Abstract
The traditional sex morbidity-mortality paradox that females have worse health but better survival than males is based on studies of major health traits. We applied a cumulative deficits approach to study this paradox, selecting 34 minor health deficits consistently measured in the 9th (1964) and 14th (1974) Framingham Heart and 5th (1991–1995) Offspring Study exams focusing on the 55–78 age range. We constructed four deficit indices (DIs) using all 34 deficits as well as subsets of these deficits characterizing males’ (DIM) and females’ (DIF) health disadvantages, and no relative sex-disadvantages. The DI34-specific age patterns are sex-insensitive within the 55-to-74 age range. The DI34, however, tends to selectively increase the risk of death for males. The DIF-associated health dimension supports the traditional morbidity paradox, whereas the DIM-associated dimension supports the inverse paradox, wherein males have worse health but better survival than females. The traditional paradox became less pronounced, whereas the inverse paradox became more pronounced from the 1960s to the 1990s. The sex-specific excess in minor health deficits may vary according to particular set of deficits, thus providing evidence for traditional and inverse morbidity paradoxes. The time-trends suggest the presence of a strong exogenous effect modifier affecting the rate of health deterioration and mortality risk.
Keywords: Sex differences, mortality, morbidity paradox, Framingham Heart Study
INTRODUCTION
Females tend to have worse health than males, but they live longer than males. This traditional sex morbidity-mortality paradox was noted in the mid 1970s (Nathanson, 1975; Verbrugge, 1982). From that time, this paradox has been further studied by focusing on various aspects of health and the effect of socio-demographic factors (see, e.g., (Arber and Cooper, 1999; Case and Paxson, 2005; Idler and Benyamini, 1997; Lawlor et al., 2001; MacIntyre et al., 1999; Verbrugge and Wingard, 1987a; b)).
The most common explanations of this paradox are based on differences in health and health behaviors, i.e., biological risks; the risks acquired through life-style-related behaviors, illness behavior, health reporting behavior, and differences in the health care utilization (MacIntyre et al., 1999; Verbrugge, 1989). Biological vulnerability and acquired risks are typical explanations when individuals are examined in clinical or laboratory settings (Verbrugge, 1982). The other three potential explanations listed above are typically used to explain sex differentials observed in surveys wherein data on health behaviors are self-reported.
Numerous studies (see e.g., (Gorman and Read, 2006) and references therein) suggest that sex differences may vary by health dimensions reflecting the process of population health deterioration (a simplified pathway of health worsening can be sketched as: pre-disease → pathology → loss of functioning → disability → death (Crimmins, 2004; Verbrugge and Jette, 1994)). Most population-based studies have focused on major health-related dimensions including self-perceived health, functional limitations, selected diseases, as well as health-care utilization and hospitalization (see, e.g., (Case and Paxson, 2005; Gorman and Read, 2006; Idler and Benyamini, 1997; Verbrugge, 1982)). Explanations of males’ excessive morbidity typically emphasize greater biological vulnerability and lower health care utilization (MacIntyre et al., 1999; Verbrugge, 1989). The excessive morbidity of females is usually attributed to illness (e.g., diabetes, heart diseases, hypertension) and health reporting behaviors (Verbrugge, 1982; 1989). Despite these studies, however, the nature of this paradox is still uncertain.
Further insights into the nature of the morbidity-mortality paradox can be gained by analyzing “minor-effect” health traits associated with the least severe health dimensions, i.e., with the pre-disease state (e.g., signs, symptoms) and certain diseases. Such analyses can shed light on possible sex differentials in pre-disease conditions that may lead to sex-targeted interventions to prevent the development of major health problems. Relatively few population-based studies, however, have focused on such traits (see (MacIntyre et al., 1999; Verbrugge, 1982) and references therein). These studies basically examined the prevalence of problem-oriented symptoms (e.g., symptoms related to headaches (Celentano et al., 1990)). They found that rates of morbidity for minor-effect health traits tend to be higher in females (Emslie et al., 1999), although the sex-specific excess in such traits may vary according to the particular setting of problems (MacIntyre et al., 1996). These studies, however, rarely examined associations of those minor traits with mortality. The challenge facing studies of such associations is the large number of various minor health traits and the small, insignificant, or inconsistent effect of each on mortality risks. The aggregate effect of several such minor traits, however, might be more informative. This is the underlying paradigm of recent developments of a promising new instrument, which is called a frailty index (Goggins et al., 2005; Mitnitski et al., 2001; Rockwood and Mitnitski, 2007) or an index of cumulative deficits (Kulminski et al., 2007c; Yashin et al., 2007a). The concept of a cumulative health deficits index (DI) appears to be useful for examining possible sex health differentials on the level of minor health traits.
