Abstract
Aims
The Urinary Incontinence Treatment Network (UITN) was established in 2000 as a multi-disciplinary, multi-institutional network by the National Institute for Diabetes, Digestive, and Kidney Diseases (NIDDK) to investigate treatments for urinary incontinence in women.
Methods
Over 8 years this network composed of urologists, urogynecologists, geriatricians, behavioral psychologists, physical therapists, nurses, epidemiologists, social scientists and statisticians from nine academic sites and a Data Coordinating Center has been effective in designing and completing prospective randomized clinical trials for treatments of urinary incontinence in women.
Results
Two major clinical trials have been completed and a third has completed recruitment. The focus of the completed trials was a comparison of surgical methods to treat stress urinary incontinence whereas the third examined the potential benefit of combined behavioral intervention and antimuscarinic drug therapy to eliminate the need for long-term use of drug therapy alone to manage urge urinary incontinence. The scientific output of the network measured by abstracts, original papers and presentations demonstrates the productivity of the network.
Conclusions
Many unique challenges are posed by a multi-disciplinary team located at sites across the United States undertaking several clinical trials. This review presents some of the logistics, barriers, tactics, and strategies used to create this successful clinical trials network focused on urinary incontinence.
Keywords: clinical trials, LUTS, urinary incontinence, Urinary Incontinence Treatment Network
ORIGIN AND HISTORY OF THE URINARY INCONTINENCE TREATMENT NETWORK
When patients seek counseling for the common and costly complaint of urinary incontinence the clinician is challenged by a paucity of data from prospective randomized trials, especially for surgical interventions. Surgical trials are particularly prone to operator bias because of the implications for patient referrals and the surgeon’s reputation. Unlike the reversible nature of most pharmaceuticals and biologics used to treat medical conditions, a surgical trial can directly influence patient referrals if results are poor. Even the best intentioned surgeon when reporting his/her results may unconsciously exclude data, select only the best surgical candidates, underreport complications, or assume durability based on last observation carried forward analysis for patients not returning for follow-up. A large multi-center trial in which de-identified surgeons and sites can accurately report out-comes without fear of professional repercussions offers the best opportunity to gain insight into surgical efficacy and associated complications. The National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) issued a request for applications (RFA) for sites to study urinary incontinence.
NIDDK is one of the Institutes of the National Institutes of Health (NIH), the lead government agency supporting biomedical research in the United States. The NIDDK has substantial responsibility for fostering research and education in urinary incontinence. Prior to the development of the urinary incontinence initiative, the NIDDK reviewed the literature on urinary incontinence. Studies on outcomes for the surgical treatment of stress urinary incontinence suffer from a number of shortcomings including poorly defined enrollment criteria and outcome measures, low rates and short duration of study participant follow-up, small sample size, and lack of appropriate comparison groups.
The establishment of the UITN was timely with the increasing prominence of large clinical research networks combining to reengineer the U.S. clinical research enterprise.1 Compared to drug studies, randomized surgical trials face unique challenges. For example, when studying complex surgical procedures, it must be determined whether the type of procedure or the surgeon’s skill, specialty training and experience represent significant outcome variables. However, large trials that standardize surgeries and require surgical experience decrease the influence of such variables. The challenges facing the implementation of national clinical trials include: (1) difficulties in recruitment, (2) conflicts of interest, (3) community involvement, (4) diversity of participants, (5) heterogeneity of requirements, (6) delays by Institutional Review Boards (IRB), (7) privacy concerns, (8) inadequate information systems, and (9) lack of trained trialists.2 This review presents some of the logistics, barriers, tactics and strategies used to create our large, multi-disciplinary, multi-site and productive clinical research network.
Selection of Participating Centers
To engage the surgical subspecialties responsible for the evaluation and surgical treatment of women with urinary incontinence, the NIDDK research solicitation required that each site include a urologist and a urogynecologist. In 2000, applications underwent peer review and four sites were selected as Continence Treatment Centers (CTC’s): William Beaumont Hospital in Royal Oak and Oakland Hospital; the University of Texas at San Antonio; and Magee Women’s Hospital at the University of Pittsburgh. Duke University was also selected as a CTC but withdrew for administrative reasons after the first Steering Committee meeting. The New England Research Institutes (Watertown, MA) was selected as the Data and Biostatistical Coordinating Center (DCC). In the subsequent year, the NIDDK released another solicitation, Urinary Incontinence Treatment Network: Continence Treatment Centers (RFA, DK-01-018) to increase the number of CTCs. In the spring of 2001, the following CTCs were added to the UITN: Loyola University; University of Maryland; University of Texas Southwestern Medical School; University of California, San Diego; University of Alabama at Birmingham; and the University of Utah. In 2006, a Limited Competition was used to extend the UITN intact for an additional 4 years to complete an ongoing trial.
