Abstract
The protein kinase Chk2, the mammalian homolog of the budding yeast Rad53 and fission yeast Cds1 checkpoint kinases, is phosphorylated and activated in response to DNA damage by ionizing radiation (IR), UV irradiation, and replication blocks by hydroxyurea (HU). Phosphorylation and activation of Chk2 are ataxia telangiectasia-mutated (ATM) dependent in response to IR, whereas Chk2 phosphorylation is ATM-independent when cells are exposed to UV or HU. Here we show that in vitro, ATM phosphorylates the Ser-Gln/Thr-Gln (SQ/TQ) cluster domain (SCD) on Chk2, which contains seven SQ/TQ motifs, and Thr68 is the major in vitro phosphorylation site by ATM. ATM- and Rad3-related also phosphorylates Thr68 in addition to Thr26 and Ser50, which are not phosphorylated to a significant extent by ATM in vitro. In vivo, Thr68 is phosphorylated in an ATM-dependent manner in response to IR, but not in response to UV or HU. Substitution of Thr68 with Ala reduced the extent of phosphorylation and activation of Chk2 in response to IR, and mutation of all seven SQ/TQ motifs blocked all phosphorylation and activation of Chk2 after IR. These results suggest that in vivo, Chk2 is directly phosphorylated by ATM in response to IR and that Chk2 is regulated by phosphorylation of the SCD.
DNA damage activates a response pathway that arrests the cell cycle to provide time for repair of damage and induces the transcription of genes that facilitate repair (1). Defects in this DNA damage response pathway can result in genomic instability, a mutagenic condition leading to cancer predisposition (2). Key regulators of the DNA damage response pathway are the phosphoinositide kinase homologs ataxia telangiectasia-mutated (ATM) and ATM- and Rad3-related (ATR), the protein kinases Chk1 and Chk2, and the tumor suppressor protein p53 (1, 3). ATM controls cell cycle arrest in G1 and G2. Control of G2 arrest by ATM is thought to involve maintenance of Cdc2 in a tyrosine-phosphorylated state (1), but precisely how ATM regulates Cdc2 has been unclear. Recently, Chk2, the mammalian homolog of the budding yeast Rad53 and fission yeast Cds1 checkpoint kinases (4–6), was cloned and found to link ATM and Cdc2 (7, 8). In response to ionizing radiation (IR), Chk2 is rapidly phosphorylated and activated in an ATM-dependent manner, and in vitro, Chk2 phosphorylates Cdc25C on Ser216 (7, 8), which interferes with Cdc25C's ability to activate Cdc2 (9). In Chk2−/− mouse embryonic stem cells, maintenance of G2 arrest and reduced Cdc2 kinase activity in response to IR is defective (10). These results suggested a model in which ATM controls G2 arrest in part by activating Chk2.
The functional role of ATR and Chk1 in the checkpoint pathway is not fully understood. Overexpression of ATR partially suppresses cells lacking ATM, and overexpression of a kinase-defective ATR mutant in wild-type cells abrogates G2/M arrest after exposure to IR (11) and increases sensitivity of cells to IR and UV (12), suggesting an overlapping function between ATM and ATR. Chk1 is modified in response to DNA damage and can phosphorylate Ser216 on Cdc25C in vitro (13). Recently, ATR−/− blastocyst cells were found to die with chromosome fragmentation, implying an essential role for ATR in the DNA replication checkpoint (14), and mouse embryonic stem cells lacking Chk1 have a defective G2/M DNA damage checkpoint (15). Furthermore, in response to UV, HU, and IR, Chk1 is found to be phosphorylated on Ser345, and the phosphorylation in response to UV is ATR dependent (15). ATR and Chk1 disruptions both lead to peri-implantation embryonic lethality in mice (14–16). Thus, Chk1 is regulated by ATR (15).
ATM-dependent G1 arrest in response to IR is achieved by activation of p53 (17), which acts as a sequence-specific DNA-binding transcription factor to induce transcription of the Cdk inhibitor p21CIP1/WAF1 (18, 19). Activation of p53 in response to DNA damage is accomplished by both increasing its protein levels and by increasing its transcriptional activity. The increase in p53 levels in response to DNA damage is caused by decreased degradation of p53, and phosphorylation of Ser20 on p53 is required for the decrease in p53 degradation (20–22). Recently, Chk2 was found to phosphorylate Ser20 on p53 in vitro (10, 23, 24), and we have found that Chk2 is required for increased stability of p53 in response to IR (10), providing a possible mechanism for regulation of p53 stability by Chk2.
Transcriptional activation of p53 is mediated by acetylation and phosphorylation of p53. Acetylation of p53 by histone acetyltransferases CBP/p300, which function as a coactivator for p53-mediated transcription (25, 26), is shown to enhance sequence-specific DNA binding of p53 (27). Phosphorylation of Ser15 on p53 was found to increase the interaction with CBP/p300 and acetylation of p53 (28). ATM phosphorylates Ser15 in vitro, and in response to IR, Ser15 is phosphorylated in an ATM-dependent manner, suggesting that Ser15 is phosphorylated by ATM in vivo (29, 30). However, Ser15 is phosphorylated in an ATM-independent manner when cells are exposed to UV, indicating that a kinase distinct from ATM phosphorylates Ser15. A candidate kinase is ATR, which phosphorylates Ser15 in vitro, and overexpression of kinase dead ATR interferes with phosphorylation of Ser15 after exposure of cells to IR or UV (31).
