Abstract
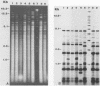
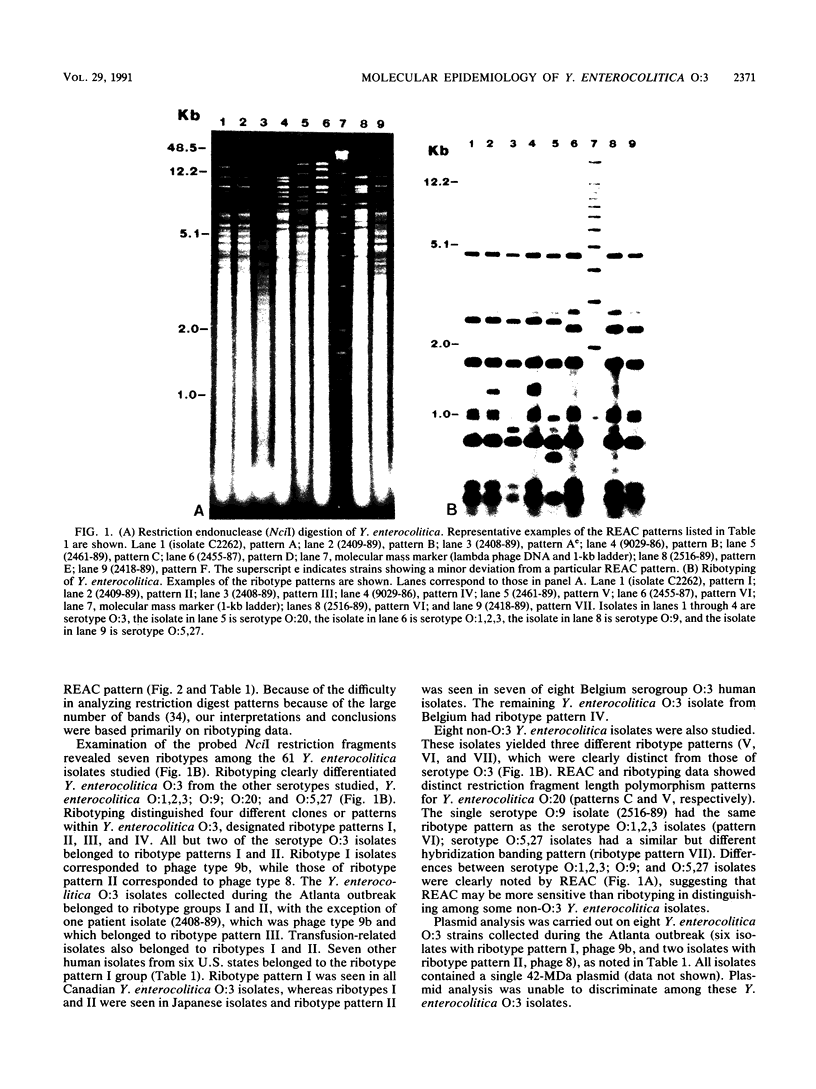
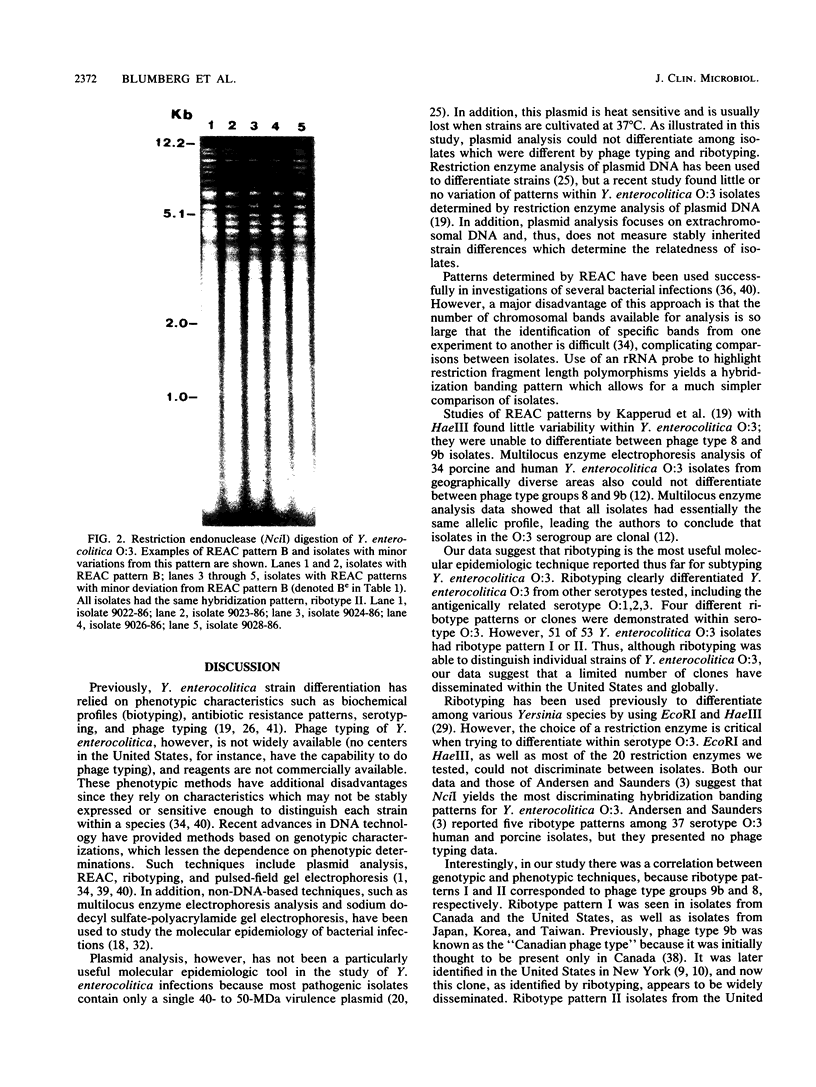
Yersinia enterocolitica is a major enteric pathogen associated with a wide variety of clinical and immunologic manifestations, including transfusion-associated disease, from which there is a high mortality. Although previously rare in the United States, in the late 1980s Y. enterocolitica O:3 emerged as the predominant serotype in the United States, as it has been in Canada, Europe, and Japan. Epidemiologic investigation of this serogroup has been hampered by the limited availability of a phage typing system and the fact that Y. enterocolitica harbors few plasmids that are useful as strain markers. We therefore analyzed whole-cell DNA restriction fragment length polymorphisms of rRNA genes (ribotyping) to study a group of 61 (50 human, 11 porcine) Y. enterocolitica isolates. Initially, 20 different restriction enzymes were used: NciI appeared to give the best discrimination of hybridization banding patterns (ribotypes) within Y. enterocolitica O:3. Ribotyping distinguished seven clones among all the study isolates and four clones within Y. enterocolitica O:3 (53 isolates studied) and clearly differentiated Y. enterocolitica O:3 from Y. enterocolitica O:9; O:1,2,3; O:20; and O:5,27. Most serogroup O:3 isolates belonged to two clones, ribotypes I and II, including 23 of 24 Y. enterocolitica O:3 (13 human, 11 porcine chitterling) isolates recovered from a recent outbreak of Y. enterocolitica in children in Atlanta associated with chitterling preparation and 3 transfusion-associated O:3 isolates from the United States. Y. enterocolitica O:3 ribotypes I and II were also isolated in Japan, ribotypes II and IV were isolated in Belgium, and ribotype I was isolated in Canada. Ribotype patterns I and II corresponded to phage types 9b and 8, respectively. Ribotyping was able to distinguish individual strains of Y. enterocolitica O:3, but suggests that a limited number of clones have disseminated within the United States and globally. The finding of identical ribotype patterns in chitterling and human specimens from the Atlanta outbreak supports epidemiologic evidence that swine were the source of infection and major reservoir for Y. enterocolitica O:3.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Bouziges N., Carles-Nurit M. J., Bourg G., Gouby A., Ramuz M. Use of low-frequency-cleavage restriction endonucleases for DNA analysis in epidemiological investigations of nosocomial bacterial infections. J Clin Microbiol. 1989 Sep;27(9):2057–2061. doi: 10.1128/jcm.27.9.2057-2061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altwegg M., Hickman-Brenner F. W., Farmer J. J., 3rd Ribosomal RNA gene restriction patterns provide increased sensitivity for typing Salmonella typhi strains. J Infect Dis. 1989 Jul;160(1):145–149. doi: 10.1093/infdis/160.1.145. [DOI] [PubMed] [Google Scholar]