This study uses the concept of the DI to address the following three major research questions: i) Are there differences in the prevalence of morbidity associated with minor health traits (e.g., signs, symptoms, health conditions with non-significant effect on mortality, etc.) between the sexes? ii) How do those minor health dimensions affect the mortality risks for each sex? and iii) Are there time trends in the morbidity paradox? To address these questions, we studied the health status of a sample of adult and elderly individuals participating in the Framingham Heart Study [FHS] and Offspring [FHSO] using the new instrument, the DI, which aggregates minor health-related variables routinely collected during the 1960-to-1990 period.
METHODS
The FHS and FHSO data
Beginning in 1948, 5,209 respondents (46% male) aged 28–62 years residing in Framingham, Massachusetts were enrolled in the FHS. The FHSO dataset consists of a sample of 3,514 biological descendants of the FHS cohort, 1,576 of their spouses and 34 adopted offspring for a total sample of 5,124 subjects; 48% male. The FHSO subjects were enrolled in 1971–1975 using research protocols similar to those of the FHS so that comparisons of the results from the FHSO and the FHS could be made. Selection criteria and study design have been described (Dawber et al., 1963; Kannel et al., 1979). These cohorts have been followed for the occurrence of certain diseases (e.g., heart disease, cancer, diabetes mellitus) and death. The periodic examinations included an interview, physical examination, and laboratory tests.
The cumulative deficits approach
In traditional analyses, the effects of minor health traits which exhibit small, inconsistent, or non-significant contributions to risks of adverse health outcomes or mortality are usually ignored. When the number of such traits is large enough, however, their cumulative effect on chances of future adverse events may become consistently significant and, thus, an integrative or cumulative measure (i.e., the DI) might be more informative compared to individual traits (Kulminski et al., 2007a; 2007b; 2007c; Yashin et al., 2007a; b). Consequently, in this study we used an approach of cumulative deficits to characterize health of individuals on the level of minor health traits. The DI was constructed as a proportion of health/well-being traits with identified deficiencies observed for an individual out of a list of all potential problems for which an individual was examined. For instance, an individual has been examined on 30 potential problems. He/she had 5 problems and for 4 potential traits information was missing. Then, the DI for the given person is 5/(30−4)=5/26. Thus, the DI theoretically ranges between 0 and 1, or, equivalently, between 0 and 100%. For this study we selected all so-called minor health-related traits which were consistently assessed in each exam and were associated with health dimensions which characterized pre-disease state (e.g., signs, symptoms) as well as certain diseases not associated with significant risks of death in this sample (e.g., pulmonary and thyroid).
Analyses
Construction of the DIs and analyses of possible sex differences in the age-specific health deterioration characterized by the respective age patterns of the DIs, as well as time trends in such patterns, are constrained by several factors. First, ideally, the DIs should be constructed using a wide set of heath-related conditions (see the previous section). Second, the range of intersecting ages should be as large as possible to better capture the age-specifics. Third, survey instruments have to be comparable over time. Fourth, the surveys/exams should be well separated in time. Finally, selected samples have to be of adequate size. To address all these constraints, the same set of 34 deficits (Table 1) with comparable diagnostic procedures across all years was selected. The selected deficits were either consistently dichotomized (yes or no deficit) or rescaled to the unit interval to reflect the degree of abnormality, e.g., the urinary sugar level was recoded as negative (0 or no deficit), doubtful (0.5) and positive (1 or yes).