ATM-dependent phosphorylation of Chk2 is seen only when cells are damaged by IR. Chk2 is phosphorylated in an ATM-independent manner in response to UV or HU (7, 8, 32–34). Thus, Chk2 phosphorylation parallels p53 phosphorylation on Ser15. These results prompted us to examine Chk2 phosphorylation by ATM and ATR. In this study we report that Chk2 is phosphorylated on Thr68 by both ATM and ATR in vitro, and that Thr68 is phosphorylated in an ATM-dependent manner in vivo when cells are exposed to IR but in an ATM-independent manner when cells are treated with UV or HU. Furthermore, Chk2 was found to be regulated by phosphorylation of the N-terminal Ser-Gln/Thr-Gln (SQ/TQ) cluster domain (SCD).
Materials and Methods
Plasmids.
Full-length Chk2 cDNA with NdeI site at its first ATG was constructed in pBluscript by replacing a KpnI–NcoI fragment of a Chk2 cDNA clone with a double-stranded oligonucleotide with a NdeI site. pBSChk2-KD, a plasmid having kinase dead Chk2 with substitution of Asp347 by Ala, was created by PCR. pGST-Chk2 and pGST-Chk2-KD were respectively constructed by ligating a NdeI-EcoRI fragment of full-length Chk2 from pBSChk2 and pBSChk2-KD into pGEX2TKcs. To make pGST-Chk2-KD2, Ala-substitution at Lys249 was introduced by PCR. pGST-SQ/TQ was made by ligating NdeI–ApaI (blunt-ended) fragment (1–96 amino acids) from pBSChk2 into NdeI–EcoRI (blunt-ended) sites of pGEX2TKcs. pGST-ΔSQ/TQ was constructed by ligating a ApaI (blunt-ended)–EcoRI fragment (99–543 amino acids) from pBSChk2-KD into NcoI(blunt-ended)–EcoRI sites on pGEX2TKcs. pRetFLAG-Chk2, retrovirus vector encoding FLAG-tagged human Chk2, was constructed in pBabe-puro (35). Site-directed mutagenesis for replacement of SQ/TQ with Ala-Gln (AQ) was performed by PCR, and all mutations were confirmed by DNA sequencing. Plasmids expressing FLAG-ATM-WT and FLAG-ATM-KD (30) were from M. Kastan (St. Judes Children's Hospital, Memphis, TN), and FLAG-ATR-WT and FLAG-ATR-KD (31) were from R. Abraham (Duke University, Durham, NC).
Production of Anti-Phospho-Chk2 Antibodies.
Rabbit polyclonal anti-phospho-Thr68 antibodies were raised against a peptide containing phospho-Thr68 [ETVST(-PO4)QELYS] and were affinity-purified using a column of the antigen peptide. The affinity-purified anti-phospho-Thr68 antibodies were further purified by passage through a column of a non-phospho-Chk2-peptide (ETVSTQELYS) to deplete contaminated antibodies reactive with unphosphorylated antigen.
Western Blotting.
Cells were lysed in 20 mM Tris⋅HCl buffer (pH 8.0) containing 100 mM NaCl, 0.5% Triton X-100, 10 mM NaF, 1 mM Na3VO4, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 2 μg/ml antipain, and 1 mM phenylmethylsulfonyl fluoride (PMSF) for 15 min at 4°C. After centrifugation at 10,000 × g for 10 min, cell extracts were incubated with anti-Chk2 antibodies and protein A–Sepharose (Amersham Pharmacia) for 1 h at 4°C. The precipitated Sepharose beads were washed four times with 20 mM Tris⋅HCl buffer (pH 8.0) containing 100 mM NaCl, 0.1% Triton X-100, 10 mM NaF, 1 mM Na3VO4, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 2 μg/ml antipain, and 1 mM PMSF and then applied to SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 5% nonfat-milk in 50 mM Tris⋅HCl (pH 7.5), 100 mM NaCl, and 0.05% Tween 20, the membranes were incubated with anti-phospho-Thr68 antibodies and 100 μg/ml non-phospho-Chk2-peptides (ETVSTQELYS), and then horseradish peroxidase-conjugated anti-rabbit IgG antibodies (Promega), followed by detection by enhanced chemiluminescence (Amersham Pharmacia). To reprobe with anti-Chk2 antibodies, membranes were washed twice with 50 mM glycine (pH 2.0) and then twice with 50 mM Tris⋅HCl (pH 7.5), 100 mM NaCl, 0.05% Tween 20 before blocking with 5% nonfat-milk in 50 mM Tris⋅HCl (pH 7.5), 100 mM NaCl, 0.05% Tween 20.
In Vitro ATM/ATR Kinase Assay.