Funding
The UITN network was supported by the Cooperative Agreement funding mechanism. Up to $3.0 million per year for the first 5 years was allocated for this effort. Allocation in certain years was linked to site productivity and resource utilization. The lead funding agency is the NIDDK, with additional support provided by the National Institute of Child Health and Human Development (NICHD) and the Office of Research on Women’s Health (ORWH), NIH.
Organizational Structure
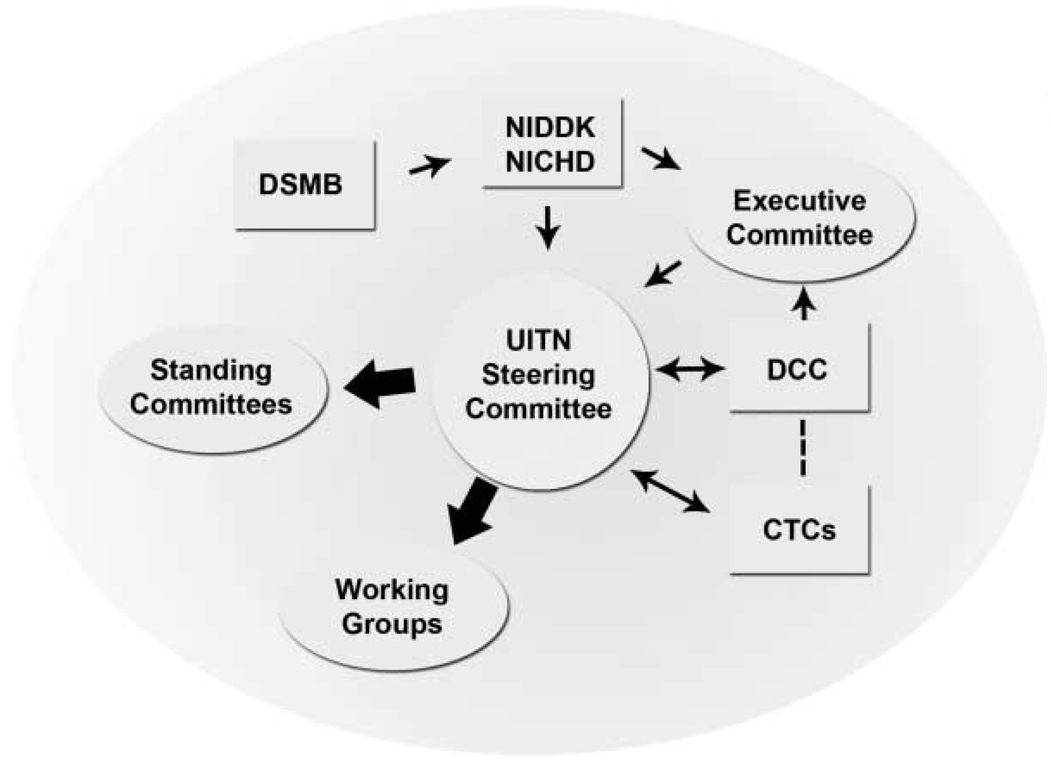
The organizational structure of the UITN is depicted in Figure 1. The UITN Steering Committee (SC) is comprised of at least one urologist and one urogynecologist from each CTC, the Principal Investigator (PI) and co-PI from the DCC as well as the Program Scientists from NIDDK and NICHD. An external Chair of the SC appointed by the NIDDK is a non-voting member of the SC, except when needed to cast tie-breaking votes. The SC is the governing board of the network responsible for developing study protocols, proposing data analysis, recruiting, providing study treatments, and conducting participant follow-up. The members of the SC also serve as chairs of the sub-committees and work groups of the UITN. The SC meets at least monthly, with three in-person meetings per year and interim meetings by conference call. In addition to the lead investigators, participation from other investigators, specialists and research coordinators is encouraged. An Executive Committee, consisting of the SC Chair, the DCC PI, and the NIH Project Scientists, address and manage issues that arise between SC meetings.
Fig. 1.
Organization diagram of UITN.
The one standing committee is the Publications and Presentation (P&P) Committee. This Committee is responsible for establishing standards and guidelines for presenting and publishing data from UITN studies, reviewing manuscripts and presentations, and advising on public information dissemination. The Committee is composed of one investigator from each CTC and the DCC, as well as an NIH representative. The SC elects a P&P Chair and Vice Chair biannually to balance urogynecology and urology representation.
