v. morphine on carrageenin evoked c-Fos expression in the superficial dorsal horn of the rat spinal cord. Brain Res. 1999;824(1):105–111. doi: 10.1016/s0006-8993(99)01207-x. [DOI] [PubMed] [Google Scholar]
- 5.Ebersberger A, Anton F, Tolle TR, Zieglgänsberger W. Morphine, 5-HT2 and 5-HT3 receptor antagonists reduce c-fos expression in the trigeminal nuclear complex following noxious chemical stimulation of the rat nasal mucosa. Brain Res. 1995;676(2):336–342. doi: 10.1016/0006-8993(95)00118-a. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto N, Kawarada K, Kamata K, Suzuki TA. Electrical stimulation of tooth pulp increases the expression of c-fos in the cat supraoptic nucleus but not in the paraventricular nucleus. Life Sci. 1993;53(15):1235–1241. doi: 10.1016/0024-3205(93)90542-b. [DOI] [PubMed] [Google Scholar]
- 7.Kovelowski CJ, Raffa RB, Porreca F. Tramadol and its enantiomers differentially suppress c-fos-like immunoreactivity in rat brain and spinal cord following acute noxious stimulus. Eur J Pain. 1998;2(3):211–219. doi: 10.1016/s1090-3801(98)90017-9. [DOI] [PubMed] [Google Scholar]
- 8.Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45(1):1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- 9.Rittner HL, Stein C. Involvement of cytokines, chemokines and adhesion molecules in opioid analgesia. Eur J Pain. 2005;9(2):109–112. doi: 10.1016/j.ejpain.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Choe H, Choi YS, Kim YH, Ko SH, Choi HG, Han YJ, Song HS. Epidural morphine plus ketamine for upper abdominal surgery: improved analgesia from preincisional versus postincisional administration. Anesth Analg. 1997;84(3):560–563. doi: 10.1097/00000539-199703000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Aida S, Yamakura T, Baba H, Taga K, Fukuda S, Shimoji K. Preemptive analgesia by intravenous low-dose ketamine and epidural morphine in gastrectomy: a randomized double-blind study. Anesthesiology. 2000;92(6):1624–1630. doi: 10.1097/00000542-200006000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Sun WZ, Shyu BC, Shieh JY. Nitrous oxide or halothane, or both, fail to suppress c-fos expression in rat spinal cord dorsal horn neurones after subcutaneous formalin. Br J Anaesth. 1996;76(1):99–105. doi: 10.1093/bja/76.1.99. [DOI] [PubMed] [Google Scholar]
- 13.Zhang SP, Zhang JS, Yung KK, Zhang HQ. Non-opioid-dependent anti-inflammatory effects of low frequency electroacupuncture. Brain Res Bull. 2004;62(4):327–334. doi: 10.1016/j.brainresbull.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Lee IO, Lee IH, Lee KC. Pre-versus post-formalin effects of intrathecal ketamine on spinal Fos-like immunoreactivity in rats. Indian J Med Res. 2004;120(6):527–533. [PubMed] [Google Scholar]
- 15.Koizuka S, Obata H, Sasaki M, Saito S, Goto F. Systemic ketamine inhibits hypersensitivity after surgery via descending inhibitory pathways in rats. Can J Anaesth. 2005;52(5):498–505. doi: 10.1007/BF03016530. [DOI] [PubMed] [Google Scholar]
- 16.Gilron I, Coderre TJ. Novel targets of pain modulation in anaesthesia: preventing painful memories. Can J Anaesth. 1997;44(5 Pt 1):457–462. doi: 10.1007/BF03011930. [DOI] [PubMed] [Google Scholar]
