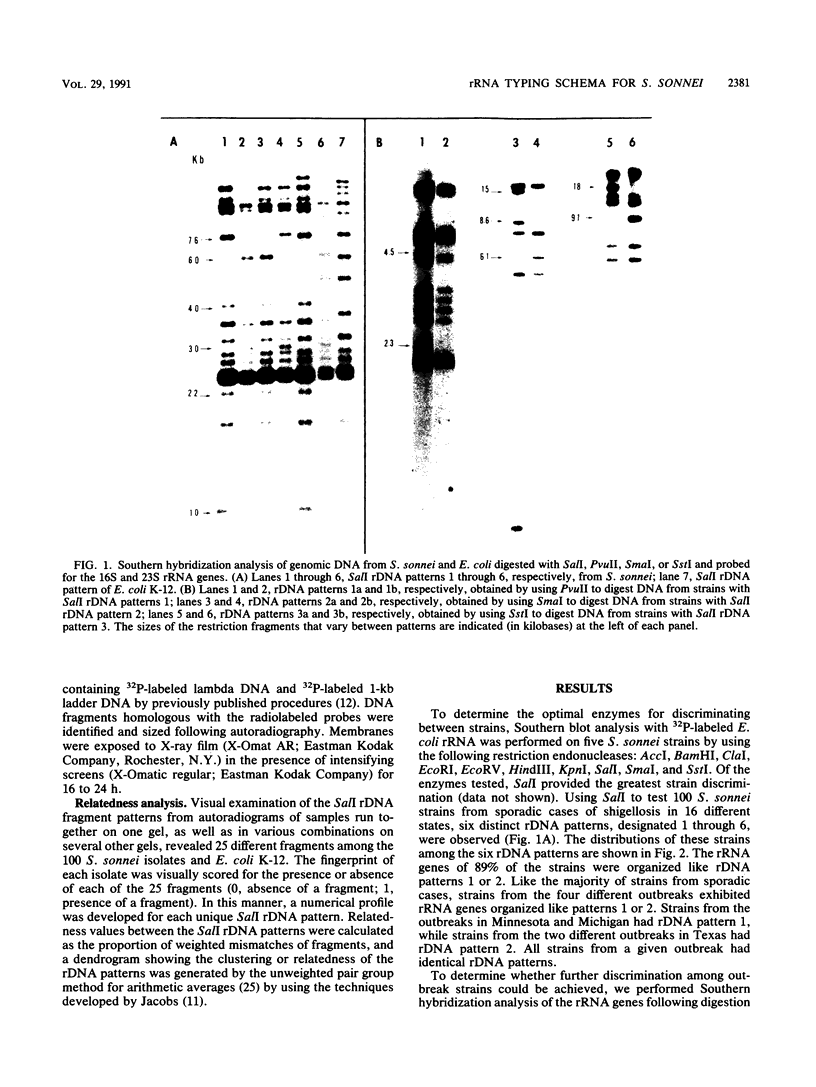
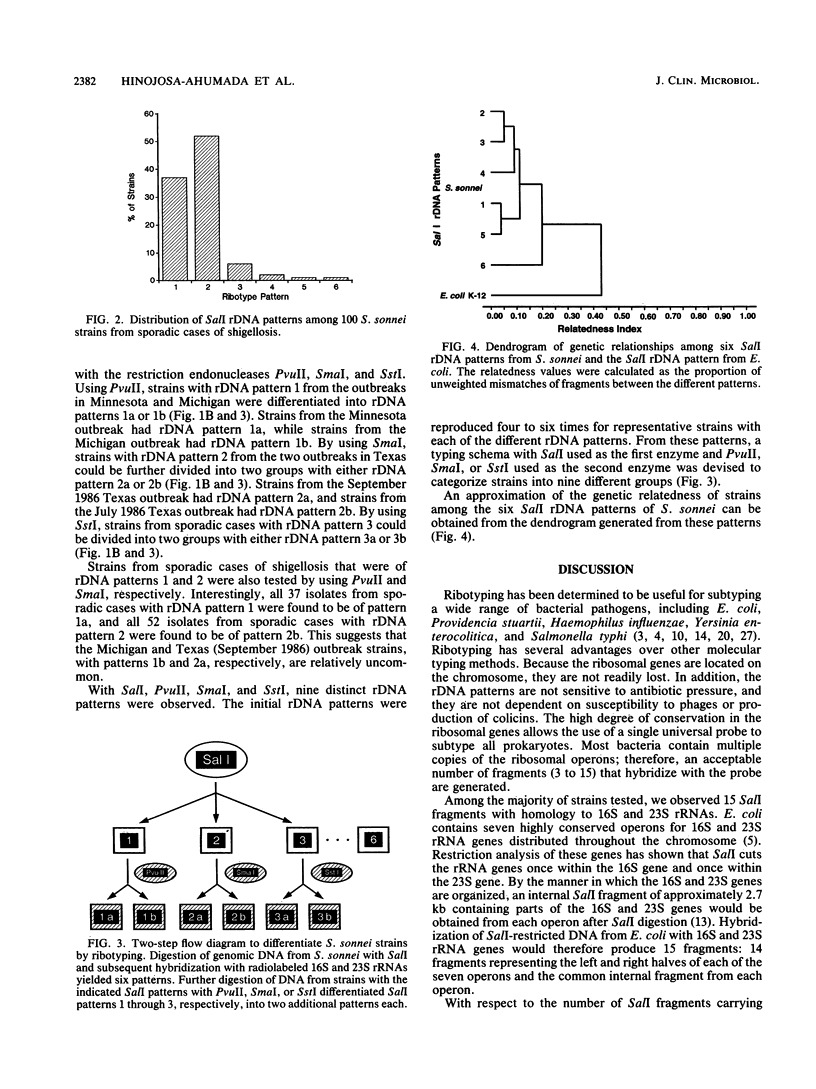
Abstract
Shigella sonnei is the most frequent cause of shigellosis in the United States. Epidemiologic studies of this organism have been hampered by the lack of adequate typing procedures. Ribosomal DNA analysis (ribotyping), a method which analyzes restriction fragment length polymorphisms in the chromosomal genes that encode rRNA, has recently been shown to be useful for microbial species identification and subtyping. To determine whether ribotyping could be used to distinguish between S. sonnei isolates, we conducted Southern hybridization studies on isolates from 16 different geographic locations and from four recent outbreaks. S. sonnei genomic DNA fragments generated following digestion with SalI hybridized with Escherichia coli 16S and 23S rRNAs to produce six distinct patterns; strains with patterns 1, 2, and 3 were each further subdivided into two additional patterns by using PvuII, SmaI, and SstI, respectively. Epidemiologically related strains had identical patterns. Ribotyping appears to be a useful tool for epidemiologic studies of shigellosis caused by S. sonnei.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT J. D., SHANNON R. A method for typing Shigella sonnei, using colicine production as a marker. J Clin Pathol. 1958 Jan;11(1):71–77. doi: 10.1136/jcp.11.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altwegg M., Altwegg-Bissig R., Demarta A., Peduzzi R., Reeves M. W., Swaminathan B. Comparison of four typing methods for Aeromonas species. J Diarrhoeal Dis Res. 1988 Jun;6(2):88–94. [PubMed] [Google Scholar]
- Altwegg M., Hickman-Brenner F. W., Farmer J. J., 3rd Ribosomal RNA gene restriction patterns provide increased sensitivity for typing Salmonella typhi strains. J Infect Dis. 1989 Jul;160(1):145–149. doi: 10.1093/infdis/160.1.145. [DOI] [PubMed] [Google Scholar]
- Andersen J. K., Saunders N. A. Epidemiological typing of Yersinia enterocolitica by analysis of restriction fragment length polymorphisms with a cloned ribosomal RNA gene. J Med Microbiol. 1990 Jul;32(3):179–187. doi: 10.1099/00222615-32-3-179. [DOI] [PubMed] [Google Scholar]