Work groups are established for more efficient finalization and oversight of all major aspects of study protocols. Typical work groups include: Protocol Committee—Eligibility (to resolve questions of study eligibility); Complications (to define protocol-specific complications; to post-hoc review and code study complications); Specialized Study Measures (to oversee quality control issues for complex measures; examples include Urodynamics and a post-operative pain diary, etc.); Study Treatments (to establish the necessary level of standardization for study interventions and develop a corresponding training and certification program); Case Report Forms; Quality Assurance; Informed Consent (to develop an informed consent template that covers all essential items); and a Budget group to estimate the cost of each proposed study.
The NIDDK established an independent Data and Safety Monitoring Board (DSMB) advisory to them that monitors progress and safety of network studies. The membership of this committee is multi-disciplinary with representation from urology, urogynecology, biostatistics and epidemiology.
PROTOCOL DEVELOPMENT
Initial Efforts
At the first meeting of the SC in June 2000, investigators from the four original CTCs and DCC were joined by invited consultants to discuss the mission of the UITN and the design of the first study. At this meeting, participants argued convincingly against the implementation of an observational cohort study, which had the potential to result in biased findings, and recommended to the NIDDK that a prospective randomized clinical trial (RCT) for stress incontinence be conducted instead. Factors that represented challenges to implement SISTR are presented in Table I. Both surgical and non-surgical treatments were considered. The PI from each CTC presented study designs from their NIH grant applications which included comparisons of two versus three surgical procedures with and without inclusion of adjuvant pelvic muscle exercises. It was at this meeting that an RCT of Burch versus a sling procedure was chosen as the first clinical to be implemented by the Network with a primary outcome assessment at a minimum of 2 years.18 The NIDDK and the DSMB concurred with this major change in study design and approved implementation. However, concerns regarding the feasibility of randomization were raised and whether to include patients with pelvic floor prolapse, or other newer procedures using synthetic midurethral and whether the number of sites was sufficient to recruit adequate numbers of subjects (Table I).
TABLE I.
Issues Arising From Randomized Surgical Trial (SISTEr)
| Is surgery as primary treatment ethical without failure of behavioral therapy? |
| Is it possible to randomize surgery patients? |
| Should patients be blinded to surgery? |
| Should surgeons be blinded to urodynamic results? |
| How long should follow up be maintained? |
| What is best way to certify surgeons? |
| How much variability in surgical technique should be allowed? |
| Should significant pelvic floor prolapse and concomitant repairs be allowed? |
| Should fellows or residents perform the procedure with guidance? |
Following expansion to nine CTCs, the first UITN study was the Stress Incontinence Surgical Treatment Efficacy Trial (SISTEr). The primary aim of this study was to compare the efficacy and safety of the autologous fascial pubovaginal sling and Burch colposuspension procedures 24 months post-surgery. Details of the study design18 and results3 have been reported. Participants are followed annually to assess status up to 7 years post-surgery (in 2011).
The second network study was the Behavior Enhances Drug Reduction of Incontinence (BE-DRI) trial. This was a multi-center randomized trial of behavioral training combined with drug therapy versus drug therapy alone for the treatment of urge incontinence. The primary aims were to determine whether adding behavioral training to drug therapy will increase the number of women who can discontinue drug therapy and sustain a significant reduction of incontinence, and to test whether the short-term effectiveness of drug therapy can be enhanced by combining it with behavioral training. The primary outcome manuscript is in final revision.9