- 17.Graven-Nielsen T, Aspegren Kendall S, Henriksson KG, Bengtsson M, Sörensen J, Johnson A, Gerdle B, Arendt-Nielsen L. Ketamine reduces muscle pain, temporal summation, and referred pain in fibromyalgia patients. Pain. 2000;85(3):483–491. doi: 10.1016/S0304-3959(99)00308-5. [DOI] [PubMed] [Google Scholar]
- 18.Warncke T, Stubhaug A, Jørum E. Preinjury treatment with morphine or ketamine inhibits the development of experimentally induced secondary hyperalgesia in man. Pain. 2000;86(3):293–303. doi: 10.1016/S0304-3959(00)00260-8. [DOI] [PubMed] [Google Scholar]
- 19.Jaksch W, Lang S, Reichhalter R, Raab G, Dann K, Fitzal S. Perioperative small-dose S(+)-ketamine has no incremental beneficial effects on postoperative pain when standard-practice opioid infusions are used. Anesth Analg. 2002;94(4):981–986. doi: 10.1097/00000539-200204000-00038. [DOI] [PubMed] [Google Scholar]
- 20.Fu ES, Miguel R, Scharf JE. Preemptive ketamine decreases postoperative narcotic requirements in patients undergoing abdominal surgery. Anesth Analg. 1997;84(5):1086–1090. doi: 10.1097/00000539-199705000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Kucuk N, Kizilkaya M, Tokdemir M. Preoperative epidural ketamine does not have a postoperative opioid sparing effect. Anesth Analg. 1998;87(1):103–106. doi: 10.1097/00000539-199807000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Zou CJ, Liu JD, Zhou YC. Roles of central interleukin-1 on stress-induced-hypertension and footshock-induced-analgesia in rats. Neurosci Lett. 2001;311(1):41–44. doi: 10.1016/s0304-3940(01)02140-1. [DOI] [PubMed] [Google Scholar]
- 23.Vendruscolo LF, Pamplona FA, Takahashi RN. Strain and sex differences in the expression of nociceptive behavior and stress-induced analgesia in rats. Brain Res. 2004;1030(2):277–283. doi: 10.1016/j.brainres.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Costa A, Smeraldi A, Tassorelli C, Greco R, Nappi G. Effects of acute and chronic restraint stress on nitroglycerin-induced hyperalgesia in rats. Neurosci Lett. 2005;383(1-2):7–11. doi: 10.1016/j.neulet.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Amit Z, Galina ZH. Stress-induced analgesia: adaptive pain suppression. Physiol Rev. 1986;66(4):1091–1120. doi: 10.1152/physrev.1986.66.4.1091. [DOI] [PubMed] [Google Scholar]
- 26.Grau JW. The central representation of an aversive event maintains the opioid and nonopioid forms of analgesia. Behav Neurosci. 1987;101(2):272–288. doi: 10.1037//0735-7044.101.2.272. [DOI] [PubMed] [Google Scholar]
- 27.Herbert J. Fortnighly review. Stress, the brain, and mental illness. BMJ. 1997;315(7107):530–535. doi: 10.1136/bmj.315.7107.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueyama T, Saika M, Koreeda C, Senba E. Water immersion-restraint stress induces expression of immediate-early genes in gastrointestinal tract of rats. Am J Physiol. 1998;275(2 Pt 1):G287–G295. doi: 10.1152/ajpgi.1998.275.2.G287. [DOI] [PubMed] [Google Scholar]
- 29.Dickenson AH, Chapman V, Green GM. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen Pharmacol. 1997;28(5):633–638. doi: 10.1016/s0306-3623(96)00359-x. [DOI] [PubMed] [Google Scholar]
- 30.Harris JA, Westbrook RF, Duffield TQ, Bentivoglio M. Fos expression in the spinal cord is suppressed in rats displaying conditioned hypoalgesia. Behav Neurosci. 1995;109(2):320–328. doi: 10.1037//0735-7044.109.2.320. [DOI] [PubMed] [Google Scholar]
