Abstract
Purpose
The purpose of this article is to present the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TOD2AY) study and a description of the implementation of the standard diabetes education (SDE) program.
Methods
A total of 218 participants (one third of the eventual sample of 750) were initially enrolled in the study. To date, the mean age of participants was 14.3 + 2.1 years, with 63% being female. Families of study participants were largely low or middle income (more than half report family income <$35 000) and about three-quarters were minority.
Results
More than three-quarters (79%) of families achieved full mastery of the entire SDE program. Mastery required on average 5.5 + 1.3 sessions. In addition, 62% of the families were able to achieve mastery of the session topic in a single visit.
Conclusions
In summary, the TOD2AY study SDE program fills the need for effective, engaging materials for youth and their families to use in mastering essential type 2 diabetes skills and knowledge.
Development of a Diabetes Education Program for Youth with Type 2 Diabetes
Obesity has dramatically increased in prevalence worldwide among children and adolescents. This increase has been accompanied by the appearance and increasing prevalence of type 2 diabetes. Before the 1990s, it was rare for most pediatric centers to have patients with type 2 diabetes. However, by 1994, type 2 diabetes patients represented up to 16% of new cases of diabetes in children in urban diabetes centers,1 and by 1999, depending on geographic location, the range of percentage of new cases due to type 2 diabetes was between 8% and 45% and disproportionately represented in minority populations.2 Indeed, over the last decade, the increase in the number of children and adolescents with type 2 diabetes has been labeled an “epidemic.”3
Twenty years of diabetes intervention research and the results of meta-analytic review have demonstrated that diabetes education is a necessary, but not sufficient, intervention to enhance self-care in people with diabetes.4-7 The majority of the previous work in children, however, has focused on children with type 1 diabetes.
The relative novelty of type 2 diabetes in children has led many treatment centers to rely on materials designed for youth with type 1 diabetes or adults with type 2 diabetes. The population of youth with type 2 diabetes, however, is more likely to be of ethnic minority background than those with type 1,3 and materials for adults fail to account for developmental issues in youth. Thus, systematic education programs for youth with type 2 diabetes are needed.
There are no studies of educational programs for youth with type 2 diabetes in the literature. Thus, the purpose of this article is to describe the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TOD2AY) study and standard diabetes education (SDE) program, and to present a description of the implementation of the program in the first 218 participants enrolled in the TOD2AY trial.
Methods
TOD2AY is a multisite National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Disease (NIH/NIDDK)-sponsored randomized parallel group clinical trial designed to evaluate the relative efficacy of 3 treatments for type 2 diabetes in youth age 10 to 18 years are (1) metformin alone, (2) metformin plus rosiglitazone, and (3) metformin plus an intensive lifestyle intervention called the TOD2AY Lifestyle Program (TLP). Methods for the study have been summarized in a previous article,8 so they will not be detailed here. Youth with type 2 diabetes diagnosed within 2 years (n = 750) and a family support person are being recruited at 15 clinical centers. Youth participants will be followed for a minimum of 3 years and up to 6 years. All subjects participate in a screening visit and a 2 to 6 month single-blind run-in period during which SDE is provided to the youth and family support persons. Individuals who are eligible at the end of the run-in period are randomized 1:1:1 to 1 of 3 treatment arms.
SDE
SDE is provided during the run-in period to assure that all of the youth in the study have equivalent diabetes education prior to randomization. The purposes of the SDE program are to provide the participants and a family support person with basic knowledge about type 2 diabetes and to teach basic survival skills and behaviors that are important for successful management of this disease. The family support person is usually a parent or guardian, but may be another adult who agrees to participate in the study with the youth.
The program includes educational content dealing with type 2 diabetes physiology and treatment and progressive skill building. Materials have been combined into interactive, developmentally appropriate, and culturally sensitive workbooks provided to the youth and their family support person. All are available in English and Spanish. Certified diabetes educators (CDEs) at each study site teach the program. Education can be provided by telephone or in person, or in groups.
Youth and their designated family support person for the study must achieve 80% mastery of the content by the time of randomization. Mastery quizzes are included in the education manual. The participants continue to receive education and take a series of quizzes until mastery is achieved.