- Boros I., Kiss A., Venetianer P. Physical map of the seven ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1979;6(5):1817–1830. doi: 10.1093/nar/6.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H., Taylor J. P., Perdue J. N., Stelma G. N., Jr, Humphreys J. M., Jr, Rowntree R., 3rd, Greene K. D. A shigellosis outbreak traced to commercially distributed shredded lettuce. Am J Epidemiol. 1988 Dec;128(6):1312–1321. doi: 10.1093/oxfordjournals.aje.a115084. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Irino K., Grimont F., Casin I., Grimont P. A. rRNA gene restriction patterns of Haemophilus influenzae biogroup aegyptius strains associated with Brazilian purpuric fever. J Clin Microbiol. 1988 Aug;26(8):1535–1538. doi: 10.1128/jcm.26.8.1535-1538.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehlbauch J. A., Plikaytis B. D., Swaminathan B., Cameron D. N., Wachsmuth I. K. Restriction fragment length polymorphisms in the ribosomal genes for species identification and subtyping of aerotolerant Campylobacter species. J Clin Microbiol. 1991 Aug;29(8):1670–1676. doi: 10.1128/jcm.29.8.1670-1676.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A., Sain B., Venetianer P. The number of rRNA genes in Escherichia coli. FEBS Lett. 1977 Jul 1;79(1):77–79. doi: 10.1016/0014-5793(77)80354-2. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Stull T. L., Dasen S. E., Pidcock K. A., Kaye D., Korzeniowski O. M. DNA polymorphisms among Escherichia coli isolated from bacteriuric women. J Infect Dis. 1989 Mar;159(3):526–532. doi: 10.1093/infdis/159.3.526. [DOI] [PubMed] [Google Scholar]
- Litwin C. M., Storm A. L., Chipowsky S., Ryan K. J. Molecular epidemiology of Shigella infections: plasmid profiles, serotype correlation, and restriction endonuclease analysis. J Clin Microbiol. 1991 Jan;29(1):104–108. doi: 10.1128/jcm.29.1.104-108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza M. C., Gonzalez A. J., Mendez F. J., Hardisson C. Plasmid typing of Shigella sonnei epidemic strains and molecular relationship of their R-plasmids. Eur J Epidemiol. 1988 Jun;4(2):158–163. doi: 10.1007/BF00144744. [DOI] [PubMed] [Google Scholar]
