Abstract
Background: Water-filtered infrared-A (wIRA) irradiation has been shown to enhance penetration of clinically used topically applied substances in humans through investigation of functional effects of penetrated substances like vasoconstriction by cortisone.
Aim of the study: Investigation of the influence of wIRA irradiation on the dermatopharmacokinetics of topically applied substances by use of optical methods, especially to localize penetrating substances, in a prospective randomised controlled study in humans.
Methods: The penetration profiles of the hydrophilic dye fluorescein and the lipophilic dye curcumin in separate standard water-in-oil emulsions were determined on the inner forearm of test persons by tape stripping in combination with spectroscopic measurements. Additionally, the penetration was investigated in vivo by laser scanning microscopy. Transepidermal water loss, hydration of the epidermis, and surface temperature were determined. Three different procedures (modes A, B, C) were used in a randomised order on three separate days of investigation in each of 12 test persons. In mode A, the two dyes were applied on different skin areas without water-filtered infrared-A (wIRA) irradiation. In mode B, the skin surface was irradiated with wIRA over 30 min before application of the two dyes (Hydrosun® radiator type 501, 10 mm water cuvette, orange filter OG590, water-filtered spectrum: 590–1400 nm with dominant amount of wIRA). In mode C, the two dyes were applied and immediately afterwards the skin was irradiated with wIRA over 30 min. In all modes, tape stripping started 30 min after application of the formulations. Main variable of interest was the ratio of the amount of the dye in the deeper (second) 10% of the stratum corneum to the amount of the dye in the upper 10% of the stratum corneum.
Results: The penetration profiles of the hydrophilic fluorescein showed in case of pretreatment or treatment with wIRA (modes B and C) an increased penetration depth compared to the non-irradiated skin (mode A): The ratio of the amount of the dye in the deeper (second) 10% of the stratum corneum to the amount of the dye in the upper 10% of the stratum corneum showed medians and interquartile ranges for mode A of 0.017 (0.007/0.050), for mode B of 0.084 (0.021/0.106), for mode C of 0.104 (0.069/0.192) (difference between modes: p=0.0112, significant; comparison mode A with mode C: p<0.01, significant). In contrast to fluorescein, the lipophilic curcumin showed no differences in the penetration kinetics, in reference to whether the skin was irradiated with wIRA or not. These effects were confirmed by laser scanning microscopy. Water-filtered infrared-A irradiation increased the hydration of the stratum corneum: transepidermal water loss rose from approximately 8.8 g m-2 h-1 before wIRA irradiation to 14.2 g m-2 h-1 after wIRA irradiation and skin hydration rose from 67 to 87 relative units. Skin surface temperature increased from 32.8°C before wIRA to 36.4°C after wIRA irradiation.
Discussion: The better penetration of the hydrophilic dye fluorescein after or during skin irradiation (modes B and C) can be explained by increased hydration of the stratum corneum by irradiation with wIRA.
Conclusions: As most topically applied substances for the treatment of patients are mainly hydrophilic, wIRA can be used to improve the penetration of substances before or after application of substances – in the first case even of thermolabile substances – with a broad clinical relevance as a contact free alternative to an occlusive dressing.
Keywords: water-filtered infrared-A (wIRA), penetration, stratum corneum, skin barrier, penetration enhancer, dermatopharmacokinetics, dye, fluorescein, curcumin, tape stripping method, spectroscopy, laser scanning microscopy, transepidermal water loss (TEWL), hydration of the epidermis, corneometry, skin surface temperature, hydrophilic, lipophilic, occlusive dressing
Abstract
Hintergrund: Für Bestrahlungen mit wassergefiltertem Infrarot A (wIRA) wurde durch die Untersuchung funktioneller Effekte penetrierter Substanzen, wie Vasokonstriktion durch Kortison, am Menschen gezeigt, dass sie die Penetration von klinisch verwendeten topisch aufgebrachten Stoffen verbessern.
Ziele der Studie: Untersuchung des Einflusses von wIRA-Bestrahlung auf die Dermatopharmakokinetik von topisch angewandten Stoffen unter Verwendung von optischen Methoden, insbesondere zum Lokalisieren von penetrierenden Stoffen, in einer prospektiven randomisierten, kontrollierten Studie am Menschen.
Methoden: Die Penetrationsprofile des hydrophilen Farbstoffs Fluoreszein und des lipophilen Farbstoffs Curcumin in separaten Standard-Wasser-in-Öl-Emulsionen wurden auf der Innenseite des Unterarms von Probanden mit der Tape-Stripping-Methode in Verbindung mit spektroskopischen Messungen bestimmt. Außerdem wurde die Penetration in vivo mittels Laser-Scan-Mikroskopie untersucht. Zudem wurden der transepidermale Wasserverlust, die Hydratation der Epidermis und die Oberflächentemperatur gemessen. Drei verschiedene Verfahren (Modi A, B, C) wurden in randomisierter Reihenfolge an drei separaten Untersuchungstagen bei jedem der 12 Testpersonen verwendet. Beim Modus A wurden die zwei Farbstoffe ohne Bestrahlung mit wassergefiltertem Infrarot A (wIRA) auf separate Hautareale aufgetragen. Beim Modus B wurde die Hautoberfläche vor dem Auftragen der zwei Farbstoffe 30 min lang mit wIRA bestrahlt (Hydrosun®-Strahler, Typ 501, 10 mm Wasserküvette, Orangefilter OG590, wassergefiltertes Spektrum: 590–1400 nm mit dominantem Anteil an wIRA). Beim Modus C wurde unmittelbar nach dem Auftragen der Farbstoffe die Haut 30 min lang mit wIRA bestrahlt. Bei allen Modi begann das Tape-Stripping 30 min nach dem Auftragen der Formulierungen. Hauptzielvariable war das Verhältnis der Menge an Farbstoff in den tieferen (zweiten) 10% des Stratum corneum zur Menge an Farbstoff in den oberen 10% des Stratum corneum.
Ergebnisse: Die Penetrationsprofile des hydrophilen Fluoreszeins zeigten bei Vorbehandlung oder Behandlung mit wIRA (Modi B und C) eine gesteigerte Penetrationstiefe im Vergleich zu nicht bestrahlter Haut (Modus A): Das Verhältnis der Menge an Farbstoff in den tieferen (zweiten) 10% des Stratum corneum zur Menge an Farbstoff in den oberen 10% des Stratum corneum zeigte Mediane und Interquartilspannen für Modus A von 0,017 (0,007/0,050), für Modus B von 0,084 (0,021/0,106), für Modus C von 0,104 (0,069/0,192) (Unterschied zwischen den Modi: p=0,0112, signifikant; Vergleich von Modus A mit Modus C: p<0,01, signifikant). Im Gegensatz zum Fluoreszein zeigte das lipophile Curcumin keine Unterschiede in der Penetrationskinetik in Abhängigkeit davon, ob die Haut mit wIRA bestrahlt wurde oder nicht. Diese Effekte wurden durch die Laser-Scan-Mikroskopie-Ergebnisse bestätigt. Bestrahlung mit wassergefiltertem Infrarot A steigerte die Hydratation des Stratum corneum: Der transepidermale Wasserverlust nahm von etwa 8,8 g m-2 h-1 vor wIRA-Bestrahlung auf 14,2 g m-2 h-1 nach wIRA-Bestrahlung zu, und die Hauthydratation stieg von 67 auf 87 relative Einheiten. Die Temperatur an der Hautoberfläche stieg von 32,8°C vor wIRA auf 36,4°C nach wIRA-Bestrahlung.