Table 1.
List and prevalence of 34 deficits used in the analyses
| Group | N | Deficit | Prevalence*, % |
Group | N | Deficit | Prevalence*, % |
||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | ||||||
| 1 | 1 | chronic cough | 13.9 | 11.1 | 2 | 6 | dyspnea or exertion | 23.1 | 27.4 |
| 2 | trouble with wheezing | 11.5 | 9.0 | 7 | xanthelasma | 1.4 | 2.5 | ||
| 3 | discomfort in lower limbs while walking | 7.3 | 5.0 | 8 | thyroid exam: scar | 1.0 | 4.5 | ||
| 4 | peripheral pulses: dorsal pedis | 10.7 | 6.2 | 9 | thyroid exam: diffuse enlargement | 0.4 | 2.5 | ||
| 5 | premature beats on ECG | 9.3 | 6.1 | 10 | thyroid exam: single nodule | 0.5 | 1.3 | ||
| 6 | urinary sugar | 3.3 | 1.8 | 11 | thyroid exam: multiple nodules | 0.6 | 1.0 | ||
| 7 | arcus senilis | 35.5 | 27.0 | 12 | other manifestation of thyroid disease | 0.1 | 0.7 | ||
| 8 | respiratory exam: increased antero- posterior diameter | 7.4 | 2.8 | 13 | left ankle edema | 5.9 | 10.8 | ||
| 9 | rales | 3.2 | 1.7 | 14 | right ankle edema | 5.4 | 10.1 | ||
| 10 | abnormal breath sounds | 8.1 | 3.7 | 3 | 1 | paroxysmal nocturnal dyspnea | 1.6 | 1.5 | |
| 11 | liver enlarged | 3.7 | 1.3 | 2 | frequent coldness in one hand/foot | 2.2 | 2.1 | ||
| 12 | pulmonary disease | 8.7 | 5.5 | 3 | peripheral pulses: radial | 0.6 | 0.6 | ||
| 2 | 1 | increase in dyspnea | 8.4 | 10.4 | 4 | peripheral pulses: femoral | 1.4 | 1.2 | |
| 2 | orthopnea | 1.9 | 3.0 | 5 | peripheral pulses: posterior tibial | 8.8 | 7.7 | ||
| 3 | ankle edema | 5.5 | 18.9 | 6 | chest discomfort | 18.9 | 16.9 | ||
| 4 | venous insufficiency or varicose veins | 9.9 | 16.7 | 7 | xanthomata | 0.2 | 0.0 | ||
| 5 | abnormal heart sounds | 4.3 | 5.3 | 8 | distended neck veins | 0.3 | 0.2 | ||
Group 1 (2): prevalence of the deficits in this group is significantly higher for males (females) than for females (males) Group 3: there is no significant difference in the prevalence of the deficits in this group between sexes
A health condition was dichotomized as presence of trait vs. no such a trait.
The deficits were selected from two representative exams of the FHS (the 9th FHS exam performed in 1964; N=3833; age range: 44–78 years; mean age (MA) ± standard error=59.0±0.13 years and the 14th FHS exam performed in 1974; N=2871; age range: 55–88 years; MA=67.5±0.14) and one representative exam of the FHSO (5th FHSO exam performed in 1991–1995; N=3799; age range: 31–78 years; MA=55.0±0.16). Because we have to compare the same age groups, the age range in all analyses was limited to that which was common for all exams, i.e., from 55 to 78 years. This considerably reduced samples: N=2437 (MA=64.0±0.12) for the 9th FHS; N=2554 (MA=65.7±0.12) for the 14th FHS, and N=1902 (MA=63.3±0.13) for the 5th FHSO exams. To ensure that missing answers do not bias the weight of deficits over time, all analyses were performed with individuals for whom information on any of the selected 34 deficits was missing excluded. These yielded samples of Nmales=916 (MA=63.8±0.20) and Nfemales=1258 (MA=64.1±0.17) for the 9th FHS; Nmales=1041 (MA=65.2±0.19) and Nfemales=1432 (MA=65.9±0.16) for the 14th FHS, and Nmales=711 (MA=63.2±0.21) and Nfemales=732 (MA=63.1±0.20) for the 5th FHSO exams. By comparing the resulting estimates with those for the complete sample (i.e., without exclusion of individuals for whom information on deficits was missing), we verified that this exclusion did not bias the results.