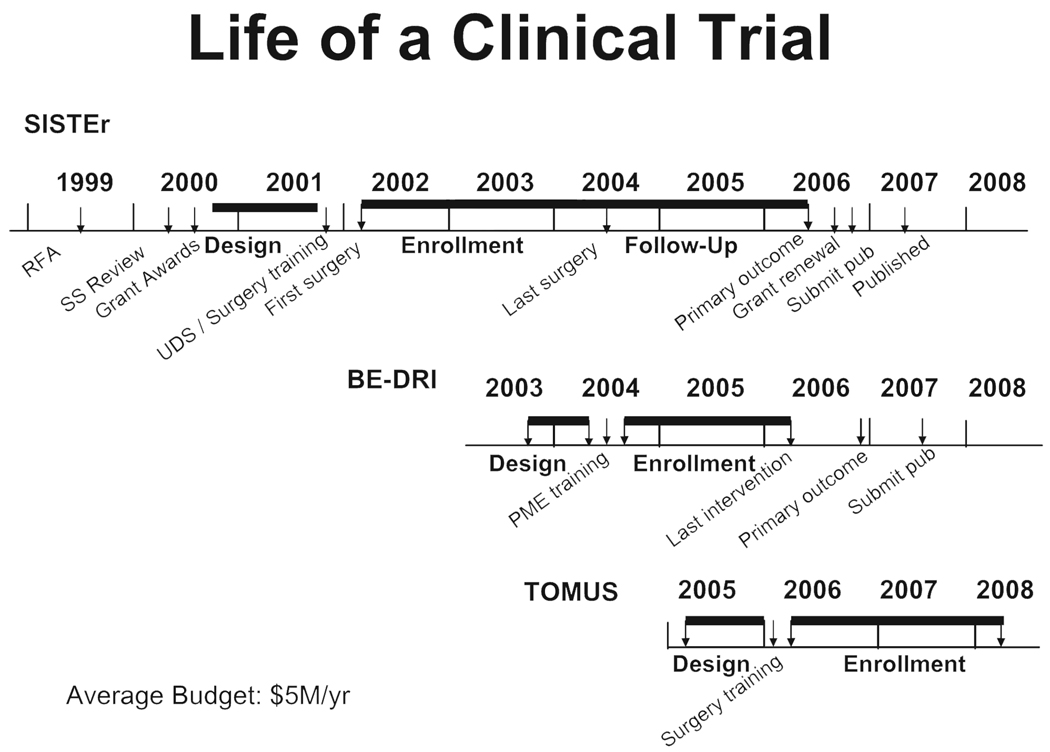
The UITN has been successful in operationalizing multiple synchronous trials (Fig. 2). The third study began in 2006. The trial of midurethral slings (TOMUS) is randomizing approximately 600 women in order to compare subjective and objective success rates for urinary incontinence (UI) at 12 and 24 months following retropubic and transobturator midurethral sling procedures.19 This study began in 2006 and will enroll 588 women. Twelve-month outcome data are expected in late 2009 and long-term annual follow-up similar to SISTEr is planned. Timelines for development and conduct of these studies is depicted in Figure 2.
Fig. 2.
Timelines for UITN trials.
Procedures for Development of Subsequent Protocols
Because incontinence is an understudied condition, many high-quality studies can be designed to test specific hypotheses. The UITN utilizes a hypothesis-based methodology that allows network-wide representation in the development of protocols.
Major hypotheses leading to the generation of index studies are typically in response to direction by the network’s executive committee. The process by which index hypotheses are generated is outlined within the UITN Publications and Presentations Policies and Procedures. During the initial phase of hypothesis generation, one or two UITN members draft a formal concept proposal. The concept proposal is a 1–3 page document that contains a summary of the proposed research and the primary research question, background, study design and comments on feasibility, limitations and estimated cost.
After a single concept or multiple concepts have been proposed, a presentation is given at an in person SC meeting where discussion ensues. Typically, multiple concepts are discussed concurrently with SC members collegially weighing the strengths, weaknesses and potential impact of resulting studies. Interest in proceeding to a mini protocol stage with the development of a protocol working group occurs with a majority vote. If multiple concepts are presented, a priority ranking is performed. Once consensus is reached, a working group is formed to further refine the hypothesis, specific aims and study design. This working group includes representatives of the CTCs and the DCC. Typically these working groups design an index study to be approved the by the full UITN SC and the DSMB prior to implementation. A mini-protocol is then developed (10–20 pages), followed by a full protocol (20–30 pages). The final protocol requires a super-majority vote (8/11). The system of graduated protocol development facilitates group consensus and avoids excess work of unfeasible studies. If the ancillary study requires substantial funding the UITN and other investigators have the option of submitting grant applications to the NIH (and other sources of funds) under a Program Announcement, PAR-07-024, Ancillary Studies to Major Ongoing NIDDK and National Heart, Lung and Blood Institute Clinical Research Studies.
In keeping with the mission to maximize data obtained with public funds and leverage index studies, the UITN also has a process to accept proposals for ancillary hypotheses related to index studies. The process by which ancillary hypotheses are generated is outlined within the UITN Publications and Presentations Policies and Procedures. Ancillary studies may be generated during or after the construction of index studies. The Ancillary Study Committee is a subset of the Publications and Presentation Committee. This group was assembled and charged with initially reviewing the proposal as well as forwarding the proposal and recommendations to the SC. If the proposal receives a favorable majority vote, a protocol work-group is then convened which is typically chaired by the originator of the concept proposal and includes representation from each participating CTC and the DCC. The process for development of the protocol is the same as for index protocols described above.