Diskussion: Die bessere Penetration des hydrophilen Farbstoffs Fluoreszein nach oder während Bestrahlung der Haut (Modi B und C) kann mit der gesteigerten Hydratation des Stratum corneum durch die wIRA-Bestrahlung erklärt werden.
Folgerungen: Da die meisten topisch aufgetragenen Substanzen für die Behandlung von Patienten hauptsächlich hydrophil sind, kann wIRA verwendet werden, um die Penetration von Stoffen vor oder nach dem Auftragen der Stoffe – im ersten Fall sogar von hitzeempfindlichen Stoffen – mit einer breiten klinischen Relevanz als kontaktfreie Alternative zum Okklusivverband zu verbessern.
Introduction
The stratum corneum acts simultaneously as a barrier and as a reservoir for topically applied substances. Topically applied drugs are mainly found in the uppermost 20% of the stratum corneum [1], [2]. The limiting factor for percutaneous absorption of a drug is the reservoir and the penetration into the stratum corneum. Therefore, penetration enhancement is an important task for modern dermatopharmacology. Previous studies, mainly based on the investigation of functional effects of penetrated substances (like anesthesia by tetracaine and vasoconstriction by cortisone), indicated that wIRA irradiation of the skin stimulates the penetration of topically applied substances [3], [4], [5].
Water-filtered infrared-A (wIRA) radiation, compared to conventional infrared radiation with a large amount of infrared B and C, feels more pleasant and allows a multiple energy transfer into tissue without irritating or overheating the skin [6], [7], [8]. wIRA increases tissue temperature [6], [7], [9], [10], tissue oxygen partial pressure [6], [7], [10], and tissue perfusion [6], [7], [9], [11], and hence tissue metabolism. wIRA with appropriate irradiances has been shown not only to be harmless to human skin [6], [7], [12], [13], but to have protective abilities against damage caused by UV radiation [6], [7], [14], [15], [16]. wIRA has been shown to improve wound healing (including reducing pain) in chronic venous leg ulcers [7], [17], [18], [19], [20], [21], [22] and in acute wounds [7], [18], [10], [22], [23]. wIRA has proven to reduce pain in scleroderma [24], [25], [26], in ankylosing spondylitis [27] and in gonarthrosis [9], and wIRA is used in physiotherapy [9], [28], sports medicine [29], [30], pediatrics/neonatology [31], oncology [9], and in the treatment of warts [8] or in combination with photodynamic therapy [8], [32], [33].
Aim of the present study was the investigation of the influence of wIRA on the penetration of hydrophilic and lipophilic substances, mainly based on optical methods to localize penetrating substances. Therefore, skin penetration experiments were performed in 12 healthy test persons. Two different model substances, hydrophilic fluorescein sodium and lipophilic curcumin, were applied on different skin areas of the inner forearm. Penetration behaviour of both substances was determined with and without application of wIRA.
Tape stripping, in combination with spectroscopic measurements for the determination of the test substances and the amount of stratum corneum, was used for the calculation of the penetration depth. This modified tape stripping procedure allows an exact localization of the applied drug in the stratum corneum [34], [35], [36], [37].
In contrast to the combined tape stripping method, in vivo laser scanning microscopy allows the online analysis of the penetration process and the determination of the distribution of the applied drug in the uppermost layers of the stratum corneum [38], [39].
In addition to these two optical methods, changes in the skin physiology during the irradiation were monitored by measurements of transepidermal water loss (TEWL), skin hydration, and temperature.
Methods and design
Test persons
Twelve healthy male volunteers aged 19–30 years were included. They did not present any sign of relevant skin disease and had no history of skin disease or allergy. The study was approved by the local ethics committee. All test persons gave their written informed consent.
Experimental conditions
All tests were performed under the same conditions, i.e. test persons rested quietly for 30 min after entering the test area with constant ambient temperature (20°C) and humidity (50%).
Chemicals and applied formulations
Fluorescein sodium, as example for a hydrophilic chemical, was used in a 0.2% standard water-in-oil formulation. Fluorescein sodium (C20H10Na2O5, molecular weight MW=376.3) is a fluorescent dye with an absorbance maximum at 500 nm. This allows an easy detection with ultraviolet/visible light (UV/VIS) spectroscopy. It can also be used for the online detection with laser scanning microscopy.
Curcumin, as example for a lipophilic chemical, was used in a 2% standard water-in-oil formulation. Curcumin (C21H20O6, MW=368.4) is a fluorescent dye with an absorbance maximum at 421 nm. It can be detected by UV/VIS spectroscopy and laser scanning microscopy in the same way as fluorescein sodium.
Water-filtered infrared-A irradiation (wIRA)
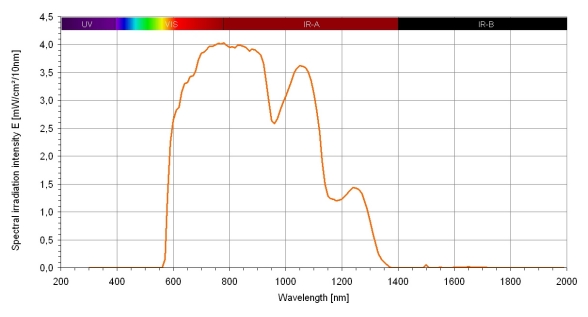
For infrared-A irradiation, a special water-filtered infrared-A radiator was used (Hydrosun® radiator, Hydrosun® Medizintechnik GmbH, Müllheim, Germany, radiator type 501, 10 mm water cuvette, orange filter OG590, water-filtered spectrum: 590–1400 nm, spectrum shown in Figure 1 (Fig. 1), with approximately 210 mW/cm2 total irradiation intensity with approximately 150 mW/cm2 wIRA and approximately 60 mW/cm2 visible orange light VIS, when used at a distance of 25 cm). Infrared-B and -C and the absorption bands within infrared-A are mainly absorbed or decreased by this radiator. Therefore, heat can be built up in deeper parts of the skin and an overheating of the skin surface can be avoided. Within an irradiation area of approximately 100 cm2 the irradiation is homogeneous.
Figure 1. Spectrum of a water-filtered infrared-A radiator (Hydrosun® 501).