- Andersen J. K., Saunders N. A. Epidemiological typing of Yersinia enterocolitica by analysis of restriction fragment length polymorphisms with a cloned ribosomal RNA gene. J Med Microbiol. 1990 Jul;32(3):179–187. doi: 10.1099/00222615-32-3-179. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett M. L., Powers C., Abbott S. L., Janda J. M. Epidemiologic investigations of Yersinia enterocolitica and related species: sources, frequency, and serogroup distribution. J Clin Microbiol. 1990 May;28(5):910–912. doi: 10.1128/jcm.28.5.910-912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjune G., Ruud T. E., Eng J. Bacterial shock due to transfusion with Yersinia enterocolitica infected blood. Scand J Infect Dis. 1984;16(4):411–412. doi: 10.3109/00365548409073970. [DOI] [PubMed] [Google Scholar]
- Boelaert J. R., van Landuyt H. W., Valcke Y. J., Cantinieaux B., Lornoy W. F., Vanherweghem J. L., Moreillon P., Vandepitte J. M. The role of iron overload in Yersinia enterocolitica and Yersinia pseudotuberculosis bacteremia in hemodialysis patients. J Infect Dis. 1987 Aug;156(2):384–387. doi: 10.1093/infdis/156.2.384. [DOI] [PubMed] [Google Scholar]
- Bottone E. J. Current trends of Yersinia enterocolitica isolates in the New York City area. J Clin Microbiol. 1983 Jan;17(1):63–67. doi: 10.1128/jcm.17.1.63-67.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottone E. J., Gullans C. R., Sierra M. F. Disease spectrum of Yersinia enterocolitica serogroup 0:3, the predominant cause of human infection in New York City. Contrib Microbiol Immunol. 1987;9:56–60. [PubMed] [Google Scholar]
- Bufill J. A., Ritch P. S. Yersinia enterocolitica serotype 0:3 sepsis after blood transfusion. N Engl J Med. 1989 Mar 23;320(12):810–810. doi: 10.1056/NEJM198903233201220. [DOI] [PubMed] [Google Scholar]
- Caugant D. A., Aleksic S., Mollaret H. H., Selander R. K., Kapperud G. Clonal diversity and relationships among strains of Yersinia enterocolitica. J Clin Microbiol. 1989 Dec;27(12):2678–2683. doi: 10.1128/jcm.27.12.2678-2683.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S. G. The Yersinia enterocolitica situation in Denmark. Contrib Microbiol Immunol. 1987;9:93–97. [PubMed] [Google Scholar]