Protection of Human Subjects and Safety Monitoring
Central to the safety of human subjects is the need for informed consent and independent oversight of a clinical trial.15 Before any subjects can be enrolled in a clinical trial, the entire protocol must be reviewed and approved by a local IRB. The local IRB may have a substantial impact on the informed consent process and recruitment materials to ensure patients have the capacity to understand risks and benefits, how information is delivered and degree of voluntariness. The variation in IRB responses to the common study protocol may delay beginning enrollment or result in making modifications to the study protocol.16 The unique challenge to the SISTEr trial was approval for randomization in the operating room while the patient was anesthetized to reduce biases in the choice of surgery both on the part of the physician and the study participant and reduce withdrawals by minimizing time between randomization and surgery.
For NIH and other sponsored clinical trials a DSMB is established to provide ongoing evaluation of study participant safety as well as enrollment, retention, and complications and interim and final results. The DSMB may be composed of independent clinicians with expertise in the relevant clinical specialties, lay members, ethicists, and statisticians, among others. Data presented by randomized groups are blinded; procedures are in place for un-blinding the Board before the planned of the study. Data are also blinded to site, interventionist, and treatment group. Procedures are in place if un-blinding becomes necessary. A fundamental responsibility of a DSMB is to make recommendations to the sponsor concerning the study continuation. Throughout the course of the UITN, the DSMB has offered valuable insights in protocol design and provided additional impetus to meet recruitment and retention goals.
CLASSIFICATION OF COMPLICATIONS
For all trials implemented by the UITN, it was considered important to attempt to utilize common outcome measures, to produce comparable results across studies and expedite the development of data collection forms (Table II). Shortly after the design of the SISTEr trial it became apparent that although surgical outcomes for SUI may be similar, procedures may differ in their morbidity or complications. Adequately powering a trial to demonstrate a difference in morbidity and complications would require a prohibitively large number of subjects. To address this need to define and grade post-operative complications Dindo6 and others7 employed a classification system that ranked complications by interventions needed. The modified Clavien/Dindo system was adopted for the SISTEr and TOMUS trials and has allowed meaningful comparisons across the two trials. However, a major dilemma arose with regard to attribution of complications when a concomitant procedure was performed. As a result, the concept of attribution was abandoned and complications were merely presented as having occurred in cases with or without concomitant surgery, when that latter variable was examined.
TABLE II.
Outcome Measures
| Instrument (O = outcome, C = collected, not outcome) |
SISTEr | BE-DRI | TOMUS |
|---|---|---|---|
| Incontinence | |||
| MESA20 | O | O | O |
| Standardized stress test (300 ml) | O | O | |
| 24-hr pad test21 | O | O | |
| Diary 3 day22 | O | O | |
| Diary 7 day22 | O | ||
| Standardized Urodynamic Testing | C | ||
| Q-tip test | C | ||
| Post-void residual | C | C | C |
| OAB-q23 | C | ||
| Retreatment | O | O | |
| Other pelvic symptoms | |||
| Pain: Surgical Pain Scale | C | ||
| Prolapse | |||
| POP-Q24 | C | C | C |
| Sexual function | |||
| PISQ-1225 | C | C | C |
| Quality of life | |||
| Incontinence Impact Questionnaire26 | C | C | C |
| Urogenital Distress Inventory26 | C | C | |
| SF-1227 | C | C | C |
| HUI-328 | C | C | C |
| ICI-Q29 | O | ||
| Exploratory measures | |||
| Satisfaction | C | C | C |
| Expectations | C | C | C |
| Other | |||
| Activities Assessment Scale | C | ||
| Complications and morbidity6 | O | C | O |
STANDARDIZATION AND TRAINING
Although most investigators at the nine sites had extensive experience with clinical trials developed by industry, with the formation of the UITN it became apparent that some had to acquire new skills necessary to develop and execute standardized protocols and to collect study data in a standardized fashion to protect the internal validity of the study.
Standardization of Surgical Procedures
To assess outcomes for two surgical procedures it is reasonable to minimize variability in the techniques and materials used. A requirement for a minimum number of each surgical procedure previously performed minimized training effect or variability in a surgeon’s learning curve. At some institutions all surgeons were facile at both procedures being evaluated. Too minimize learning curve variability at some sites, two surgeons were on standby at the time of randomization, ready to undertake the procedure they were best trained in. During the SISTEr study detailed step by step diagrams of Burch colposuspension and autologous fascial sling plus a teaching video tape were used. The suture, fascial length, and positioning of fascial suture and sling were standardized. Surgeons were proctored during the procedure to ensure a standard technique was utilized. For TOMUS, standardization was met by using the company’s procedures and proctoring by experienced surgeons. Repeat surgery following a procedure for SUI may be necessary to correct pelvic floor defects not addressed in the primary operation.8 Therefore, concomitant surgery was allowed, although limits were set on the number and type in TOMUS as compared to SISTEr.