Calculated for Hydrosun® 501 with 10 mm water cuvette and orange filter OG590 at 210 mW cm-2 (= 2.1 x 103 W m-2) total irradiation intensity (wIRA+VIS) with approx. 150 mW cm-2 water-filtered infrared-A (wIRA) and approx. 60 mW cm-2 visible orange light (VIS) (in a distance of 25 cm), from Measurement of University of Applied Sciences Munich, dated June 30, 1999
A previous investigation concerning penetration enhancement, comparing wIRA+VIS versus VIS, had shown – in accordance with other controlled clinical trials [8], [10] – wIRA to be the enhancing factor [3]. Therefore, a control radiator (emitting VIS only) was dispensible.
Determination of the skin variables
Transepidermal water loss (TEWL)
As a variable for the barrier function of the skin, transepidermal water loss was used [40], [41]. The evaluations were performed with the Tewameter TM 210® (Courage + Khazaka electronic GmbH, Cologne, Germany) on the skin of the test persons before and immediately after irradiation.
Naturally evaporated water from the skin represents a water vapour pressure in the surrounding air, which can be measured on the surface of the skin. The principles of measurement of the Tewameter® are based on Fick’s Law of Diffusion. The diffusion flow dm/dt shows the amount of evaporated water per time-unit. This diffusion flow is in proportion with the surface A and the thickness gradient dc/dx: In this case, D is the diffusion coefficient of the water vapour (dm/dt = D x A x dc/dx).
The water evaporating from the skin surface passes through the probe of the Tewameter, the resulting density gradient is measured by the pair of sensors to be found in the probe cylinder and, subsequently, evaluated by the micro-processor.
Corneometry
In order to assess the hydration of the epidermis, corneometric measurements were performed with the Corneometer CM 820® (Courage + Khazaka electronic GmbH, Cologne, Germany) before and immediately after irradiation.
The evaluation of the moisture of the skin was undertaken with the so-called Corneometer, whereby a capacity measurement through the stratum corneum is used. This method is based on the existence of highly significant differences in dielectric errors present in water. Varying capacities of the tissue, depending upon the amount of water, can be measured.
Temperature measurements
Before and after irradiation, skin surface temperature was taken with a laser skin surface thermometer (Raynger® MX, Raytek®, Schlender Messtechnik INS, Berlin, Germany) without touching the skin surface.
Tape stripping
Tape stripping in combination with spectroscopic measurements is a well-suited and established method to localize penetrating substances quantitatively and is described in detail by Lademann et al. and Weigmann et al. [34], [35], [36], [37]. The measurement of the pseudo-absorbance of the proteins and the absorbance of the applied dyes removed by the single tape strips allows the exact calculation of the distribution from the surface to the depth [34], [35], [36], [37].
Laser scanning microscopy (LSM)
A dermatological laser scanning confocal microscope “Stratum” (OptiScan Ltd., Melbourne, Australia) was used to determine the emission signal of the fluorescent dyes. Blue laser light radiation of an argon-laser at 488 nm (with a power of 3 mW) made an excitation of fluorescein sodium and curcumin possible. The radiation was transferred into a fiber-optic system. The depth scan was realized by changing the focus plane manually, using the image depth control fixed on a hand piece. A laser at 488 nm has a maximum penetration depth of about 110 µm in human cutaneous tissue. This means, primarily skin surface, stratum corneum and parts of the upper epidermis layers and the distribution of the fluorescent dyes in the uppermost layers of the stratum corneum can be investigated by this method.
Study protocol
Experiments were carried out on 12 healthy test persons once a week for 3 consecutive weeks. Each week, four different skin areas, two on the left inner forearm and two on the right inner forearm, were chosen for the study. One formulation, fluorescein sodium or curcumin, was applied at the same concentration on the two skin areas of the same forearm, the other formulation was applied on the two skin areas of the other forearm. For each test person it was randomised, as to which formulation was used on the left forearm and which on the right forearm and which forearm was investigated first during a day of investigation, keeping these randomisations valid for the whole study period. The irradiation conditions (modes A, B or C) differed in a randomised order between the three days of investigation. In mode A (“no irradiation”), 40 µg/cm2 curcumin was applied on two areas of one forearm and 4 µg/cm2 fluorescein sodium was applied on two areas of the other forearm. After a penetration time of 30 min, tape stripping was performed on one area of each forearm, and laser scanning microscopy was carried out on the other skin area of each forearm. On another day of investigation, the skin areas were irradiated with wIRA from a distance of 25 cm (approximately 210 mW/cm2 total irradiation intensity with approximately 150 mW/cm2 wIRA and approximately 60 mW/cm2 visible orange light VIS) over 30 min before topical application of the dyes, starting one hour before tape stripping and 30 min before application of the dyes (mode B, “irradiation before application”). In mode C (“irradiation after application”), skin areas were irradiated with wIRA from a distance of 25 cm (approximately 210 mW/cm2 total irradiation intensity with approximately 150 mW/cm2 wIRA and approximately 60 mW/cm2 visible orange light VIS) immediately after topical application of the dyes and for the whole penetration time of 30 min. Tape stripping and laser scanning microscopy were accomplished in the same way as in mode A.
Statistics
Already in the planning phase it was decided to use – beside the possibility to present individual penetration profiles exemplarily – only non-parametric statistical methods (both descriptive and confirmatory) in order to avoid all problems of parametric statistics (even descriptive parametric statistics) [8], as variables in clinical medicine often show an unsymmetrical distribution and as this was especially expected concerning the main confirmatory variable of interest. Descriptive analysis should be done with median, percentiles of 25 and 75, minimum, and maximum (box and whiskers graphs). Regarding necessity of alpha error adjustment in cases of multiple testing, especially concerning more than one main variable of interest, confirmatory analysis was focused on the clinically most important variable of interest.
Taking into account, that typically the distribution of substances within the first 20% of the stratum corneum is of great importance [42], “the ratio of the amount of dye in the deeper (second) 10% of the stratum corneum to the amount of dye in the upper 10% of the stratum corneum” was defined as the single main confirmatory variable of interest.
The total alpha error probability was set at 0.05 (5%). Friedman test was used to compare the three irradiation modes A, B and C of the single main confirmatory variable of interest; in case of a significant result, Wilcoxon-Wilcox test was used as post-hoc test (this procedure needs no alpha error adjustment). As the analysis of the main variable of interest was done separately for the two dyes, an alpha error adjustment by Bonferroni was used with alpha* = alpha/2 = 0.025 (2.5%).
SAS® software (SAS®, Statistical Analysis System Institute Inc., North Carolina, USA), BiAS® for Windows software (http://www.bias-online.de) and Graph Pad Prism 4.0 for Windows package (San Diego, USA) were used.