The SDE content and prototype training materials were developed in a large Houston diabetes center for children and adolescents. The curriculum had been used successfully for more than a year with children and families dealing with type 2 diabetes. Additionally, more than 20 families from various ethnicities received the SDE according to protocol in a prestudy feasibility study. In this feasibility study, the curriculum and preliminary materials were used by CDEs with youth with newly diagnosed type 2 diabetes. The CDEs collected qualitative information (satisfaction with the curriculum) from families and provided this data to the committee. Findings from the feasibility study were used to revise the materials and sequence of sessions.
The SDE curriculum was designed to blend cognitive and psychomotor content, and to build on the child’s current diabetes knowledge. The educator assessed knowledge and skills using a competency checklist (Table 1). The initial session provided a broad overview, focusing on the survival skills glycemic targets, monitoring skills, health eating, and record keeping.
Table 1.
Educational Competencies for SDE Prerandomization
| Mastery Demonstrated by Participant/Family | Tool | |
|---|---|---|
| Cognitive | ||
| What is diabetes? | Ability to state why blood sugar goes high in type 2 diabetes | Q & A |
| Ability to state what causes type 2 diabetes | ||
| Oral medications | Ability to state that type 2 diabetes is treated with pills and insulin (as well as activity and nutrition) | Q & A |
| Ability to state that diabetes pills are not insulin | ||
| Ability to state that treatment changes as the problems of type 2 diabetes change | ||
| Ability to state that metformin causes the body to use insulin better | ||
| Ability to state that metformin should be taken before meals | ||
| Ability to state what to do if a dose of metformin is missed | ||
| What are CHO? | Ability to identify types of carbohydrates | Q & A |
| Ability to state that foods high in fats and calories contribute to weight gain | ||
| Ability to make nutritious snack choices | ||
| Portion sizes | Ability to select acceptable portions of food | Q & A |
| Effect of exercise on BG | Ability to state that activity makes blood sugar get closer to target | Q & A |
| When to speed up/slow down activity | Ability to show how to decide when to move faster or slower when active | Demonstrate pulse check or Borg perceived exertion scale |
| Hypoglycemic symptoms and treatment | Ability to list common symptoms of hypoglycemia | Q & A |
| Ability to state how to treat a low blood sugar | ||
| Target BG and HbA1C | Ability to state that goals of blood sugar are 70-150 and goals of HbA1C are less than 7% | Q & A |
| Ability to state that HbA1C measures the average blood sugar for the past 3 months/is measured every 3 months | ||
| Problem solving | Ability to state that ketones should be checked when BG is high (>300 mg/dl) | Q & A |
| Ability to state that 15 gm of CHO is used to treat a low BG | ||
| Risk reduction | Ability to list the body systems affected by years of hyperglycemia (eyes, kidneys, heart, nerves) | Q & A |
| Living with diabetes | Ability to state that stress can raise BG | Q & A |
| Ability to state that having negative feelings about diabetes is normal and can be shared with family, friends, and study team members | ||
| Psychomotor | ||
| BG monitoring | Ability to perform BG test with acceptable skill | Demonstrate fingerstick, use of meter, logbook |
| Ability to complete a logbook accurately | ||
| Ability to use BG results to tell effect of activity and food | ||
| Ketone testing | Ability to perform urine ketone test with acceptable skill | Demonstrate with Ketostix |
| Food label reading | Ability to determine quantity of a food permitted in meal plan using the food’s label | Demonstrate portion, fat, and CHO amount |
CHO, carbohydrates; BG, blood glucose.
Each subsequent session began with a brief assessment of content covered in the previous session. Session 2 focused on aspects of medical nutrition therapy (MNT) (food types, role of carbohydrate, food pyramid, meal plans) and medications (types and when to take). Session 3 further covered MNT, focusing on portion control and label reading. In addition, concepts about physical activity were introduced, including types of activities and how to obtain the most benefit. Session 4 further built on MNT providing information about low fat eating and eating out. Stress management and building social support was also addressed. Furthermore, for children 12 years and older, additional age-specific content was included. Session 5 built on the physical activity focus with additional content about footwear, hydration, and exercise intensity. Hypoglycemia and hyperglycemia management were also covered, including ketone testing skills. Session 6 reviewed goal setting and school issues, and decision-making strategies for teens. The first 3 sessions were conducted face-to-face, either one-to-one or in small groups. Later sessions were also typically conducted face-to-face, but could be conducted by phone.