- 31.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4(2):161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 32.Vanderah TW, Wild KD, Takemori AE, Sultana M, Portoghese PS, Bowen WD, Mosberg HI, Porreca F. Mediation of swim-stress antinociception by the opioid delta 2 receptor in the mouse. J Pharmacol Exp Ther. 1992;262(1):190–197. [PubMed] [Google Scholar]
- 33.Vaccarino AL, Marek P, Liebeskind JC. Stress-induced analgesia prevents the development of the tonic, late phase of pain produced by subcutaneous formalin. Brain Res. 1992;572(1-2):250–252. doi: 10.1016/0006-8993(92)90478-r. [DOI] [PubMed] [Google Scholar]
- 34.Gogas KR, Cho HJ, Botchkina GI, Levine JD, Basbaum AI. Inhibition of noxious stimulus-evoked pain behaviors and neuronal fos-like immunoreactivity in the spinal cord of the rat by supraspinal morphine. Pain. 1996;65(1):9–15. doi: 10.1016/0304-3959(95)00141-7. [DOI] [PubMed] [Google Scholar]
- 35.Sawamura S, Fujinaga M, Kingery WS, Belanger N, Davies MF, Maze M. Opioidergic and adrenergic modulation of formalin-evoked spinal c-fos mRNA expression and nocifensive behavior in the rat. Eur J Pharmacol. 1999;379(2-3):141–149. doi: 10.1016/s0014-2999(99)00463-x. [DOI] [PubMed] [Google Scholar]
- 36.Hao S, Takahata O, Mamiya K, Iwasaki H. Sevoflurane suppresses noxious stimulus-evoked expression of Fos-like immunoreactivity in the rat spinal cord via activation of endogenous opioid systems. Life Sci. 2002;71(5):571–580. doi: 10.1016/s0024-3205(02)01704-6. [DOI] [PubMed] [Google Scholar]
- 37.Gilron I, Quirion R, Coderre TJ. Pre- versus postformalin effects of ketamine or large-dose alfentanil in the rat: discordance between pain behavior and spinal Fos-like immunoreactivity. Anesth Analg. 1999;89(1):128–135. doi: 10.1097/00000539-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 38.Jennings E, Fitzgerald M. C-fos can be induced in the neonatal rat spinal cord by both noxious and innocuous peripheral stimulation. Pain. 1996;68(2-3):301–306. doi: 10.1016/s0304-3959(96)03194-6. [DOI] [PubMed] [Google Scholar]
- 39.Ji RR, Rupp F. Phosphorylation of transcription factor CREB in rat spinal cord after formalin-induced hyperalgesia: relationship to c-fos induction. J Neurosci. 1997;17(5):1776–1785. doi: 10.1523/JNEUROSCI.17-05-01776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang W, Simpson RK., Jr Ketamine suppresses c-fos expression in dorsal horn neurons after acute constrictive sciatic nerve injury in the rat. Neurosci Lett. 1999;269(3):165–168. doi: 10.1016/s0304-3940(99)00344-4. [DOI] [PubMed] [Google Scholar]
- 41.Bontempi B, Sharp FR. Systemic morphine-induced Fos protein in the rat striatum and nucleus accumbens is regulated by mu opioid receptors in the substantia nigra and ventral tegmental area. J Neurosci. 1997;17(21):8596–8612. doi: 10.1523/JNEUROSCI.17-21-08596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol. 1984;230(1):133–141. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- 43.Nakao S, Miyamoto E, Masuzawa M, Kambara T, Shingu K. Ketamine-induced c-Fos expression in the mouse posterior cingulate and retrosplenial cortices is mediated not only via NMDA receptors but also via sigma receptors. Brain Res. 2002;926(1-2):191–196. doi: 10.1016/s0006-8993(01)03338-8. [DOI] [PubMed] [Google Scholar]