Results
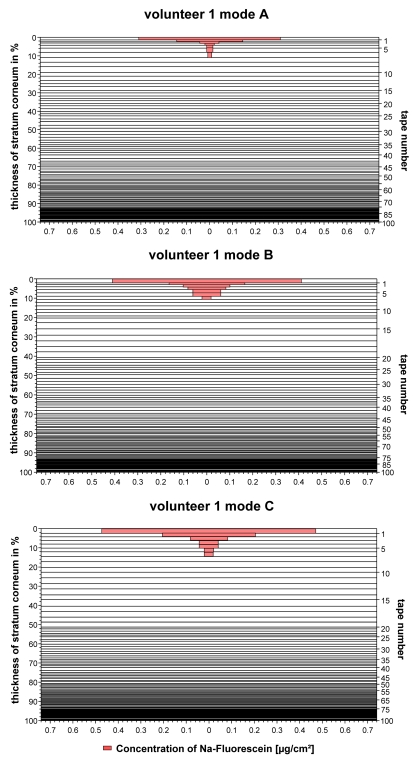
Typical penetration profiles of the hydrophilic fluorescein sodium obtained from the non-treated skin area (mode A), the skin area irradiated with wIRA before the topical application (mode B), and the skin area irradiated after the topical application of a formulation (mode C) are presented in Figure 2 (Fig. 2).
Figure 2. Penetration profiles of the fluorescein containing formulation.
a) obtained from the non-irradiated skin (mode A)
b) obtained from the skin irradiated before the topical application (mode B)
c) obtained from the skin irradiated after the topical application (mode C)
In all cases, the fluorescent dye fluorescein was located in the upper 20% of the stratum corneum where a reservoir was formed. However, in these upper skin layers, fluorescein showed a significantly different distribution, depending upon the used mode. While the dye was located mostly around the first cell layer of corneocytes, if the skin was not irradiated, it penetrated into deeper parts of the stratum corneum in the case of the skin being irradiated. This means, that the penetration is more efficient, if the skin is irradiated before or after the application of fluorescein.
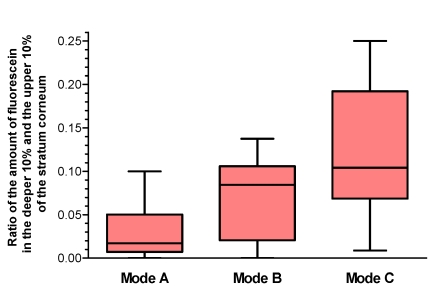
To quantify these differences in the distribution of fluorescein sodium in the stratum corneum, the first 20% of the stratum corneum were investigated and the ratio of the amount of dye in the deeper (second) 10% of the stratum corneum in one square centimeter of the test area to the amount of dye in the upper 10% of the stratum corneum was the main variable of interest. The results are summarized in Figure 3 (Fig. 3). There was a significant difference between the modes (p=0.0112 in the Friedman test) with a significant difference between mode A and mode C in the Wilcoxon-Wilcox test as posthoc test (p<0.01), showing a significant deeper penetration into the stratum corneum in mode C compared to mode A. As a trend mode B showed a deeper penetration compared to mode A.
Figure 3. Distribution of fluorescein in the upper 20% of the stratum corneum Ratio of the amount of fluorescein in the deeper (second) 10% of the stratum corneum to the amount in the upper 10% of the stratum corneum.
Mode A: without wIRA irradiation, Mode B: wIRA irradiation before application of the dye, Mode C: wIRA irradiation after application of the dye (given as minimum, percentiles of 25, median, percentiles of 75, and maximum (box and whiskers graph with the box representing the interquartile range IQR)), n=12
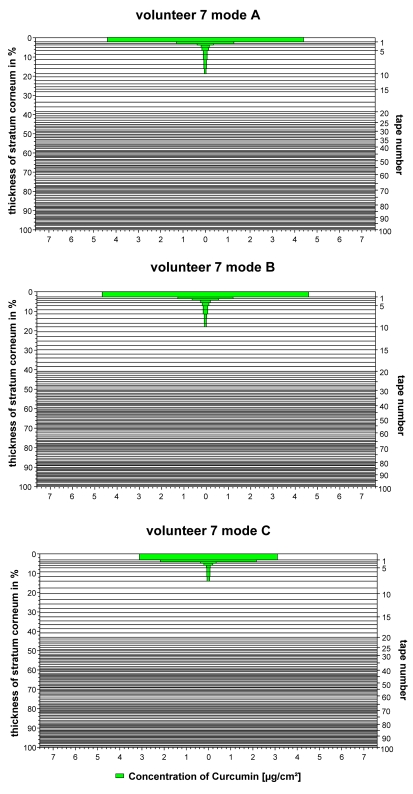
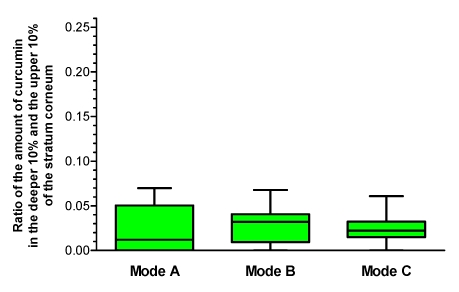
The results obtained for the lipophilic dye curcumin containing formulation vary to a large extent. No differences in the distribution could be found between the irradiated and non-irradiated skin. Typical findings are presented in Figure 4 (Fig. 4).
Figure 4. Penetration profiles of the curcumin containing formulation.
a) obtained from the non-irradiated skin (mode A)
b) obtained from the skin irradiated before the topical application (mode B)
c) obtained from the skin irradiated after the topical application (mode C)
Similar results were obtained for all test persons investigated. The data are presented in Figure 5 (Fig. 5). A comparison of mode A, B and C showed no significant differences (p=0.86 in the Friedman test).
Figure 5. Distribution of curcumin in the upper 20% of the stratum corneum Ratio of the amount of curcumin in the deeper (second) 10% of the stratum corneum to the amount in the upper 10% of the stratum corneum.
Mode A: without wIRA irradiation, Mode B: wIRA irradiation before application of the dye, Mode C: wIRA irradiation after application of the dye (given as minimum, percentiles of 25, median, percentiles of 75, and maximum (box and whiskers graph with the box representing the interquartile range IQR)), n=12
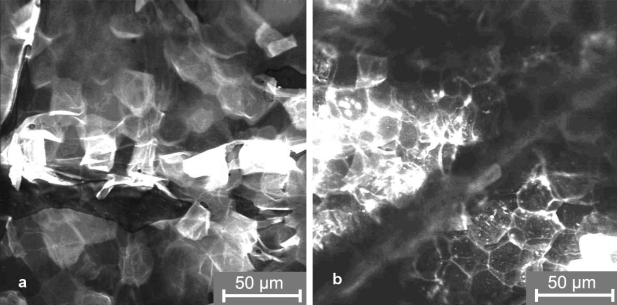
The results obtained by tape stripping in combination with spectroscopic measurements for the determination of the amount of stratum corneum on the removed tape strips were confirmed by laser scanning microscopy (LSM). The fluorescent dyes fluorescein and curcumin could be well detected in the skin in vivo. A typical image of the stratum corneum after application of the fluorescein sodium containing formulation, without wIRA irradiation, is presented in Figure 6a (Fig. 6). The fluorescent dye is located in the lipid layers around the corneocytes. While the dye could be detected only up to a depth of 1 µm in the stratum corneum without irradiation, it was found up to a depth of 4 µm in the case of the stratum corneum being irradiated. This was determined by moving the laser focus deeper into the skin starting from the surface of the stratum corneum (Figure 6b (Fig. 6)).