Sex differences in the DIs were assessed for 5-year age segments. First, we evaluated the sex-specific age patterns for the DI constructed using set of all 34 deficits. Then we compared the mean values of individual deficits for males and females and classified them according to their relative prevalence. The most common deficits for males were gathered into group 1 (see selection criteria in footnote of Table 1). The most common deficits for females comprised group 2. Deficits which showed no statistically significant difference between sexes were gathered into group 3. Accordingly, three deficit-specific DIs (DIM, DIF, and DIN) were constructed and used in our analyses.
Cox proportional hazard regression models were used to evaluate the effects of the four constructed DIs—all as measured in the baseline exams—on the hazard of death considering deaths that occurred within the maximum follow-up period for the 5th FHSO exam, i.e., within 6 years (the last known vital status assessment was at the 6th FHSO exam performed in 1996–1997 and the 25th FHS exam performed in 1998). The regression models were estimated for 10% increases in the respective DIs and were age adjusted.
RESULTS
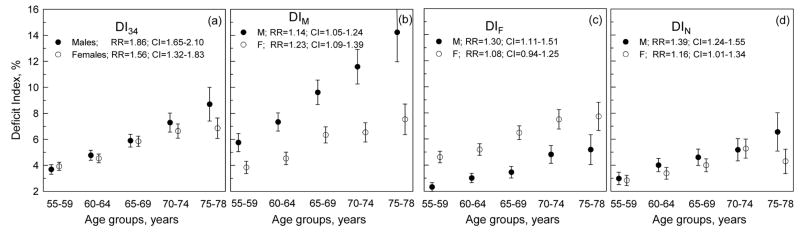
For the pooled sample of participants of the 9th and 14th FHS and 5th FHSO exams, Figure 1a shows no significant differences in the mean values of the DI constructed using a set of all 34 deficits (DI34) except in the last age group (75–78 years). For certain age groups, however, the DI34 for males tend to be higher than for females. Disaggregation of the DI34 into the DIs associated with males’ health disadvantages (i.e., DIM; prevalence of individual deficits is significantly higher for males than for females, Table 1, group 1), females’ health disadvantages (i.e., DIF; prevalence of individual deficits is significantly higher for females than for males, Table 1, group 2), and no relative disadvantage between sexes (i.e., DIN; there is no significant differences in the prevalence of individual deficits between sexes, Table 1, group 3) disentangles qualitatively different behaviors associated with the respective health dimensions (Figures 1b–d). Specifically, the DIM-specific age pattern is significantly higher for males than for females. The opposite situation is seen for the DIF-specific age pattern. Although there is no significant difference in the mean vales of the DIN in the respective 5-year age groups (except the 75–78 age group), the mean DIN values tend to be systematically higher for males than for females.
Figure 1.
The sex-specific age patterns of the deficit indices constructed using the set of (a) all 34 deficits used in the analyses (DI34) as well as selected sub-sets of deficits (see Table 1), i.e., (b) DIM, (c) DIF, and (d) DIN for the pooled sample of participants of the 9th (1964) and 14th (1974) FHS and 5th (1991–1995) FHSO exams. Bars show 95% Confidence Intervals (CIs). Insets show the sex-specific relative risks (RRs) of death for 10% increase of the respective deficit index for all ages combined.