Behavioral Interventionist Training
One challenge in the BE-DRI study was the standardization of the delivery of the behavioral intervention. A multi-dimensional training protocol was implemented in BE-DRI which included: (1) teaching pelvic floor muscle control and exercises, (2) behavioral strategies to diminish urgency, suppress bladder contractions and prevent incontinence,9 (3) delayed voiding to increase voiding intervals for those who void greater than eight times per day, and (4) in addition to a fluid management handout, individualized fluid management for those subjects with excessive urine output (>70 ounces per day). As this was a comprehensive and labor intensive protocol, it required 2-day centralized training and certification of interventionists. Training included didactic material and hands-on training with live models followed by certification based on demonstrated skills. To monitor fidelity to the treatment protocol and to minimize procedure drift, mandatory bi-monthly conference calls were conducted with the interventionists to review patient encounters and provide technical assistance.
Urodynamic Studies, Central Reading, Intra- and Inter-Rater Reliability
Although good practice guidelines for urodynamics (UDS) have been published, no large-scale studies demonstrating reproducibility of complex urodynamics in multi-institutional trials among different specialists has been performed and linked to outcomes.11,12 Therefore an early challenge for the UITN was to coordinate and standardize UDS and to determine reproducibility; a work group was developed for this purpose. UDS testers at each site were certified in relation to these procedures. All UDS tracings were sent to an electronic repository at the DCC. A major question for UDS was whether interpretation of the UDS tracings, specifically during the filling (CMG, VLPP) and emptying (pressure-flow) phases, could be done by a local reviewer at the clinical site or whether a central reviewer should be utilized. To address this question, after development and implementation of UDS Interpretation Guidelines, a study was conducted to compare UDS CMG-tracing interpretation by local versus central reviewers.13,14 There was excellent inter-rater reliability and therefore, it was determined that local reviewers could interpret the filling CMG tracings. The pressure-flow study inter-rater reliability analyses are underway. Lastly, whether UDS predict UI outcomes is the subject of several reports from SISTEr and TOMUS.
Recruitment and Retention
On average only 21% of respondents for any clinical trial are eligible, 7% enroll and only 5% complete the study.2 Aggregate data for surgical trials are unavailable but it is reasonable to assume that it may be more difficult to recruit to a trial involving invasive therapies. Challenges with recruitment and retention are closely linked to the specific protocol and influenced by a wide range of factors including access to treatment in the community, associated risks, and level of burden (travel, number and length of follow-up visits, and invasiveness of testing). Recruitment for all UITN trials has been completed within a few months of planned closure, with early experience used to refine recruitment strategies (Fig. 2). Recruitment and retention strategies are listed in Table III and varied by site. At SC meetings, CTCs shared productive strategies, but successful extension to other sites was limited. Subject retention in surgical trials may be more challenging than in studies providing medications or continuous interventions, because of the lack of an incentive to return to the study site once surgery has been done. To help keep patients engaged in the trials a UITN newsletter was instituted to reach out to participants on an informational and social level. Cards, letters, and phone calls have maintained acceptable retention rates, especially for long-term follow-up in SISTEr (E-SISTEr) and BE-DRI (E-BE-DRI). These latter two studies represent long-term follow-up studies to assess durability of outcomes. Finally, compensation for visit completion was recognized as an important retention strategy, with the amount of compensation directly related to the associated burden of each study visit and to cover travel expenses.
TABLE III.
Recruitment and Retention Strategies
| Notification of PI’s institution/practice (most from MDs’ practice) |
| Publicity in newspapers, TV and radio ads |
| UITN public website: www.uitn.net |
| Fliers in laundries, buses, restrooms |
| Institutional notifications |
| Letters to community physicians |
| Payment for parking, transportation |
| UITN newsletter |
| Cards and phone reminders |
Paper/Abstract Proposals
For each approved UITN study, a list of primary outcome papers and likely secondary outcome papers are developed by the SC. Manuscript development as well as authorship is monitored and distributed equitably across the participating institutions. All UITN investigators and professional staff are encouraged to submit written proposals for abstracts or papers to the UITN P&P Committee (Table IV). It is expected that abstracts accepted for a presentation or poster will have been derived from a manuscript in development or subsequently will be developed as a manuscript for publication. Every effort is made to broadly distribute papers and presentations among both gynecology and urology journals and professional societies.
TABLE IV.