Figure 6. Laser scanning microscopy images of the stratum corneum after application of the fluorescein containing formulation.
a) without wIRA irradiation (“mode A”)
b) after wIRA irradiation of the skin (“mode C”)
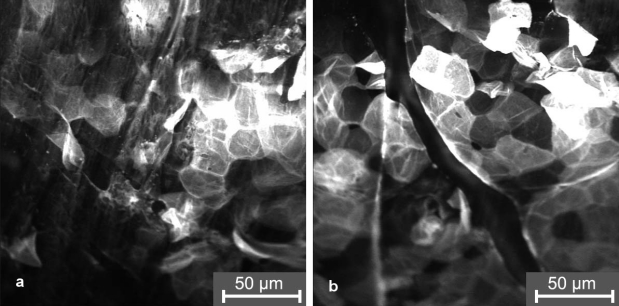
When the curcumin containing formulation was applied, the fluorescent dye could be detected only up to a depth of about 1 µm in the stratum corneum for the non-irradiated and irradiated skin (Figure 7 (Fig. 7)).
Figure 7. Laser scanning microscopy images of the distribution of the fluorescent dye curcumin in the stratum corneum.
a) without wIRA irradiation (“mode A”)
b) with wIRA irradiation of the skin (“mode B”)
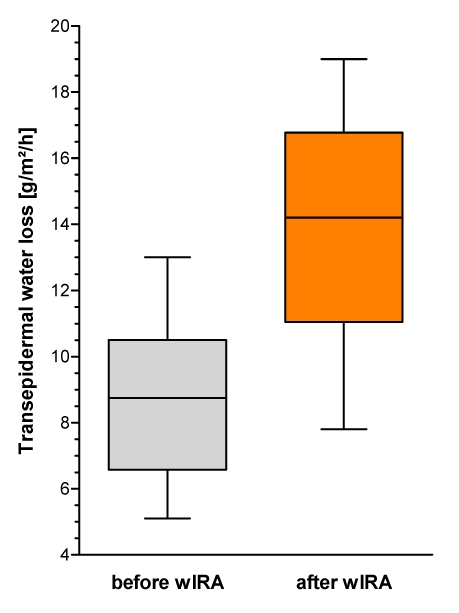
Additionally to the assessment of the penetration depth of the fluorescent dyes and the distribution in the upper skin layers, skin physiology measurements were carried out: transepidermal water loss TEWL as a variable for the barrier function of the skin, skin hydration by corneometry and skin temperature were taken (all TEWL measurements were taken fivefold, all hydration and temperature measurements threefold). The results are given in Figure 8 (Fig. 8), Figure 9 (Fig. 9) and Figure 10 (Fig. 10).
Figure 8. Transepidermal water loss TEWL (given as minimum, percentiles of 25, median, percentiles of 75, and maximum (box and whiskers graph with the box representing the interquartile range IQR)), n=12.
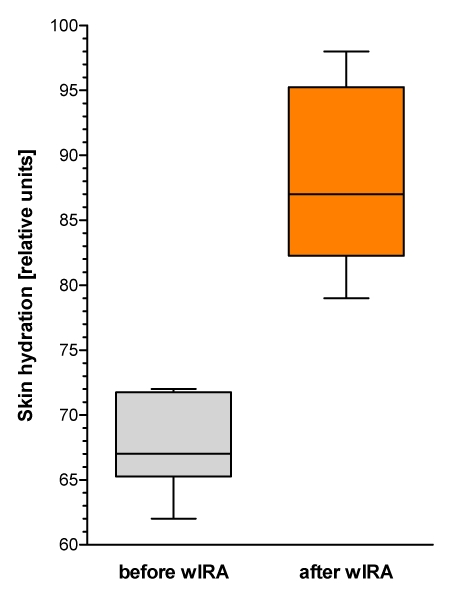
Figure 9. Skin hydration (corneometry) (given as minimum, percentiles of 25, median, percentiles of 75, and maximum (box and whiskers graph with the box representing the interquartile range IQR)), n=12.
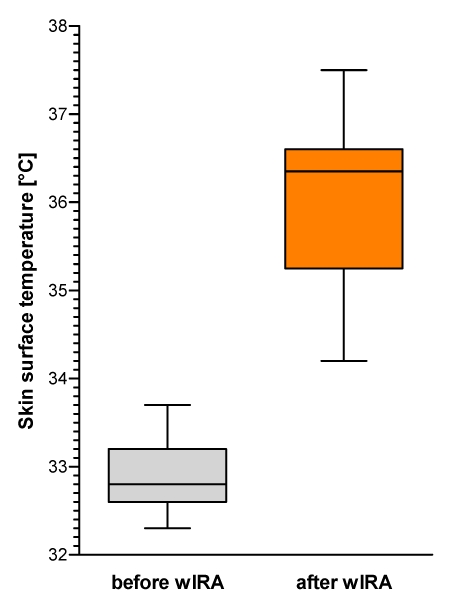
Figure 10. Skin surface temperature (given as minimum, percentiles of 25, median, percentiles of 75, and maximum (box and whiskers graph with the box representing the interquartile range IQR)), n=12.
Transepidermal water loss rose from approximately 8.8 g m-2 h-1 before wIRA irradiation to 14.2 g m-2 h-1 after wIRA irradiation (medians) (Figure 8 (Fig. 8)). Without any exception all test persons showed an increase of transepidermal water loss: median increase was 6.1 g m-2 h-1 (interquartile range: 2.3–7.3 g m-2 h-1, total range: 0.2–8.4 g m-2 h-1).
Skin hydration (in relative (arbitrary) units) rose from 67 before wIRA to 87 after wIRA irradiation (medians) (Figure 9 (Fig. 9)). Without any exception all test persons showed an increase of skin hydration: median increase was 20 (interquartile range: 15–26, total range: 9–34).
Skin surface temperature rose from 32.8°C before wIRA to 36.4°C after wIRA irradiation (medians) (Figure 10 (Fig. 10)). (Highest temperature measured among all test persons after wIRA was 37.5°C.) Without any exception all test persons showed an increase of skin surface temperature: median increase was 2.9°C (interquartile range: 2.8–3.7°C, total range: 1.4–4.7°C).