- Cover T. L., Aber R. C. Yersinia enterocolitica. N Engl J Med. 1989 Jul 6;321(1):16–24. doi: 10.1056/NEJM198907063210104. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Irino K., Grimont F., Casin I., Grimont P. A. rRNA gene restriction patterns of Haemophilus influenzae biogroup aegyptius strains associated with Brazilian purpuric fever. J Clin Microbiol. 1988 Aug;26(8):1535–1538. doi: 10.1128/jcm.26.8.1535-1538.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J. F., Jr Molecular analysis of nosocomial epidemics. Infect Dis Clin North Am. 1989 Dec;3(4):683–700. [PubMed] [Google Scholar]
- Kapperud G., Nesbakken T., Aleksic S., Mollaret H. H. Comparison of restriction endonuclease analysis and phenotypic typing methods for differentiation of Yersinia enterocolitica isolates. J Clin Microbiol. 1990 Jun;28(6):1125–1131. doi: 10.1128/jcm.28.6.1125-1131.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay B. A., Wachsmuth K., Gemski P., Feeley J. C., Quan T. J., Brenner D. J. Virulence and phenotypic characterization of Yersinia enterocolitica isolated from humans in the United States. J Clin Microbiol. 1983 Jan;17(1):128–138. doi: 10.1128/jcm.17.1.128-138.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehlbauch J. A., Plikaytis B. D., Swaminathan B., Cameron D. N., Wachsmuth I. K. Restriction fragment length polymorphisms in the ribosomal genes for species identification and subtyping of aerotolerant Campylobacter species. J Clin Microbiol. 1991 Aug;29(8):1670–1676. doi: 10.1128/jcm.29.8.1670-1676.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. A., Gerber A. R., Lonsway D. R., Smith J. D., Carter G. P., Puhr N. D., Parrish C. M., Sikes R. K., Finton R. J., Tauxe R. V. Yersinia enterocolitica O:3 infections in infants and children, associated with the household preparation of chitterlings. N Engl J Med. 1990 Apr 5;322(14):984–987. doi: 10.1056/NEJM199004053221407. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Stull T. L., Dasen S. E., Pidcock K. A., Kaye D., Korzeniowski O. M. DNA polymorphisms among Escherichia coli isolated from bacteriuric women. J Infect Dis. 1989 Mar;159(3):526–532. doi: 10.1093/infdis/159.3.526. [DOI] [PubMed] [Google Scholar]
- Maruyama T. Yersinia enterocolitica infection in humans and isolation of the microorganism from pigs in Japan. Contrib Microbiol Immunol. 1987;9:48–55. [PubMed] [Google Scholar]
- Nesbakken T., Kapperud G., Sørum H., Dommarsnes K. Structural variability of 40-50 Mdal virulence plasmids from Yersinia enterocolitica. Geographical and ecological distribution of plasmid variants. Acta Pathol Microbiol Immunol Scand B. 1987 Jun;95(3):167–173. doi: 10.1111/j.1699-0463.1987.tb03107.x. [DOI] [PubMed] [Google Scholar]