- 44.Shibata M, Ohkubo T, Takahashi H, Inoki R. Modified formalin test: characteristic biphasic pain response. Pain. 1989;38:347–352. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]
- 45.Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 46.Sawynok J, Reid A. Modulation of formalin-induced behaviors and edema by local and systemic administration of dextromethorphan, memantine and ketamine. Eur J Pharmacol. 2002;450(2):153–162. doi: 10.1016/s0014-2999(02)02119-2. [DOI] [PubMed] [Google Scholar]
- 47.Lee IO, Lee IH. Systemic, but not Intrathecal, Ketamine Produces Preemptive Analgesia in the Rat Formalin Model. Acta Anaesthesiol Sin. 2001;39(3):123–127. [PubMed] [Google Scholar]
- 48.Yamamoto T, Yaksh TL. Comparison of the antinociceptive effects of pre- and post-treatment with intrathecal morphine and MK801, an NMDA antagonist, on the formalin test in the rat. Anesthesiology. 1992;77(4):757–763. doi: 10.1097/00000542-199210000-00021. [DOI] [PubMed] [Google Scholar]
- 49.Annetta MG, Iemma D, Garisto C, Tafani C, Proietti R. Ketamine: new indications for an old drug. Curr Drug Targets. 2005;6(7):789–794. doi: 10.2174/138945005774574533. [DOI] [PubMed] [Google Scholar]
- 50.Hao JX, Sjölund BH, Wiesenfeld-Hallin Z. Electrophysiological evidence for an antinociceptive effect of ketamine in the rat spinal cord. Acta Anaesthesiol Scand. 1998;42(4):435–441. doi: 10.1111/j.1399-6576.1998.tb05138.x. [DOI] [PubMed] [Google Scholar]
- 51.Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280:829–838. [PubMed] [Google Scholar]
- 52.Kawamata T, Omote K, Sonoda H, Kawamata M, Namiki A. Analgesic mechanisms of ketamine in the presence and absence of peripheral inflammation. Anesthesiology. 2000;93(2):520–528. doi: 10.1097/00000542-200008000-00032. [DOI] [PubMed] [Google Scholar]
- 53.Shimoyama M, Shimoyama N, Gorman AL, Elliott KJ, Inturrisi CE. Oral ketamine is antinociceptive in the rat formalin test: role of the metabolite, norketamine. Pain. 1999;81(1-2):85–93. doi: 10.1016/s0304-3959(98)00269-3. [DOI] [PubMed] [Google Scholar]
- 54.Sevostianova N, Danysz W, Bespalov AY. Analgesic effects of morphine and loperamide in the rat formalin test: Interactions with NMDA receptor antagonists. Eur J Pharmacol. 2005;525(1-3):83–90. doi: 10.1016/j.ejphar.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Benrath J, Brechtel C, Stark J, Sandkühler J. Low dose of S+-ketamine prevents long-term potentiation in pain pathways under strong opioid analgesia in the rat spinal cord in vivo. Br J Anaesth. 2005;95(4):518–523. doi: 10.1093/bja/aei215. [DOI] [PubMed] [Google Scholar]
- 56.Kamei J, Hitosugi H, Misawa M, Nagase H, Kasuya Y. Delta-Opioid receptor-mediated forced swimming stress-induced antinociception in the formalin test. Psychopharmacology (Berl.) 1993;113(1):15–18. doi: 10.1007/BF02244327. [DOI] [PubMed] [Google Scholar]
- 57.Deutsch SI, Mastropaolo J, Riggs RL, Rosse RB. The antiseizure efficacies of MK-801, phencyclidine, ketamine, and memantine are altered selectively by stress. Pharmacol Biochem Behav. 1997;58(3):709–712. doi: 10.1016/s0091-3057(97)90014-9. [DOI] [PubMed] [Google Scholar]
- 58.Tateyama S, Ikeda T, Kosai K, Nakamura T, Kasaba T, Takasaki M, Nishimori T. Endomorphins suppress nociception-induced c-Fos and Zif/268 expression in the rat spinal dorsal horn. Eur J Pharmacol. 2002;451(1):79–87. doi: 10.1016/s0014-2999(02)02196-9. [DOI] [PubMed] [Google Scholar]