Discussion
The stratum corneum is an efficient reservoir for topically applied substances. The reservoir is characterized by the specific distribution of the applied formulation inside the stratum corneum. This distribution correlates to the efficacy of the formulation to pass the barrier, thus, reaching the living cells [43]. In the case of the hydrophilic fluorescein being applied onto the skin without irradiation, nearly the whole amount of the dye was detected only around the first cell layers of corneocytes. Small amounts of dye were found on the tape strip with the numbers 4–10, previous studies have shown that these little amounts are basically located in the hair follicles [42]. The penetration into deeper cell layers is not efficient and requires a longer time. However, time is a limiting factor for this superficial reservoir, because the upper layer of the stratum corneum recedes within 24 h on account of desquamation. Furthermore, most of the topically applied formulations recede without having reached the living cells. A biological response cannot be expected in this case. Quite different is the situation when the skin is irradiated. In this case, the hydrophilic dye penetrates much more efficiently into the stratum corneum. The reservoir is extended up to the 5th cell layer of the corneocytes within 30 min. In this case, the penetration is much faster and is not relevantly disturbed by desquamation. In relation to drug delivery, a biological response of the living tissue has to be expected.
In contrast to the hydrophilic dye fluorescein sodium, the lipophilic curcumin shows no differences in the penetration behavior between non-irradiated and irradiated skin. These results are confirmed by in vivo laser scanning microscopy, which demonstrated that the formulations were located in the lipid layers around the corneocytes and in the orifices of the hair follicles.
The better penetration of the hydrophilic dye fluorescein in the case of skin irradiation (modes B and C) can be explained by the increased hydration of the stratum corneum during and after skin irradiation with wIRA. This was demonstrated by the increase of transepidermal water loss (TEWL) and of the hydration status (corneometry values) after irradiation of the skin. This indicates that the environment had been improved for the diffusion of the hydrophilic dye, while the lipophilic curcumin was not influenced by this procedure.
This assumption is confirmed by differences in distribution of fluorescein sodium in the stratum corneum in cases of the skin being irradiated before or after the topical application of the formulation. If the skin was irradiated before the topical application, the hydrophilic dye had improved conditions for penetration in comparison to the non-irradiated skin. As the diffusion inside the lipid layers is a slow process, a pre-irradiation (which already stopped ahead of the application of the dye) could not maintain the improved conditions to an optimal extend until the beginning of the tape stripping procedure. Therefore the penetration process was even slightly better, when the irradiation started after topical application of the dye, maintaining improved conditions to an optimal extend until the beginning of the tape stripping procedure.
The results clearly show that wIRA irradiation acts as a penetration enhancer for the hydrophilic dye fluorescein sodium.
Conclusions
Optical methods such as combined tape stripping and laser scanning microscopy are well suited to analyze the penetration efficacy and pathways of topically applied substances. The penetration efficacy of a hydrophilic substance can be increased by wIRA irradiation before and after topical application of the formulation. In such cases, the skin becomes more hydrated, thus, the penetration of the hydrophilic dye is increased. The loss of the topically applied formulation by desquamation is relevantly reduced. As typical topically applied substances in therapy are mainly hydrophilic, wIRA can be used to improve penetration.
The immediate broad relevance for clinical application (e.g. for the therapeutic application of the patient in dermatology, internal medicine, orthopedics, rheumatology etc.) is based on the fact, that water-filtered infrared-A (wIRA) represents a contact free “alternative to the occlusive dressing” [3] for the improvement of the penetration of topically applied substances and that water-filtered infrared-A – unlike other penetration increasing procedures (which are based e.g. on a mechanical or chemical alteration of the skin) – maintains the integrity of the skin (including structure integrity of the skin, which is of importance to fulfill protection functions). Even though transepidermal water loss (TEWL) and skin hydration were not measured in the presented study as long term effects, the extended clinical observation over months and years shows that skin, which is irradiated daily with wIRA, presents an improved appearance with good skin turgor and no signs of desiccation. As already a pure pre-irradiation with wIRA (without irradiation after application of the substance) improves penetration, wIRA can be used to improve penetration even of thermolabile substances.
Notes
Acknowledgements
We would like to thank the Dr. med. h.c. Erwin Braun foundation, Basel, for technical and financial support.
Conflicts of interest
None declared.
References
- 1.Rougier A, Dupuis D, Lotte C, Roguet R, Schaefer H. In vivo correlation between stratum corneum reservoir function and percutaneous absorption. J Invest Dermatol. 1983;81(3):275–278. doi: 10.1111/1523-1747.ep12518298. DOI: 10.1111/1523-1747.ep12518298. Available from: http://dx.doi.org/10.1111/1523-1747.ep12518298. [DOI] [PubMed] [Google Scholar]
- 2.Hueber F, Wepierre J, Schaefer H. Role of transepidermal and transfollicular routes in percutaneous absorption of hydrocortisone and testosterone: in vivo study in the hairless rat. Skin Pharmacol. 1992;5(2):99–107. doi: 10.1159/000211026. [DOI] [PubMed] [Google Scholar]
- 3.Bankova L, Heinemann C, Fluhr JW, Hoffmann G, Elsner P. Improvement of penetration of a topical corticoid by water filtered infrared A (wIRA). 1st Joint Meeting 14th International Congress for Bioengineering and the Skin & 8th Congress of the International Society for Skin Imaging; 2003 May 21-24; Hamburg. 2003. P100 [Google Scholar]