UITN Publications and Presentations
| Papers | ||||
|---|---|---|---|---|
| Study | Published | In press | In development |
Abstracts |
| SISTEr (2002–2006) | 14 | 3 | 13 | 36 |
| BE-DRI (2004–2006) | 1 | 9 | 4 | |
| TOMUS (2006–present) | 1 | |||
Rules for Authorship
For primary and secondary outcome papers (i.e., addressing primary or secondary study aims), authorship is stated as “The Urinary Incontinence Treatment Network” if allowed by the journal. A credit roster of the SC, CTC Investigators and Study Coordinators, and the DSMB appears at the end of each main paper. Alternately, main papers and presentations identify members of the writing group as authors followed by the phrase “for the Urinary Incontinence Treatment Network.” The Chair of the Writing Group determines the order of authorship. A major criterion for order of authorship is the effort and contribution made by each member of the writing group. The Uniform Requirements for Manuscripts Submitted to Biomedical Journals17 are used to define authorship.
COLLABORATIVE STUDIES-URINARY INCONTINENCE TREATMENT NETWORK (UITN) AND THE PELVIC FLOOR DISORDERS NETWORK (PFDN)
There are substantial benefits to a liaison of two large networks committed to research in female pelvic floor disorders, and such collaboration is consistent with the NIH roadmap.4 Like the UITN, the Pelvic Floor Disorders Network (PFDN) sponsored by the NICHD was established after a request for applications (RFA) in July 2000. The PFDN’s mandate is to investigate problems in women with pelvic floor disorders including pelvic organ prolapse, urinary incontinence, fecal incontinence, and other sensory and emptying abnormalities of the lower urinary and gastrointestinal tracts. The PFDN currently consists of seven clinical sites and a Data Coordinating Center (DCC). Five sites participating in the UITN are also members of the PFDN.
Initially, investigators wished to develop meaningful, clinically relevant co-analyses of common data elements from populations of women with related or common pelvic floor abnormalities such as stress urinary incontinence (SUI), urge urinary incontinence (UUI) and pelvic organ prolapse. A number of challenges arose including issues of leadership, communication, website issues, and the need for additional conference calls, adoption or ownership by “initiating” network, logistics of proposing concept proposals and analyses, data management and funding. Four main challenges to the integrity and success of research alliances have been identified: (1) maintaining a unified purpose despite multiple and challenging environments, (2) achieving and sustaining integration across organizational boundaries, (3) meeting complex information needs created by multi-organizational arrangement, and (4) fostering a cooperative spirit among alliance partners who may have been competitors.5 Despite the best intentions a tangible product of cross-network collaboration has not yet been achieved. Currently, research protocols initiated within each network have been extended to the other network for potential participation and development.
INDUSTRY COLLABORATION
For the second (Behavioral Enhances Drug Reduction of Incontinence, BE-DRI) trial developed by the UITN, a working relationship with a pharmaceutical (Detrol, Pfizer) manufacturer was established. Part of the due diligence in protocol assessment was selection of drugs to be used, based on a working group assessment. These decisions were based not only on the scientific rationale, but on the market position of current products for generalizability of results. Early in discussions of trial development the critical need for independent trials without industry influence was agreed upon. Indeed, many investigators consider that a major strength of the UITN was the desire to perform independent design and outcome assessment for commonly used drugs and devices. Modest support from industry was received for the BE-DRI study conducted by the UITN in its first phase to support data collection. Drugs were donated by Pfizer who did not have a role in data analysis or preparation of the manuscript describing the primary results. Industry collaboration was not established for SISTEr and TOMUS.
CONCLUSION
The UITN was formed because of the need for standardized and scientifically rigorous studies in an area of increasing public health importance, that is, treatment of urinary incontinence in women. Our multi-disciplinary team of investigators located across the US has demonstrated that it is possible to conduct such important trials, thereby gathering information that will allow us to optimize patient care.