ATM kinase assay with immunoprecipitated ATM from normal and AT lymphoblast cells was carried out as described (29). ATM-WT, ATM-KD, ATR-WT, and ATR-KD proteins were expressed as FLAG-tagged protein in 293T cells by transfection of the expression vector for ATM (28) or ATR (29). DNA transfection into 293T cells was performed with lipofectamine and Opti-MEM (GIBCO/BRL). Forty-eight hours after transfection, cells were lysed by sonication in 50 mM Tris⋅HCl buffer (pH 7.5) containing 50 mM β-glycerophosphate, 150 mM NaCl, 10% glycerol, 1% Tween 20, 1 mM NaF, 1 mM Na3VO4, 1 mM PMSF, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 2 μg/ml antipain, and 1 mM DTT. After centrifugation at 10,000 × g for 10 min, FLAG-tagged proteins were immunoprecipitated with anti-FLAG M2 antibodies (Sigma) and protein A/G-Sepharose (Santa Cruz Biotechnology) and washed four times with the lysis buffer, twice with 50 mM Tris⋅HCl buffer (pH 7.5) containing 0.5 M LiCl, and then three times with 20 mM Hepes (pH 7.4), 10 mM MgCl2, and 10 mM MnCl2. Kinase reactions contained immunoprecipitated FLAG-tagged proteins bound to protein A/G–Sepharose and GST-Chk2 substrates in 20 mM Hepes (pH 7.4), 10 mM MgCl2, 10 mM MnCl2, 25 μM ATP, and 15 μCi [γ-32P]ATP for 30 min at 30°C. Proteins were separated by SDS-PAGE and visualized by autoradiography.
Retrovirus-Mediated Expression of Human Chk2 in Chk2−/− Mouse Embryonic Fibroblast (MEF) Cells.
pRetFLAG-Chk2 or the empty vector was transfected into Phoenix-eco cells (36), and, after 48 h, viral supernatants were harvested and used to infect immortalized Chk2−/− MEF cells (10). Forty-eight hours after infection, cells were treated with or without 20 Gy of ionizing radiation (IR), and cell extracts were prepared as described previously (7).
Results and Discussion
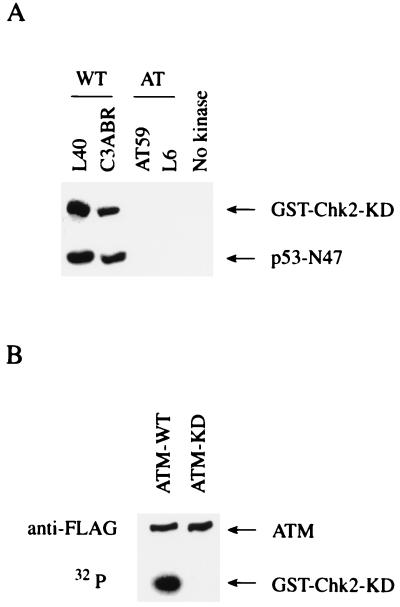
To examine Chk2 phosphorylation by ATM, an in vitro ATM kinase assay was performed, with GST-Chk2-KD as substrate. GST-Chk2-KD is defective for kinase activity because of substitution of the catalytically essential Asp347 residue with Ala. This mutation prevents autophosphorylation of Chk2, which might complicate detection of phosphorylation by ATM. ATM immunoprecipitates from the normal lymphoblastoid cell lines L40 and C3ABR phosphorylated GST-Chk2 as well as p53-N47 (29), whereas immunoprecipitates obtained from AT lymphoblastoid cell lines AT59 and L6, which lack the ATM protein, did not phosphorylate GST-Chk2 and p53-N47 (Fig. 1A). To eliminate the possibility that an ATM-associated kinase phosphorylated Chk2, transfected FLAG-tagged wild type (WT) or kinase dead (KD) ATM was immunoprecipitated and used in a Chk2 phosphorylation assay. Immunoprecipitates from ATM-WT expressing cells phosphorylated GST-Chk2, but immunoprecipitates from cells expressing ATM-KD did not (Fig. 1B). These results demonstrate that Chk2 is phosphorylated by ATM in vitro.
Figure 1.
Phosphorylation of Chk2 by ATM. (A) Chk2 phosphorylation by ATM from normal lymphoid cells. Immunoprecipitates with anti-ATM antibodies from normal lymphoblastoid cell lines (L40 and C3ABR) or AT lymphoblastoid cell lines (AT59 and L6) were incubated with [γ-32P]ATP and KD Chk2 fused to GST (GST-Chk2-KD) or p53-N47 (29). The control reaction was carried out without immunoprecipitate (No kinase). Proteins were resolved by SDS-PAGE and visualized by autoradiography. (B) Chk2 phosphorylation by transfected ATM. Immunoprecipitates with anti-FLAG from 293T cells transfected with the vector expressing FLAG-tagged wild-type ATM (ATM-WT) or kinase dead ATM (ATM-KD) were incubated with GST-Chk2-KD and [γ-32P]ATP. Proteins were resolved by SDS-PAGE and visualized by autoradiography or Western blotting with anti-FLAG antibodies.