Both curriculum-specific materials and public domain materials were used throughout the program. For example, in session 2, the food guide pyramid was a fundamental teaching tool. Other materials from the American Diabetes Association and the National Diabetes Education Program were employed. Content specific education sheets were created for the curriculum with emphasis on age appropriateness and diversity, as shown in Figure 1.
Figure 1.
Example of an education sheet.
A main purpose in creating and delivering the SDE program was to make sure that children and families had a firm foundation in diabetes knowledge and skills. Competencies were evaluated throughout the sessions, beginning with baseline assessments of knowledge. Mastery checklists were created to track building skill levels and to guide any need for remedial training (Figure 2). Assessments were designed to be simple, concrete, and engaging. Mastery assessments linked directly to program goals. Most of the assessment questions asked for application of new knowledge. For instance, given a meal plan, the child and family member were asked to create a lunch and a snack. In another example, the child was given a list of events for a day (blood glucose levels, food eaten, activity) and asked to complete a logbook page.
Figure 2.
Example of a mastery checklist.
Follow-up education was provided after study randomization to offer continuous basic education to both randomized groups. Five topics were offered: focus, not an ordinary day, nutrition nibbles, teen issues, and living with diabetes. Educators tracked completion of these topics for each participant and offered the content in no specific sequence.
Results
The first 218 participants randomized in the study, who comprise the sample for this report, ranged in age from 10 to 18 years at recruitment, with a mean of 14.3 years (±2.1 years), and with 63% female. Families of study participants were largely low or middle income (55% report family income <$35 000) and about three-quarters were minority (73% are not white, with 29% black, 27% white, 21% Latino, and 6% Native American).
A total of 79% achieved mastery for the entire SDE program in an average of 5.5 ± 1.3 sessions. The majority of sessions (97%) were accomplished by the CDE individually with a family, and 62% families achieved mastery of a single session topic at a single visit. Figure 3 shows the distribution of the number of sessions to mastery. Although the majority achieved mastery of the SDE program in 5 sessions, about 10% achieved mastery in less than 5 sessions, and about 25% took longer than 5 sessions, with maximum of 12 sessions.
Figure 3.
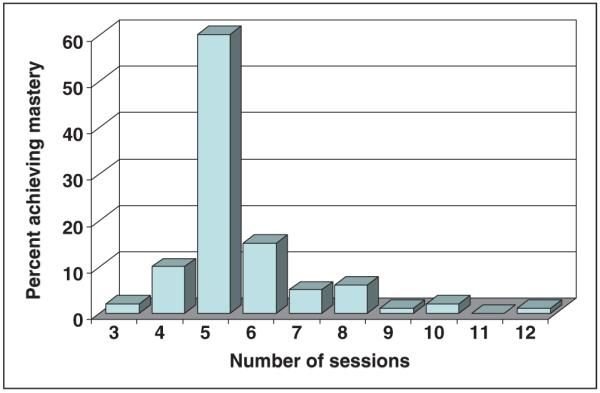
Number of sessions to mastery.
Factors were examined that may affect time to mastery by comparing mean number of sessions to mastery by factors such as language, gender, and education. These data are shown in Figure 4. As might be expected, because they had received initial education more recently, those who had diabetes less than 1 year took less time to mastery than those who were diagnosed more than 1 year before. If a translator was necessary to cover the material, an average of 8 sessions was necessary compared with 5 sessions for those who did not need a translator. There were no obvious differences in time to mastery by gender; primary language of the youth and family support person; whether materials were presented in individual or group sessions; whether SDE was presented at the clinical center, home, or a community site; or whether the youth was on insulin at the time of screening.
Figure 4.
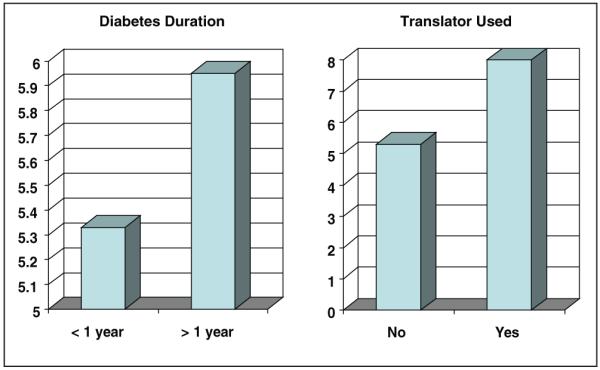
Differences in time to mastery.