- 59.Labuz D, Chocyk A, Wedzony K, Toth G, Przewlocka B. Endomorphin-2, deltorphin II and their analogs suppress formalin-induced nociception and c-Fos expression in the rat spinal cord. Life Sci. 2003;73(4):403–412. doi: 10.1016/s0024-3205(03)00309-6. [DOI] [PubMed] [Google Scholar]
- 60.Abbadie C, Besson JM. C-fos expression in rat lumbar spinal cord following peripheral stimulation in adjuvant-induced arthritic and normal rats. Brain Res. 1993;607(1-2):195–204. doi: 10.1016/0006-8993(93)91507-o. [DOI] [PubMed] [Google Scholar]
- 61.Liu RJ, Qiang M, Qiao JT. Nociceptive c-fos expression in supraspinal areas in avoidance of descending suppression at the spinal relay station. Neuroscience. 1998;85(4):1073–1087. doi: 10.1016/s0306-4522(97)00662-3. [DOI] [PubMed] [Google Scholar]
- 62.Kosek E, Lundberg L. Segmental and plurisegmental modulation of pressure pain thresholds during static muscle contractions in healthy individuals. Eur J Pain. 2003;7(3):251–258. doi: 10.1016/S1090-3801(02)00124-6. [DOI] [PubMed] [Google Scholar]
- 63.Woda A, Blanc O, Voisin DL, Coste J, Molat JL, Luccarini P. Bidirectional modulation of windup by NMDA receptors in the rat spinal trigeminal nucleus. Eur J Neurosci. 2004;19(8):2009–2016. doi: 10.1111/j.0953-816X.2004.03328.x. [DOI] [PubMed] [Google Scholar]
- 64.Bruijnzeel AW, Stam R, Compaan JC, Croiset G, Akkermans LM, Olivier B, Wiegant VM. Long-term sensitization of Fos-responsivity in the rat central nervous system after a single stressful experience. Brain Res. 1999;819(1-2):15–22. doi: 10.1016/s0006-8993(98)01350-x. [DOI] [PubMed] [Google Scholar]
- 65.de Lange RP, Geerse GJ, Dahlhaus M, van Laar TJ, Wiegant VM, Stam R. Altered brain stem responsivity to duodenal pain after a single stressful experience. Neurosci Lett. 2005;381(1-2):144–148. doi: 10.1016/j.neulet.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 66.Crane JW, French KR, Buller KM. Patterns of neuronal activation in the rat brain and spinal cord in response to increasing durations of restraint stress. Stress. 2005;8(3):199–211. doi: 10.1080/10253890500333817. [DOI] [PubMed] [Google Scholar]
- 67.Frenkel C, Urban BW. Molecular actions of racemic ketamine on human CNS sodium channels. Br J Anaesth. 1992;69(3):292–297. doi: 10.1093/bja/69.3.292. [DOI] [PubMed] [Google Scholar]
- 68.Castro AR, Pinto M, Lima D, Tavares I. Imbalance between the expression of NK1 and GABAB receptors in nociceptive spinal neurons during secondary hyperalgesia: a c-Fos study in the monoarthritic rat. Neuroscience. 2005;132(4):905–916. doi: 10.1016/j.neuroscience.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 69.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28(3):370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 70.Hoffman GE, Lyo D. Anatomical markers of activity in neuroendocrine systems: Are we all 'fos-ed out'? J Neuroendocrinol. 2002;14(4):259–268. doi: 10.1046/j.1365-2826.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- 71.De Bruin JT, Schäfer MK, Krohne HW, Dreyer A. Preoperative anxiety, coping, and intraoperative adjustment: Are there mediating effects of stress-induced analgesia? Psychol Health. 2001;16(3):253–271. [Google Scholar]