Do the respective DIs contribute selectively to the mortality risks for males and females? To address this question, we evaluated the relative risks of death attributable to the DI34 as well as to the DIM, DIF, and DIN in Cox regression analyses of the pooled sample. The analyses were performed for all ages combined to increase statistical power and separately for the DI34 and the other three DIs. In the latter case, the DIM, DIF, and DIN were included into the model all together. The relative risk attributable to the DI34 (i.e., to the entire set of deficits) tends to be higher for males than for females (Figure 1a, inset). The same is observed for the DIN except the fact that the respective relative risk is highly significant for males, while it is barely significant for females. Despite worse health for males as characterized by the DIM, the relative risk attributable to this index tends to be higher for females than for males (although this difference is not significant; see Figure 1b, inset). Figure 1c exhibits opposite situation, i.e., males have better health, as characterized by the DIF, although the respective relative risk is significant for males while the DIF does not increase the risk for females.
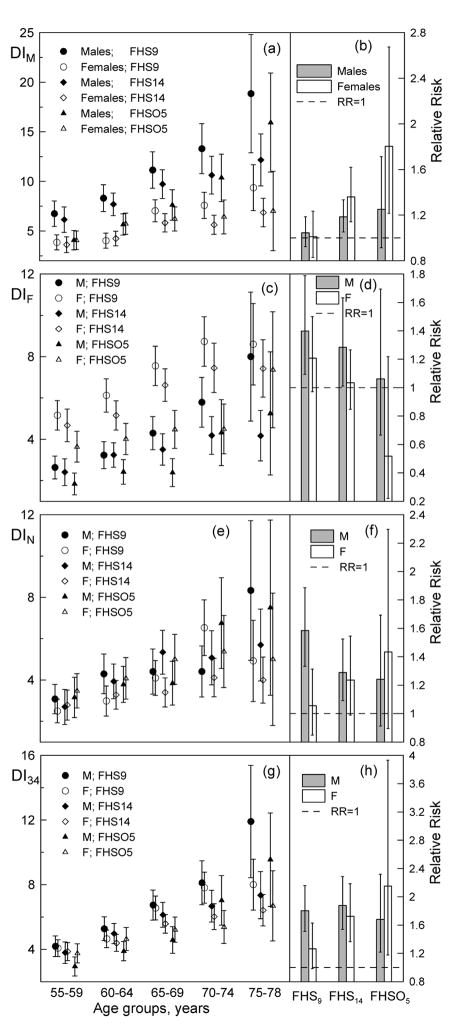
Figure 2 shows that the observed sex-specific trade-offs between morbidity and mortality vary over time. In the early years (mid 1960s and 1970s), males have higher mean values of the age-specific DIM than females, whereas in the later years (mid 1990s) mean values for the younger ages clearly converge (Figure 2a). The relative risk attributable to the DIM, however, exhibits an oposite time behavior, i.e., in the mid 1960s neither males’ nor females’ health, as characterized by the DIM, was associated with increased risks of death. The relative risks, however, increase over time for both sexes although this increase is larger for females than for males (Figure 2b).
Figure 2.
The sex-specific age patterns of the deficit indices constructed using the set of (g) all 34 deficits used in the analyses (DI34) as well as selected sub-sets of deficits (see Table 1), i.e., (a) DIM, (c) DIF, and (e) DIN for participants of the 9th (1964) and 14th (1974) FHS and 5th (1991–1995) FHSO exams. Bars show 95% Confidence Intervals (CIs). (b,d,f,h) The sex-specific relative risks (RRs) of death for 10% increase of the respective deficit index for all ages combined in each FHS/FHSO exam.
Similar behavior on convergence of the age patterns over time (although not as pronounced as in the case of the DIM) is observed for the DIF (Figure 2c). The relative mortality risk associated with this health dimension, however, declines over time for both sexes (Figure 2d). No essential change over time is observed for the DIN age pattern (Figure 2e). Systematic trends of increase of the relative mortality risk associated with the respective health dimension for females and its decrease for males are, however, evident (Figure 2f). Aggregating these compositional changes attributable to the deficit-specific DIs to the DI34 shows no net trends in the relative risks of death for males, whereas the respective risk for females increases over time (Figure 2h). This, however, is accompanied by a decline in the mean values of the DI34 over time for all age groups (more rigorous analysis of the time trends in the DIs age patterns will be published elsewhere) for each sex (Figure 2g).