- Olson D. N., Finch W. R. Reactive arthritis associated with Yersinia enterocolitica gastroenteritis. Am J Gastroenterol. 1981 Dec;76(6):524–526. [PubMed] [Google Scholar]
- Owen R. J., Beck A., Dayal P. A., Dawson C. Detection of genomic variation in Providencia stuartii clinical isolates by analysis of DNA restriction fragment length polymorphisms containing rRNA cistrons. J Clin Microbiol. 1988 Oct;26(10):2161–2166. doi: 10.1128/jcm.26.10.2161-2166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard-Pasquier N., Picard B., Heeralal S., Krishnamoorthy R., Goullet P. Correlation between ribosomal DNA polymorphism and electrophoretic enzyme polymorphism in Yersinia. J Gen Microbiol. 1990 Aug;136(8):1655–1666. doi: 10.1099/00221287-136-8-1655. [DOI] [PubMed] [Google Scholar]
- Salvi R. J., Ahroon W., Saunders S. S., Arnold S. A. Evoked potentials: computer-automated threshold-tracking procedure using an objective detection criterion. Ear Hear. 1987 Jun;8(3):151–156. [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Tauxe R. V., Vandepitte J., Wauters G., Martin S. M., Goossens V., De Mol P., Van Noyen R., Thiers G. Yersinia enterocolitica infections and pork: the missing link. Lancet. 1987 May 16;1(8542):1129–1132. doi: 10.1016/s0140-6736(87)91683-7. [DOI] [PubMed] [Google Scholar]
- Tenover F. C. Diagnostic deoxyribonucleic acid probes for infectious diseases. Clin Microbiol Rev. 1988 Jan;1(1):82–101. doi: 10.1128/cmr.1.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipple M. A., Bland L. A., Murphy J. J., Arduino M. J., Panlilio A. L., Farmer J. J., 3rd, Tourault M. A., Macpherson C. R., Menitove J. E., Grindon A. J. Sepsis associated with transfusion of red cells contaminated with Yersinia enterocolitica. Transfusion. 1990 Mar-Apr;30(3):207–213. doi: 10.1046/j.1537-2995.1990.30390194338.x. [DOI] [PubMed] [Google Scholar]
- Wachsmuth I. K., Kiehlbauch J. A., Bopp C. A., Cameron D. N., Strockbine N. A., Wells J. G., Blake P. A. The use of plasmid profiles and nucleic acid probes in epidemiologic investigations of foodborne, diarrheal diseases. Int J Food Microbiol. 1991 Jan;12(1):77–89. doi: 10.1016/0168-1605(91)90049-u. [DOI] [PubMed] [Google Scholar]
- Wachsmuth K. Genotypic approaches to the diagnosis of bacterial infections: plasmid analyses and gene probes. Infect Control. 1985 Mar;6(3):100–109. doi: 10.1017/s0195941700062767. [DOI] [PubMed] [Google Scholar]