- Morris G. K., Wells J. G. Colicin typing of Shigella sonnei. Appl Microbiol. 1974 Feb;27(2):312–316. doi: 10.1128/am.27.2.312-316.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Whittam T. S., Caugant D. A., Selander R. K. Enzyme polymorphism and genetic population structure in Escherichia coli and Shigella. J Gen Microbiol. 1983 Sep;129(9):2715–2726. doi: 10.1099/00221287-129-9-2715. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Beck A., Dayal P. A., Dawson C. Detection of genomic variation in Providencia stuartii clinical isolates by analysis of DNA restriction fragment length polymorphisms containing rRNA cistrons. J Clin Microbiol. 1988 Oct;26(10):2161–2166. doi: 10.1128/jcm.26.10.2161-2166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Borman P. A rapid biochemical method for purifying high molecular weight bacterial chromosomal DNA for restriction enzyme analysis. Nucleic Acids Res. 1987 Apr 24;15(8):3631–3631. doi: 10.1093/nar/15.8.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruneda R. C., Farmer J. J., 3rd Bacteriophage typing of Shigella sonnei. J Clin Microbiol. 1977 Jan;5(1):66–74. doi: 10.1128/jcm.5.1.66-74.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve G., Martin D. L., Pappas J., Thompson R. E., Greene K. D. An outbreak of shigellosis associated with the consumption of raw oysters. N Engl J Med. 1989 Jul 27;321(4):224–227. doi: 10.1056/NEJM198907273210404. [DOI] [PubMed] [Google Scholar]
- Slopek S., Durlakowa I., Kucharewicz-Krubkowska A., Krzywy T., Slopek A., Weber B. Phage typing of Shigella sonnei. Arch Immunol Ther Exp (Warsz) 1973;21(1):1–161. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Tacket C. O., Cohen M. L. Shigellosis in day care centers: use of plasmid analysis to assess control measures. Pediatr Infect Dis. 1983 Mar-Apr;2(2):127–130. [PubMed] [Google Scholar]
- Yogev D., Halachmi D., Kenny G. E., Razin S. Distinction of species and strains of mycoplasmas (mollicutes) by genomic DNA fingerprints with an rRNA gene probe. J Clin Microbiol. 1988 Jun;26(6):1198–1201. doi: 10.1128/jcm.26.6.1198-1201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]