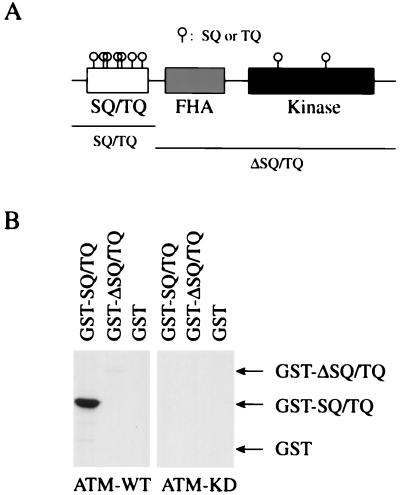
ATM is known to phosphorylate Ser15 on p53 (29, 30), and this Ser residue is followed by Gln. Recently, ATM was found to phosphorylate multiple Ser residues in the SCD on BRCA1 (37). Chk2 has nine SQ/TQ motifs; seven of them are located in its N terminus, and two are in its kinase domain (Fig. 2A). This N-terminal SCD, in addition to the kinase and fork head-associated domains, is conserved in Chk2, Rad53, and Cds1. To determine the ATM phosphorylation sites on Chk2, we made two deletion mutants of Chk2: GST-SQ/TQ, which contains seven SQ/TQ motifs in the N terminus, and GST-ΔSQ/TQ, which lacks the N-terminal SQ/TQ motifs but contains the fork head-associated domain and the kinase domain, which is inactivated by the replacement of Asp347 with Ala (Fig. 2A). As shown in Fig. 2B, GST-SQ/TQ was readily phosphorylated by ATM, whereas GST-ΔSQ/TQ was very poorly phosphorylated, suggesting that ATM primarily phosphorylates Ser and/or Thr residues in the N-terminal SQ/TQ motifs of Chk2.
Figure 2.
Mapping ATM phosphorylation sites on Chk2. (A) Domain structure of human Chk2. SQ/TQ, Ser-Gln and Thr-Gln cluster domain; FHA, fork-head associated domain; Kinase, kinase domain; O, SQ and TQ motifs. (B) Phosphorylation of the SQ/TQ-cluster domain on Chk2 by ATM. Immunoprecipitates with anti-FLAG from 293T cells transfected with FLAG-ATM were incubated with [γ-32P]ATP and GST-SQ/TQ, GST-ΔSQ/TQ, or GST. Regions of SQ/TQ and ΔSQ/TQ fused to GST are shown in A. Proteins were resolved by SDS-PAGE and visualized by autoradiography.
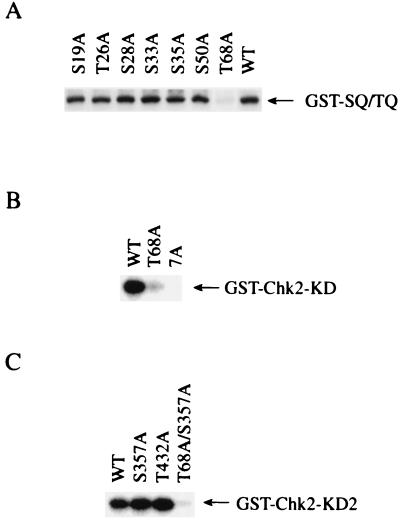
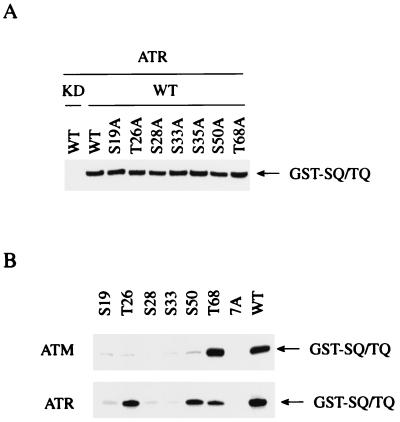
To further map the ATM phosphorylation sites on Chk2, a SQ/TQ to AQ substitution was introduced on each SQ/TQ motif by site-directed mutagenesis. Whereas substitution of Ser19, Thr26, Ser28, Ser33, Ser35, or Ser50 by Ala did not show significant reduction in 32P incorporation into GST-SQ/TQ, substitution of Thr68 by Ala largely reduced 32P incorporation into GST-SQ/TQ (Fig. 3A). Quantitative analysis of radioactivity in GST-SQ/TQ proteins revealed that GST-SQ/TQ(T68A) was phosphorylated to an extent that was less than 20% of the level observed for WT GST-SQ/TQ. Furthermore, introduction of Ala substitution at Thr68 into full-length KD Chk2 fused to GST (GST-Chk2-KD(T68A)) resulted in a large reduction of 32P incorporation into GST-Chk2, compared with “wild type” KD GST-Chk2 (WT) (Fig. 3B), indicating that Thr68 is the major phosphorylation site by ATM. It should be noted that 32P incorporation into GST-Chk2-KD(T68A) is more than GST-Chk2-KD(7A), in which all Ser and Thr in the N-terminal SQ/TQ motifs were changed to Ala. Therefore, other Ser or Thr residues in addition to Thr68 in the N-terminal SQ/TQ motifs are phosphorylated by ATM (Fig. 3B), although to a significantly lesser degree than Thr68. Finally, Ala substitutions were introduced into SQ/TQ motifs in the kinase domain of Chk2. Because Asp347 is close to Ser357, substitution at Asp347 might conceivably affect the phosphorylation of Ser357. Therefore, we made a second kinase defective mutant, GST-Chk2-KD2, in which Lys249 was replaced by Ala, and three SQ/TQ mutants of GST-Chk2-KD2, which have Ala substitution at Ser357 (S357A), Thr432 (T432A), or at both Thr68 and Ser357 (T68A/S357A). Fig. 3C shows that a similar amount of 32P was incorporated into “wild type” GST-Chk2-KD2 (WT) and mutants of Ser357 (S357A) and Thr432 (T432A). However, 32P incorporation was largely reduced in the mutant with Ala substitution at both Thr68 and Ser375 (T68A/S357A), compared with a mutant with single Ala substitution at Ser357 (S357A) (Fig. 3C). From these findings we concluded that Thr68 is the major in vitro site of phosphorylation by ATM on Chk2.