- 4.Haupenthal H. In vitro- und in vivo-Untersuchungen zur temperatur-gesteuerten Arzneistoff-Liberation und Permeation [Thesis] [In vitro and in vivo investigations of temperature dependent drug liberation and permeation] Mainz: Johannes Gutenberg-Universität;; 1997. [Google Scholar]
- 5.Thillou C. Influence d'une pré-irradiation infrarouge de fibroblastes dermiques humains sur la toxicité et l'inflammation induites par les ultraviolets-B. Université Paris - Val de Marne: Diplôme Européen d'Etudes Supérieures Spécialisées: "Modèles cellulaires in vitro: Application à l'évaluation des xénobiotiques". Monaco: 1999. [Google Scholar]
- 6.Hoffmann G. Principles and working mechanisms of water-filtered infrared-A (wIRA) in relation to wound healing [review] GMS Krankenhaushyg Interdiszip. 2007;2(2):Doc54. Available from: http://www.egms.de/en/journals/dgkh/2007-2/dgkh000087.shtml. [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann G. Wassergefiltertes Infrarot A (wIRA) zur Verbesserung der Wundheilung [Übersichtsarbeit] [Water-filtered infrared A (wIRA) for the improvement of wound healing] [review] GMS Krankenhaushyg Interdiszip. 2006;1(1):Doc20. Available from: http://www.egms.de/en/journals/dgkh/2006-1/dgkh000020.shtml. [Google Scholar]
- 8.Fuchs SM, Fluhr JW, Bankova L, Tittelbach J, Hoffmann G, Elsner P. Photodynamic therapy (PDT) and waterfiltered infrared A (wIRA) in patients with recalcitrant common hand and foot warts. Ger Med Sci. 2004;2:Doc08. Available from: http://www.egms.de/en/gms/2004-2/000018.shtml. [PMC free article] [PubMed] [Google Scholar]
- 9.Vaupel P, Krüger W, editors. Wärmetherapie mit wassergefilterter Infrarot-A-Strahlung [Thermal therapy with water-filtered infrared-A radiation]. Grundlagen und Anwendungsmöglichkeiten [The fundamentals and applications] 2nd ed. Stuttgart: Hippokrates; 1995. [Google Scholar]
- 10.Hartel M, Hoffmann G, Wente MN, Martignoni ME, Büchler MW, Friess H. Randomized clinical trial of the influence of local water-filtered infrared A irradiation on wound healing after abdominal surgery. Br J Surg. 2006;93(8):952–960. doi: 10.1002/bjs.5429. DOI: 10.1002/bjs.5429. Available from: http://dx.doi.org/10.1002/bjs.5429. [DOI] [PubMed] [Google Scholar]
- 11.Mercer JB, de Weerd L. The effect of water-filtered infrared-A (wIRA) irradiation on skin temperature and skin blood flow as evaluated by infrared thermography and scanning laser Doppler imaging. Thermology Int. 2005;15(3):89–94. [Google Scholar]
- 12.Burri N, Gebbers N, Applegate LA. Chronic infrared-A radiation repair: Implications in cellular senescence and extracellular matrix. In: Pandalai SG, editor. Recent Research Developments in Photochemistry & Photobiology. Vol. 7. Trivandrum: Transworld Research Network; 2004. pp. 219–231. [Google Scholar]
- 13.Gebbers N, Hirt-Burri N, Scaletta C, Hoffmann G, Applegate LA. Water-filtered infrared-A radiation (wIRA) is not implicated in cellular degeneration of human skin. GMS Ger Med Sci. 2007;5:Doc08. Available from: http://www.egms.de/en/gms/2007-5/000044.shtml. [PMC free article] [PubMed] [Google Scholar]
- 14.Menezes S, Coulomb B, Lebreton C, Dubertret L. Non-coherent near infrared radiation protects normal human dermal fibroblasts from solar ultraviolet toxicity. J Invest Dermatol. 1998;111(4):629–633. doi: 10.1046/j.1523-1747.1998.00338.x. DOI: 10.1046/j.1523-1747.1998.00338.x. Available from: http://dx.doi.org/10.1046/j.1523-1747.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 15.Applegate LA, Scaletta C, Panizzon R, Frenk E, Hohlfeld P, Schwarzkopf S. Induction of the putative protective protein ferritin by infrared radiation: implications in skin repair. Int J Mol Med. 2000;5(3):247–251. doi: 10.3892/ijmm.5.3.247. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann G, Meffert H. Letter to the Editor: Apparent contradiction between negative effects of UV radiation and positive effects of sun exposure. GMS Ger Med Sci. 2005;3:Doc01. Available from: http://www.egms.de/en/gms/2005-3/000019.shtml. [PMC free article] [PubMed] [Google Scholar]
- 17.von Felbert V, Schumann H, Mercer JB, Strasser W, Daeschlein G, Hoffmann G. Therapy of chronic wounds with water-filtered infrared-A (wIRA) [review] GMS Krankenhaushyg Interdiszip. 2007;2(2):Doc52. Available from: http://www.egms.de/en/journals/dgkh/2008-2/dgkh000085.shtml. [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann G. Wassergefiltertes Infrarot A (wIRA) zur Verbesserung der Wundheilung bei akuten und chronischen Wunden [Water-filtered Infrared-A (wIRA) for the improvement of wound healing of acute and chronic wounds] Wundmanagement. 2008;2:72–80. Available from: http://publikationen.ub.uni-frankfurt.de/volltexte/2008/5429/ [Google Scholar]
- 19.Hoffmann G. Improvement of wound healing in chronic ulcers by hyperbaric oxygenation and by waterfiltered ultrared A induced localized hyperthermia. Adv Exp Med Biol. 1994;345:181–188. doi: 10.1007/978-1-4615-2468-7_24. [DOI] [PubMed] [Google Scholar]
- 20.Biland L, Barras J. Die wassergefilterte Infrarot-A-Hyperthermie zur Behandlung venöser Ulcera [Water-filtered infrared A induced hyperthermia used as therapy of venous ulcers] Hefte Wundbehand. 2001;5:41. [Google Scholar]
- 21.Mercer JB, Nielsen SP, Hoffmann G. Improvement of wound healing by water-filtered infrared-A (wIRA) in patients with chronic venous leg ulcers including evaluation using infrared thermography. GMS Ger Med Sci. 2008;6:Doc11. Available from: http://www.egms.de/en/gms/2008-6/000056.shtml. [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann G. Wassergefiltertes Infrarot A (wIRA) zur Verbesserung der Wundheilung bei akuten und chronischen Wunden. Z Wundheilung - J Wound Healing. 2005;special issue 2:130. [Google Scholar]
- 23.Hartel M, Illing P, Mercer JB, Lademann J, Daeschlein G, Hoffmann G. Therapy of acute wounds with water-filtered infrared-A (wIRA) [review] GMS Krankenhaushyg Interdiszip. 2007;2(2):Doc53. Available from: http://www.egms.de/en/journals/dgkh/2007-2/dgkh000086.shtml. [PMC free article] [PubMed] [Google Scholar]
- 24.Meffert H, Buchholtz I, Brenke A. Milde Infrarot-A-Hyperthermie zur Behandlung der systemischen Sklerodermie [Mild infrared A hyperthermia in treatment of systemic scleroderma] Dermatol Monatsschr. 1990;176(11):683–686. [PubMed] [Google Scholar]
- 25.von Felbert V, Streit M, Weis J, Braathen LR. Anwendungsbeobachtungen mit wassergefilterter Infrarot-A-Strahlung in der Dermatologie [Application observations with water-filtered infrared-A radiation in dermatology] Dermatologica. 2004;7:32–34. [Google Scholar]