DISCUSSION AND CONCLUSIONS
The results show the capacity of the cumulative deficits approach for characterizing sex differentials in morbidity and mortality outcomes when considering the effect of minor health-related traits. The aggregation of such minor traits into an integrative or cumulative measure (i.e., the DI) establishes a background for reliable conclusions which are often difficult to achieve considering individual minor traits.
Analysis of the pooled sample of the participants of the 9th and 14th FHS and 5th FHSO exams shows that females’ health, characterized by the cumulative index constructed on the basis of all 34 minor health deficits used in the analysis, is the same as that of males in a wide range of ages between 55 and 74 years. Therefore, these age patterns contradict the belief that females’ morbidity at the level of minor deficits tends to be higher than that of males (Emslie et al., 1999). The sex-insensitivity of the health age pattern also is accompanied by a systematic trend towards a larger relative contribution of these deficits to the risk of death for males than for females (see Figure 1a with inset).
These observed patterns, however, are an aggregate picture that reflects the net effect over different health dimensions (associated with minor traits). More detailed analyses (Figures 1b–d) support the contrary view that the sex-specific excess in minor deficits may vary according to the particular set of potential deficits used for the analysis (MacIntyre et al., 1996). Disaggregating the health domain characterized by the DI34 to different subsets associated with a larger prevalence of deficits for males (DIM) and females (DIF), respectively, and with no difference in prevalence of deficits for either sex (DIN) leads to different conclusions. First, the DIF-related (see Figure 1c with inset) health dimension supports the traditional morbidity-mortality paradox associated with the larger morbidity and lower contribution of this morbidity to the respective mortality risks for females than for males. Another set of deficits associated with DIM-related (Figure 1b) health dimension provides evidence for the inverse morbidity paradox, wherein males have greater DIM levels, but the contribution of this index to mortality risk is smaller than for females.
Figures 2c–d demonstrate that the traditional sex morbidity-mortality paradox becomes less pronounced over time (i.e., from the 1960s to the 1990s). Contrarily, the inverse morbidity paradox (Figures 2a,b) becomes more pronounced in the mid 1990s than in the early years, i.e., the DIM-related health dimension might be associated with an increasing its role in the mortality risks for females, while the prevalence of those conditions becomes either lower or remains the same as in males. These counterbalancing trends give rise to a situation wherein the overall health of males and females, as characterized by the DI34, tends to be the same, while the contribution of the DI34 to mortality risks increases for females and does not change over time for males (Figures 2g,h).
Our findings support the view that the sex-specific excess in minor health deficits may vary according to the particular set of potential deficits used, thus providing evidence for both traditional and inverse morbidity-mortality paradoxes. The time-trends suggest the presence of a strong exogenous effect modifier affecting both the rate of health deterioration and mortality risk.