Figure 3.
Phosphorylation of Thr68 on Chk2 by ATM. (A) Immunoprecipitates with anti-FLAG from 293T cells transfected with the vector expressing FLAG-ATM were incubated with [γ-32P]ATP and wild-type GST-SQ/TQ or GST-SQ/TQ with Ala substitution at Ser19 (S19A), Thr26 (T26A), Ser28 (S28A), Ser33 (S33A), Ser35 (S35A), Ser50 (S50A), or Thr68 (T68A). Proteins were resolved by SDS-PAGE and visualized by autoradiography. (B) FLAG-ATM was incubated with [γ-32P]ATP and full-length “wild type” kinase dead Chk2 fused to GST (WT), GST-Chk2-KD with Ala substitution at Thr68 (T68A), or Ala substitution at all seven Thr and Ser in the N-terminal SQ/TQ motifs (7A). Proteins were resolved by SDS-PAGE and visualized by autoradiography. (C) FLAG-ATM was incubated with [γ-32P]ATP and GST-Chk2-KD2 (WT), a full-length “wild-type” kinase dead Chk2 fused to GST, or GST-Chk2-KD2 with Ala substitution at Ser357 (S357A), Thr432 (T432A), or at both Thr68 and Ser357 (T68A/S357A). Proteins were resolved by SDS-PAGE and visualized by autoradiography.
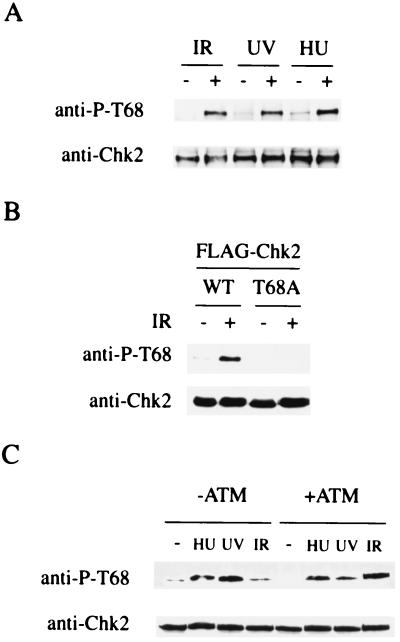
To determine whether Thr68 is a phosphorylation site for Chk2 in vivo, polyclonal rabbit anti-phospho-Thr68 antibodies were raised against a Chk2 peptide containing phosphorylated Thr68. Results obtained by ELISA indicated that the anti-phospho-Thr68 antibodies recognize a Chk2 peptide containing phosphorylated Thr68, but not unphosphorylated Thr68, and that the antibodies do not cross-react with Chk2 peptides containing other SQ/TQ motifs in the phosphorylated state (data not shown). Chk2 protein immunoprecipitated with anti-Chk2 antibodies from 293T cells treated with IR, UV, or HU and untreated 293T cells was immunoblotted with anti-phospho-Thr68 antibodies. Fig. 4A shows that Thr68 on Chk2 is phosphorylated in response to IR, UV, or HU in vivo. The anti-phospho-Thr68 antibody did not react with the T68A Chk2 mutant that was expressed in cells damaged by IR, indicating that the antibody is highly specific to phosphorylated Thr68 on Chk2 (Fig. 4B). Although phosphorylation and activation of Chk2 in response to IR is ATM dependent, Chk2 is phosphorylated in an ATM-independent manner when cells are treated with UV or HU (7, 8, 32–34). To investigate whether phosphorylation of Thr68 is ATM dependent or not, Chk2 was immunoprecipitated from AT cells into which a functional ATM gene or the vector was reintroduced (7, 38) and subjected to Western blotting with anti-phospho-Thr68 antibodies. In response to IR, Thr68 is phosphorylated in cells expressing wild-type ATM cDNA, but not in cells lacking ATM, and phosphorylation of Thr68 is seen after UV or HU treatment, regardless of the status of ATM (Fig. 4C). These results indicate that Thr68 is phosphorylated by ATM in response to IR in vivo and suggest the existence of kinase(s) other than ATM, which phosphorylates Thr68 on Chk2 in response to UV or HU treatment.
Figure 4.
Phosphorylation of Thr68 in vivo. (A) Phosphorylation of Thr68 in response to DNA damage in vivo. 293T cells were untreated or treated with 20 Gy IR or 50 J/m2 UV radiation and harvested after 2 h, or grown in 1 mM HU for 24 h before harvesting. Chk2 protein immunoprecipitated with anti-Chk2 antibodies from these cells was immunoblotted with anti-phospho-Thr68 and anti-Chk2 antibodies. (B) 293T cells were transfected with plasmids expressing FLAG-tagged wild-type Chk2 (WT), or the T68A mutant Chk2 (T68A) and Chk2 proteins were immunoprecipitated with anti-FLAG antibody from untreated cells (−) or cells treated (+) with 20 Gy IR. Chk2 was immunoblotted with anti-phospho-T68 and anti-Chk2 antibodies. (C) ATM-dependent phosphorylation of Thr68 in response to IR. AT cells containing the vector alone (−ATM) and the same cells containing the vector expressing ATM (+ATM) were untreated or treated with 10 Gy IR or 50 J/m2 UV radiation and harvested after 1 h or were grown in 1 mM HU for 24 h before harvesting. Chk2 immunoprecipitated with anti-Chk2 antibodies from cells was immunoblotted with anti-phospho- and anti-Chk2 antibodies.