References
- 1.Wagner WH, Green SM, Hurt G, et al. Building a research consortium of large health systems: The cancer research network. JNCI Monographs. 2005;35:3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 2.Sung NS, Cowley XX, Genel M, Salber P, et al. Central challenges facing a nation clinical research enterprise. JAMA. 2007;289:1278–1287. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 3.Albo ME, Richter HE, Brubaker L, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. New Engl J Med. 2007;356:2143–2155. doi: 10.1056/NEJMoa070416. [DOI] [PubMed] [Google Scholar]
- 4.Zerhouni E. The NIH Roadmap. Science. 2003;302:63–64. doi: 10.1126/science.1091867. [DOI] [PubMed] [Google Scholar]
- 5.Klabunde CN, Lacey LM, Kalunzy AD. Managing care in the community. In: Kalunzy AD, Warnecke RB, editors. Managing a health care alliance: Improving community cancer care. San Francisco: Jossey-Bass; 1996. pp. 83–103. [Google Scholar]
- 6.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veen EJ, Steenbruggen J, Roukema JA. Classifying surgical complications. Arch Surg. 2005;140:1078–1083. doi: 10.1001/archsurg.140.11.1078. [DOI] [PubMed] [Google Scholar]
- 8.Anger J, Litwin M, Wang Q, et al. Variations in stress incontinence and prolapse surgery by surgeon specialty. J Urol. 2007;178:1411–1417. doi: 10.1016/j.juro.2007.05.149. [DOI] [PubMed] [Google Scholar]
- 9.Burgio KL, Kraus SR, Menefee S, et al. Combined behavior and drug therapy enable drug discontinuation in the treatment of urge incontinence in women: A randomized controlled trial. Ann Int Med. 2008 (Submitted) [Google Scholar]
- 10.The Urinary Incontinence Treatment Network. Design of the Behavior Enhances Drug Reduction of Incontinence (BE-DRI) study. Contemp Clin Trials. 2007;28:48–58. doi: 10.1016/j.cct.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Subcommittee of the International Continence Society. Neurourol Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 12.Schafer W, Abrams P, Liao L, et al. Good urodynamic practices: Uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21:261–274. doi: 10.1002/nau.10066. [DOI] [PubMed] [Google Scholar]
- 13.Nager M, Albo M, FitzGerald S, et al. Zimmern process for development of multicenter urodynamic studies. Urology. 2006;69:63–67. doi: 10.1016/j.urology.2006.08.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmern P, Nager CW, Albo M, et al. Interrater reliability of filling cystometrogram interpretation in a multicenter study. J Urol. 2006;175:2174–2177. doi: 10.1016/S0022-5347(06)00343-0. [DOI] [PubMed] [Google Scholar]
- 15.Etchells E. Informed consent in surgical trials. World J Surg. 1999;23:1215–1219. doi: 10.1007/s002689900650. [DOI] [PubMed] [Google Scholar]
- 16.Mansbach J, Acholonu U, Sunday Clark BA, et al. Variation in institutional review board responses to a standard, observational pediatric research protocol. Soc Acad Emerg Med. 2007;14:377–380. doi: 10.1197/j.aem.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 17.Uniform Requirements for Manuscripts Submitted to Biomedical Journals. NEJM. 2001;324:424–428. doi: 10.1056/NEJM199102073240624. [DOI] [PubMed] [Google Scholar]
- 18.The Urinary Incontinence Treatment Network. Design of the SISTEr (Stress Incontinence Surgical Treatment Efficacy Trial) Study: A Randomized Surgical Trial Comparing the Burch Colposuspension and the Autologous Rectus Fascial Sling. Urology. 2005;66:1213–1217. [Google Scholar]
- 19.The Urinary Incontinence Treatment Network. The Trial of Midurethral Slings (TOMUS) Design and Durability. J Appl Res. 2008 (in press) [Google Scholar]
- 20.Herzog AR, Diokno AC, Brown MB, et al. Two-year incidence, remisison and change patterns of urinary incontinence in noninstitutionalized older adults. J Gerontol. 1990;45:M67–M74. doi: 10.1093/geronj/45.2.m67. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen L, Lose G, Thunedborg P. Diagnosis of mild incontinence in females: 24-hour home pad test versus the 1-hour ward test. Neurourol Urodynam. 1987;6:165. [Google Scholar]
- 22.Wyman JF, Choi SC, Harkins SW, et al. The urinary fiary in evaluation of incontinent women: A test-retest analysis. Obstet Gynecol. 1988;71:812–817. [PubMed] [Google Scholar]
- 23.Coyne KA. Summary of the validation of the OAB-q: A disease-specific overactive bladder questionnaire. Bethesda, MD: MEDTAP International Inc; 2002. May 15, Unpublished report for Pharmacia Corp. [Google Scholar]
- 24.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 25.Rogers R, Qualls C, Kammerer-Doak D, et al. Short form to assess sexual function in women with urinary incontinence and/or pelvic organ prolapse: The PISQ-11. Intl Urogyn J Pelvic Floor Dysfunct. 2001;12:S2. doi: 10.1007/s001920170012. [DOI] [PubMed] [Google Scholar]
- 26.Shumaker SA, Wyman JF, Uebersax JS, et al. Health-related quality of life measures for women with urinary incontinence: The incontinence impact questionnaire and the urogenital distress inventory. Qual Life Res. 1994;3:291–306. doi: 10.1007/BF00451721. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Kosinski M, Keller Sd. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Furlong W, et al. McMaster University Centre for Health Economics and Policy Analysis; Multiplicative multi-attribute utility function for healt utilities index mark 3 (HUI3) system: A technical report. 1998
- 29.Avery K, et al. ICI-Q: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurol Urodyn. 2004;23:322–330. doi: 10.1002/nau.20041. [DOI] [PubMed] [Google Scholar]