Acknowledgments
The research reported in this paper was supported by the National Institute on Aging grants 1R01 AG028259, 1R01-AG-027019, 5R01-AG-030612, and 5P01-AG-008761. The Framingham Heart Study (FHS) is conducted and supported by the NHLBI in collaboration with the FHS Investigators. This Manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the FHS or the NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arber S, Cooper H. Gender differences in health in later life: the new paradox? Soc Sci Med. 1999;48:61–76. doi: 10.1016/s0277-9536(98)00289-5. [DOI] [PubMed] [Google Scholar]
- Case A, Paxson C. Sex differences in morbidity and mortality. Demography. 2005;42:189–214. doi: 10.1353/dem.2005.0011. [DOI] [PubMed] [Google Scholar]
- Celentano DD, Linet MS, Stewart WF. Gender differences in the experience of headache. Soc Sci Med. 1990;30:1289–1295. doi: 10.1016/0277-9536(90)90309-g. [DOI] [PubMed] [Google Scholar]
- Crimmins EM. Trends in the health of the elderly. Annu Rev Public Health. 2004;25:79–98. doi: 10.1146/annurev.publhealth.25.102802.124401. [DOI] [PubMed] [Google Scholar]
- Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- Emslie C, Hunt K, Macintyre S. Problematizing gender, work and health: the relationship between gender, occupational grade, working conditions and minor morbidity in full-time bank employees. Soc Sci Med. 1999;48:33–48. doi: 10.1016/s0277-9536(98)00287-1. [DOI] [PubMed] [Google Scholar]
- Goggins WB, Woo J, Sham A, Ho SC. Frailty index as a measure of biological age in a Chinese population. J Gerontol A Biol Sci Med Sci. 2005;60:1046–1051. doi: 10.1093/gerona/60.8.1046. [DOI] [PubMed] [Google Scholar]
- Gorman BK, Read JG. Gender disparities in adult health: an examination of three measures of morbidity. J Health Soc Behav. 2006;47:95–110. doi: 10.1177/002214650604700201. [DOI] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- Kulminski A, Ukraintseva SV, Akushevich I, Arbeev KG, Land K, Yashin AI. Accelerated accumulation of health deficits as a characteristic of aging. Exp Gerontol. 2007a doi: 10.1016/j.exger.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski A, Yashin A, Arbeev K, Akushevich I, Ukraintseva S, Land K, Manton K. Cumulative index of health disorders as an indicator of aging-associated processes in the elderly: Results from analyses of the National Long Term Care Survey. Mech Ageing Dev. 2007b;128:250–258. doi: 10.1016/j.mad.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski AM, Ukraintseva SV, Akushevich IV, Arbeev KG, Yashin AI. Cumulative index of health deficiencies as a characteristic of long life. J Am Geriatr Soc. 2007c;55:935–940. doi: 10.1111/j.1532-5415.2007.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Ebrahim S, Davey Smith G. Sex matters: secular and geographical trends in sex differences in coronary heart disease mortality. Bmj. 2001;323:541–545. doi: 10.1136/bmj.323.7312.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre S, Ford G, Hunt K. Do women ‘over-report’ morbidity? Men’s and women’s responses to structured prompting on a standard question on long standing illness. Soc Sci Med. 1999;48:89–98. doi: 10.1016/s0277-9536(98)00292-5. [DOI] [PubMed] [Google Scholar]
- MacIntyre S, Hunt K, Sweeting H. Gender differences in health: are things really as simple as they seem? Soc Sci Med. 1996;42:617–624. doi: 10.1016/0277-9536(95)00335-5. [DOI] [PubMed] [Google Scholar]
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scientific World Journal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson CA. Illness and the feminine role: a theoretical review. Soc Sci Med. 1975;9:57–62. doi: 10.1016/0037-7856(75)90094-3. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- Verbrugge LM. Sex differentials in health. Public Health Rep. 1982;97:417–437. [PMC free article] [PubMed] [Google Scholar]
- Verbrugge LM. The twain meet: empirical explanations of sex differences in health and mortality. J Health Soc Behav. 1989;30:282–304. [PubMed] [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Verbrugge LM, Wingard DL. Sex differentials in health and mortality. Health Matrix. 1987a;5:3–19. [PubMed] [Google Scholar]
- Verbrugge LM, Wingard DL. Sex differentials in health and mortality. Women Health. 1987b;12:103–145. doi: 10.1300/J013v12n02_07. [DOI] [PubMed] [Google Scholar]
- Yashin AI, Arbeev KG, Kulminski A, Akushevich I, Akushevich L, Ukraintseva SV. Cumulative index of elderly disorders and its dynamic contribution to mortality and longevity. Rejuvenation Res. 2007a;10:75–86. doi: 10.1089/rej.2006.0500. [DOI] [PubMed] [Google Scholar]
- Yashin AI, Arbeev KG, Kulminski A, Akushevich I, Akushevich L, Ukraintseva SV. Health decline, aging and mortality: how are they related? Biogerontology. 2007b;8:291–302. doi: 10.1007/s10522-006-9073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]