Because Ser15 on p53 is phosphorylated by not only ATM but also by ATR in vitro (29–31), we examined in vitro Chk2 phosphorylation by ATR. ATR-WT or ATR-KD was expressed and immunoprecipitated from 293T cells and used in a Chk2 phosphorylation assay. GST-SQ/TQ was phosphorylated by ATR-WT but not by ATR-KD (Fig. 5A). Whereas Ala substitution at Thr68 resulted in a significant reduction of 32P incorporation into GST-SQ/TQ by ATM (Fig. 3A), substitution of any single SQ or TQ with AQ did not show a significant reduction of phosphorylation by ATR (Fig. 5A). This finding suggests that ATR phosphorylates multiple Ser and Thr residues in the N-terminal SCD. To determine the sites of in vitro phosphorylation by ATR, kinase assays were carried out with GST-SQ/TQ mutants, which have an Ala substitution at six of the seven Ser and Thr residues in the SQ/TQ motif, so that each mutant has only a single SQ or TQ motif. ATM efficiently phosphorylated wild-type GST-SQ/TQ (WT) and GST-SQ/TQ(T68), a mutant that has intact Thr68 and six Ala substitutions at other SQ/TQ motifs, but 32P incorporation into GST-SQ/TQ mutants that have intact Ser or Thr at Ser19 (S19), Thr26 (T26), Ser28 (S28), Ser33 (S33), or Ser50 (S50) was as poor as GST-SQ/TQ(7A), in which all seven SQ/TQ motifs are changed to AQ (Fig. 5B). These results are completely consistent with the notion that Thr68 is the major in vitro phosphorylation site by ATM.
Figure 5.
Phosphorylation of Chk2 by ATR. (A) FLAG-tagged wild-type ATR (ATR-WT) or kinase dead ATR (ATR-KD) was incubated with [γ-32P]ATP and wild-type GST-SQ/TQ (WT) or GST-SQ/TQ with Ala-substitution at Ser19 (S19A), Thr26 (T26A), Ser28 (S28A), Ser33 (S33A), Ser35 (S35A), Ser50 (S50A), or Thr68 (T68A). Proteins were resolved by SDS-PAGE and visualized by autoradiography. (B) FLAG-ATM or FLAG-ATR was incubated with [γ-32P]ATP and wild-type GST-SQ/TQ (WT), GST-SQ/TQ with Ala substitution at six Ser and Thr residues in SQ/TQ motifs except Ser19 (S19), Thr26 (T26), Ser28 (S28), Ser33 (S33), Ser50 (S50), or Thr68 (T68); or GST-SQ/TQ with Ala substitution at all seven Ser and Thr residues in SQ/TQ motifs (7A). Proteins were resolved by SDS-PAGE and visualized by autoradiography.
ATR phosphorylated GST-SQ/TQ mutants that have intact Thr26 (T26), Ser50 (S50), or Thr68 (T68), at levels that were as much as 20–40% of the phosphorylation of wild-type GST-SQ/TQ (WT), but other mutants that have intact Ser19 (S19), Ser28 (S28), and Ser33 (S33) were phosphorylated as poorly as GST-SQ/TQ(7A) (Fig. 5B). Because Ala substitution at Thr26, Ser28, Ser33, or Ser35 could possibly affect phosphorylation of the adjacent Ser and/or Thr residues in the SQ/TQ motif by altering the recognition sequence for ATR, we are unable to exclude the possibility of phosphorylation of Ser28, Ser33, and Ser35 by ATR. However, these results indicate that Thr26, Ser50, and Thr68 could be phosphorylated by ATR in vitro and demonstrate that ATM and ATR have overlapping but distinct substrate specificities in vitro.
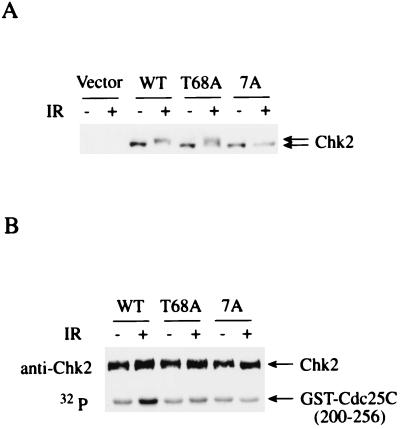
Finally, to explore the function of SQ/TQ phosphorylation, retroviruses expressing wild-type human Chk2, the T68A Chk2 mutant, or a mutant in which all seven SQ/TQ motifs in the SCD were changed to AQ, were introduced into Chk2−/− MEF cells (10). In these retroviruses human Chk2 is expressed at a level similar to that of endogenous Chk2 in human cell lines (data not shown). Wild-type human Chk2 expressed in Chk2−/− MEF cells shows the same mobility shift on SDS-PAGE as the endogenous Chk2 does in human cells (7), suggesting that it is correctly phosphorylated in response to IR (Fig. 6A). Substitution of Thr68 with Ala reduced the amount of fully phosphorylated Chk2 by 50%, and Ala substitution at all seven SQ/TQ motifs abolished the mobility shift altogether, indicating that phosphorylation of Thr68 is not solely responsible for the shift in electrophoretic mobility after IR (Fig. 6A). Kinase assays with Chk2 immunoprecipitated from these cells revealed that T68A Chk2 mutants displayed a significantly reduced IR induction of kinase activity (75% reduction relative to wild-type activation). The mutant Chk2 lacking all SQ/TQ motifs in the SCD did not show any activation by IR (Fig. 6B). These results suggest that phosphorylation of the SCD regulates Chk2 activation in response to IR.