- 26.von Felbert V, Simon D, Braathen LR, Megahed M, Hunziker T. Behandlung einer linearen Sklerodermie mit wassergefilterter Infrarot-A-Strahlung [Treatment of linear scleroderma with water-filtered infrared-A irradiation] Hautarzt. 2007;58:923–924. doi: 10.1007/s00105-007-1413-y. DOI: 10.1007/s00105-007-1413-y. Available from: http://dx.doi.org/10.1007/s00105-007-1413-y. [DOI] [PubMed] [Google Scholar]
- 27.Falkenbach A, Dorigoni H, Werny F, Gütl S. Wassergefilterte Infrarot-A-Bestrahlung bei Morbus Bechterew und degenerativen Wirbelsäulenveränderungen: Effekte auf Beweglichkeit und Druckschmerzhaftigkeit [Water-filtered infrared-A irradiation in Morbus Bechterew and degenerative vertebral column diseases: effects on flexibility and feeling of pressure] Österr Z Physikal Med Rehab. 1996;6:96–102. [Google Scholar]
- 28.Hoffmann G, Siegfried I. Volkskrankheit Rückenschmerz: neue Sichtweisen [Common illness backache: new ways of looking at]. Seminar des Arbeitskreises Sportmedizin der Akademie für ärztliche Fortbildung und Weiterbildung der Landesärztekammer Hessen. Bad Nauheim, 05.06.2004. Düsseldorf, Köln: German Medical Science; 2005. Doc 04ruecken1. Available from: http://www.egms.de/en/meetings/ruecken2004/04ruecken1.shtml. [Google Scholar]
- 29.Hoffmann G. Improvement of regeneration by local hyperthermia induced by waterfiltered infrared A (wIRA) Int J Sports Med. 2001;23 Suppl 2:S145. [Google Scholar]
- 30.Möckel F, Hoffmann G, Obermüller R, Drobnik W, Schmitz G. Influence of water-filtered infrared-A (wIRA) on reduction of local fat and body weight by physical exercise. GMS Ger Med Sci. 2006;4:Doc05. Available from: http://www.egms.de/en/gms/2006-4/000034.shtml. [PMC free article] [PubMed] [Google Scholar]
- 31.Singer D, Schröder M, Harms K. Vorteile der wassergefilterten gegenüber herkömmlicher Infrarot-Strahlung in der Neonatologie [Benefits of water-filtered as compared to conventional infrared radiation in neonatology] Z Geburtshilfe Neonatol. 2000;204(3):85–92. doi: 10.1055/s-2000-10202. DOI: 10.1055/s-2000-10202. Available from: http://dx.doi.org/10.1055/s-2000-10202. [DOI] [PubMed] [Google Scholar]
- 32.Foss P. Einsatz eines patentierten, wassergefilterten Infrarot-A-Strahlers (Hydrosun) zur photodynamischen Therapie aktinischer Dyskeratosen der Gesichts- und Kopfhaut. [Application of a patented water-filtered infrared-A radiator (Hydrosun) for photodynamic therapy of actinic keratosis of the skin of the face and the scalp] Z naturheilkundl Onkologie krit Komplementärmed. 2003;6(11):26–28. [Google Scholar]
- 33.Dickreiter B. Phototherapie - Therapeutische Möglichkeiten von Infrarotstrahlung und sichtbarem Licht [Phototherapy - therapeutic possibilities of infrared radiation and visible light] Gesundes Leben. 2002;79(6):52–57. [Google Scholar]
- 34.Lindemann U, Wilken K, Weigmann HJ, Schaefer H, Sterry W, Lademann J. Quantification of the horny layer using tape stripping and microscopic techniques. J Biomed Opt. 2003;8(4):601–607. doi: 10.1117/1.1609200. DOI: 10.1117/1.1609200. Available from: http://dx.doi.org/10.1117/1.1609200. [DOI] [PubMed] [Google Scholar]
- 35.Weigmann HJ, Lademann J, Schanzer S, Lindemann U, von Pelchrzim R, Schaefer H, Sterry W, Shah V. Correlation of the local distribution of topically applied substances inside the stratum corneum determined by tape-stripping to differences in bioavailability. Skin Pharmacol Appl Skin Physiol. 2001;14 Suppl 1:98–102. doi: 10.1159/000056397. DOI: 10.1159/000056397. Available from: http://dx.doi.org/10.1159/000056397. [DOI] [PubMed] [Google Scholar]
- 36.Lindemann U, Weigmann HJ, Schaefer H, Sterry W, Lademann J. Evaluation of the pseudo-absorption method to quantify human stratum corneum removed by tape stripping using protein absorption. Skin Pharmacol Appl Skin Physiol. 2003;16(4):228–236. doi: 10.1159/000070845. DOI: 10.1159/000070845. Available from: http://dx.doi.org/10.1159/000070845. [DOI] [PubMed] [Google Scholar]
- 37.Weigmann HJ, Lindemann U, Antoniou C, Tsikrikas GN, Stratigos AI, Katsambas A, Sterry W, Lademann J. UV/VIS absorbance allows rapid, accurate, and reproducible mass determination of corneocytes removed by tape stripping. Skin Pharmacol Appl Skin Physiol. 2003;16(4):217–227. doi: 10.1159/000070844. DOI: 10.1159/000070844. Available from: http://dx.doi.org/10.1159/000070844. [DOI] [PubMed] [Google Scholar]
- 38.Swindle LD, Thomas SG, Freeman M, Delaney PM. View of normal human skin in vivo as observed using fluorescent fiber-optic confocal microscopic imaging. J Invest Dermatol. 2003;121(4):706–712. doi: 10.1046/j.1523-1747.2003.12477.x. DOI: 10.1046/j.1523-1747.2003.12477.x. Available from: http://dx.doi.org/10.1046/j.1523-1747.2003.12477.x. [DOI] [PubMed] [Google Scholar]
- 39.Alborova A, Lademann J, Meyer L, Kramer A, Patzelt A, Sterry W, Antoniou C. Einsatz der Laser-Scan-Mikroskopie zur Charakterisierung der Wundheilung [Application of laser scanning microscopy for the characterization of wound healing] GMS Krankenhaushyg Interdiszip. 2007;2(2):Doc37. Available from: http://www.egms.de/en/journals/dgkh/2007-2/dgkh000070.shtml. [Google Scholar]
- 40.Scott RC, Oliver GJ, Dugard PH, Singh HJ. A comparison of techniques for the measurement of transepidermal water loss. Arch Dermatol Res. 1982;274(1-2):57–64. doi: 10.1007/BF00510358. DOI: 10.1007/BF00510358. Available from: http://dx.doi.org/10.1007/BF00510358. [DOI] [PubMed] [Google Scholar]
- 41.Lotte C, Rougier A, Wilson DR, Maibach HI. In vivo relationship between transepidermal water loss and percutaneous penetration of some organic compounds in man: effect of anatomic site. Arch Dermatol Res. 1987;279(5):351–356. doi: 10.1007/BF00431230. DOI: 10.1007/BF00431230. Available from: http://dx.doi.org/10.1007/BF00431230. [DOI] [PubMed] [Google Scholar]
- 42.Lademann J, Weigmann H, Rickmeyer C, Barthelmes H, Schaefer H, Mueller G, Sterry W. Penetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orifice. Skin Pharmacol Appl Skin Physiol. 1999;12(5):247–256. doi: 10.1159/000066249. DOI: 10.1159/000066249. Available from: http://dx.doi.org/10.1159/000066249. [DOI] [PubMed] [Google Scholar]
- 43.Weigmann H, Lademann J, von Pelchrzim R, Sterry W, Hagemeister T, Molzahn R, Schaefer M, Lindscheid M, Schaefer H, Shah VP. Bioavailability of clobetasol propionate - quantification of drug concentrations in the stratum corneum by dermatopharmacokinetics using tape stripping. Skin Pharmacol Appl Skin Physiol. 1999;12(1-2):46–53. doi: 10.1159/000029845. DOI: 10.1159/000029845. Available from: http://dx.doi.org/10.1159/000029845. [DOI] [PubMed] [Google Scholar]