Figure 6.
Regulation of Chk2 by phosphorylation of N-terminal SQ/TQ motifs. (A) Chk2−/− MEF cells infected with retroviruses encoding empty vector (Vector), wild-type human Chk2 (WT) or the Chk2 T68A mutant (T68A), or a mutant with Ala substitution at all seven N-terminal SQ/TQ motifs (7A) were not treated (−) or were treated (+) with 20 Gy ionizing radiation (IR) and harvested after 1 h. Protein from these cells was fractionated by SDS-PAGE and immunoblotted with anti-human Chk2 antibody. (B) Chk2 was immunoprecipitated with anti-Chk2 antibodies from cells in A. Immunoprecipitates were incubated with GST-Cdc25C () and [γ-32P]ATP. Proteins were resolved by SDS-PAGE and visualized by autoradiography or Western blotting with anti-human Chk2 antibodies.
The results shown here indicate that Chk2 is a direct substrate of ATM both in vitro and in vivo, and that Thr68 is the major site of phosphorylation in vitro and is phosphorylated in response to DNA damage in vivo. Although substitution at Thr68 largely reduced 32P incorporation into GST-Chk2-KD, further reduction in 32P incorporation into GST-Chk2-KD was observed when all seven SQ/TQ motifs in the N terminus were changed to AQ (Fig. 3B). Thus, there must be additional “minor” in vitro phosphorylation sites in the N-terminal SQ/TQ motifs. These results are consistent with those obtained from the in vivo experiments on the expression of wild-type and mutant Chk2 in Chk2−/− MEF cells, in which the Chk2 T68A mutant showed partial phosphorylation and activation in response to IR that was dependent upon the remaining six SQ/TQ motifs in the SCD (Fig. 6). Although we were unable to identify these “minor” sites, by the use of additional anti-phospho antibodies, Ser28 was also found to be phosphorylated in an ATM-dependent manner when cells were damaged by IR (data not shown). This finding indicates that sites other than Thr68 could be phosphorylated by ATM or an unidentified ATM-dependent kinase in vivo.
We have previously shown that phosphatase treatment of Chk2 from IR-treated cells reduces its kinase activity, which is activated upon IR (7). Thus, phosphorylation of Chk2 can activate Chk2 kinase activity. However, we were not able to detect activation of Chk2 when the wild-type GST-Chk2 was phosphorylated by ATM in vitro (data not shown). We may have failed to detect activation of Chk2 in this instance because only a small fraction of the Chk2 was phosphorylated by ATM in this reaction. It is also possible that an additional protein that binds and activates phosphorylated Chk2 in vivo is absent from the in vitro reaction. Alternatively, it is possible that all of the phosphorylation sites necessary for Chk2 activation are not accessible to ATM and therefore are not phosphorylated in vitro when Chk2 is fused to GST and/or ATM is bound to Sepharose beads.
In response to UV or HU treatment, Thr68 is phosphorylated in an ATM-independent manner, indicating phosphorylation of Thr68 by a kinase other than ATM. ATR phosphorylates Chk2 on Thr68 in vitro, and phosphorylation of Thr68 in response to IR occurs later in cells lacking ATM (data not shown). Phosphorylation of Ser15 on p53 in response to IR is also delayed in ATM mutant cells (39), and ATR is suggested to phosphorylate Ser15 in late phase in response to IR (31). Therefore, we believe ATR is likely to phosphorylate Thr68 on Chk2 in vivo. ATR also phosphorylates Thr26 and Ser50 in vitro. Further experiments will be required to determine whether these sites are phosphorylated by ATR in vivo.
Acknowledgments
This paper is dedicated to Dr. Akio Matsukage on the occasion of his 60th birthday. We thank B. Zhou and C. McGowan for sharing their results before publication; M. Kastan and R. Abraham for ATM and ATR expression vectors, respectively; A. Hirao and T. Mak for the Chk2 mutant MEF cells; K. Matsuoka for encouragement and help with figure preparation; and C. Cherry and S. Parker for secretarial support. This work was supported by National Institutes of Health Grant GM44664 and a grant from the Welch foundation (Q1187) to S.J.E. S.J.E. is an Investigator with the Howard Hughes Medical Institute.
Abbreviations
- IR
ionizing radiation
- HU
hydroxyurea
- GST
glutathione S-transferase
- SQ/TQ
Ser-Gln/Thr-Gln
- SCD
SQ/TQ cluster domain
- MEF cells
mouse embryonic fibroblast cells
- WT
wild type
- KD
kinase dead
- ATM
ataxia telangiectasia-mutated
- ATR
ATM- and Rad3-related
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.190030497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.190030497
References
- 1.Elledge S J. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 2.Hartwell L H, Kastan M B. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 3.Rotman G, Shiloh Y. Oncogene. 1999;18:6135–6144. doi: 10.1038/sj.onc.1203124. [DOI] [PubMed] [Google Scholar]
- 4.Allen J B, Zhou Z, Siede W, Friedberg E C, Elledge S J. Genes Dev. 1994;8:2401–2415. doi: 10.1101/gad.8.20.2401. [DOI] [PubMed] [Google Scholar]
- 5.Weinert T A, Kiser G L, Hartwell L H. Genes Dev. 1994;8:652–665. doi: 10.1101/gad.8.6.652. [DOI] [PubMed] [Google Scholar]
- 6.Murakami H, Okayama H. Nature (London) 1995;374:817–819. doi: 10.1038/374817a0. [DOI] [PubMed] [Google Scholar]
- 7.Matsuoka S, Huang M, Elledge S J. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 8.Blasina A, de Weyer I V, Laus M C, Luyten W H, Parker A E, McGowan C H. Curr Biol. 1999;9:1–10. doi: 10.1016/s0960-9822(99)80041-4. [DOI] [PubMed] [Google Scholar]
- 9.Peng C Y, Graves P R, Thoma R S, Wu Z, Shaw A S, Piwnica-Worms H. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 10.Hirao A, Kong Y Y, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge S J, Mak T W. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 11.Cliby W A, Roberts C J, Cimprich K A, Stringer C M, Lamb J R, Schreiber S L, Friend S H. EMBO J. 1998;17:159–169. doi: 10.1093/emboj/17.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright J A, Keegan K S, Herendeen D R, Bentley N J, Carr A M, Hoekstra M F, Concannon P. Proc Natl Acad Sci USA. 1998;95:7445–7450. doi: 10.1073/pnas.95.13.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez Y, Wong C, Thoma R S, Richman R, Wu Z, Piwnica-Worms H, Elledge S J. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 14.Brown E J, Baltimore D. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Q, Guntuku S, Cui X, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, et al. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 16.Takai H, Tominaga K, Motoyama N, Minamishima Y A, Nagahama H, Tsukiyama T, Ikeda K, Nakayama K, Nakanishi M, Nakayama K. Genes Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- 17.Kastan M B, Zhan Q, El-Deiry W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fournace A J., Jr Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 18.Deng C, Zhang P, Harper J W, Elledge S J, Leder P J. Cell. 1995;82:675–680. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 19.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Nature (London) 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 20.Unger T, Juven-Gershon T, Moallem E, Berger M, Vogt Sionov R, Lozano G, Oren M, Haupt Y. EMBO J. 1999;18:1805–1814. doi: 10.1093/emboj/18.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shieh S Y, Taya Y, Prives C. EMBO J. 1999;18:1815–1823. doi: 10.1093/emboj/18.7.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chehab N H, Malikzay A, Stavridi E S, Halazonetis T D. Proc Natl Acad Sci USA. 1999;96:13777–13782. doi: 10.1073/pnas.96.24.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shieh S Y, Ahn J, Tamai K, Taya Y, Prives C. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 24.Chehab N H, Malikzay A, Appel M, Halazonetis T D. Genes Dev. 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- 25.Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 26.Lill N L, Grossmand S R, Ginsberg D, DeCaprio J, Livingston D M. Nature (London) 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 27.Gu W, Roeder R G. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 28.Lambert P F, Kashanchi F, Radonovich M F, Shiekhattar R, Brady J N. J Biol Chem. 1998;273:33048–33053. doi: 10.1074/jbc.273.49.33048. [DOI] [PubMed] [Google Scholar]
- 29.Banin S, Moyal L, Shieh S, Taya Y, Anderson C W, Chessa L, Smorodinsky N I, Prives C, Reiss Y, Shiloh Y, et al. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 30.Canman C E, Lim D S, Cimprich K A, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan M B, Siliciano J D. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 31.Tibbetts R S, Brumbaugh K M, Williams J M, Sarkaria J N, Cliby W A, Shieh S Y, Taya Y, Prives C, Abraham R T. Genes Dev. 1999;13:152–157. doi: 10.1101/gad.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown A L, Lee C H, Schwarz J K, Mitiku N, Piwnica-Worms H, Chung J H. Proc Natl Acad Sci USA. 1999;96:3745–3750. doi: 10.1073/pnas.96.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaturvedi P, Eng W K, Zhu Y, Mattern M R, Mishra R, Hurle M R, Zhang X, Annan R S, Lu Q, Faucette L F, et al. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- 34.Tominaga K, Morisaki H, Kaneko Y, Fujimoto A, Tanaka T, Ohtsubo M, Hirai M, Okayama H, Ikeda K, Nakanishi M. J Biol Chem. 1999;274:31463–31467. doi: 10.1074/jbc.274.44.31463. [DOI] [PubMed] [Google Scholar]
- 35.Morgenstern J P, Land H. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinsella T M, Nolan G P. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- 37.Cortez D, Wang Y, Qin J, Elledge S J. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 38.Ziv Y, Bar-Shira A, Pecker I, Russell P, Jorgensen T J, Tsarfati I, Shiloh Y. Oncogene. 1997;15:159–167. doi: 10.1038/sj.onc.1201319. [DOI] [PubMed] [Google Scholar]
- 39.Siliciano J D, Canman C E, Taya Y, Sakaguchi K, Appella E, Kastan M B. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]