Discussion
This article describes the development and initial evaluation of a SDE program for youth with type 2 diabetes and their families. This comprehensive program allowed youth and their families to fairly quickly master multiple components of education about type 2 diabetes. Furthermore, the majority of families were able to master the materials in 5 sessions, with an average of 1 session necessary to achieve mastery of a topic. These data suggest that the TOD2AY SDE program fills the need for effective, engaging materials for youth with type 2 diabetes.
The SDE program represents the first comprehensive program developed to assist youth with type 2 diabetes in mastering basic diabetes knowledge and skills. With youth, most diabetes education is provided at or near the time of diagnosis and focuses on diabetes survival skills. Participants had been diagnosed with diabetes within the past 2 years, and each had received diabetes education at diagnosis. Nonetheless, no child or family support person achieved mastery of the entire SDE program in 1 or 2 sessions. Less than 10% were able to achieve mastery in fewer than 5 sessions. These findings suggest that for most youth with type 2 diabetes and their families, initial diabetes education is not sufficient to assure mastery of essential knowledge and skills. Furthermore, the longer the duration of diabetes, the more SDE sessions were necessary to achieve mastery. It may be that survival skills can be effectively taught at diagnosis, but full mastery needs to occur after these survival skills are mastered and will require a substantial additional effort.
This study also examined whether the time needed to achieve SDE program mastery was related to medication status, demographic features, or language factors. It might be expected that youth on insulin at the time they begin SDE would require less time to mastery since their families may have viewed the diagnosis of diabetes as more serious due to the necessity for insulin administration. This was not the case. Only 69 of the youth were on insulin at the time of recruitment. A requirement of the study design was that youth had to be managed without insulin at the time of randomization. Thus, it is possible that this requirement resulted in youth who did not view their diabetes as serious, compared to those who were not able to be managed without insulin and thus were not randomized.
The population of youth in the project closely resembles those reported to have type 2 diabetes in childhood,2,9,10 with the majority being minority, female, and pubertal. Nonetheless, gender, race/ethnicity, or age did not predict longer time to mastery.
Those who required a translator to provide SDE took approximately 1.5 times the sessions to mastery than those who did not require a translator, but primary language of the youth and family support person did not appear to affect time to mastery. It makes sense that mastery would take longer if a translator is used, as the time to deliver the materials would be longer, and thus lead to a longer time to mastery. Similarly, it would be not be surprising that more time for mastery would be required by those for whom English is not the first language. However, we did not find that this was the case, which may be due to the availability of materials in both English and Spanish.
Finally, the initial experience indicates that SDE can be provided successfully to individual families at a convenient site. The literature has not provided clear answers as to whether diabetes education when provided individually is more effective than when provided in groups.11,12 Previous studies have had mixed results, with several suggesting that individual approaches are more effective because they can be tailored to the individual, but others suggest that group approaches are more effective because of the social support offered. These studies were done primarily with adults with type 2 diabetes or youth with type 1 diabetes and may not be able to be generalized to this population of youth with type 2 diabetes. In addition, although it might be assumed that providing diabetes education at a convenient site (eg, home or community site vs clinical center) would be easier for youth and their families, it was found that time to mastery did not differ by site of education. It is important to note, however, that the great majority of educational sessions were provided individually and at the clinical center, so that the variance in these factors is limited and may have affected our ability to find differences.
Although the data suggest that the SDE program developed for the TOD2AY trial is useful for youth with type 2 diabetes and their families, limitations are acknowledged. First, because only the first 218 participants randomized in the trial were studied, statistical analyses were not performed to determine if the differences in time to mastery were statistically significant. The report focuses on differences that may be clinically significant and assist CDEs to predict how long mastery of SDE might take in these high risk youth. Second, mastery was determined by a series of mastery quizzes administered by the CDEs. Although these instruments have face and content validity, and were developed by experienced CDEs, no data are available on reliability.
Nonetheless, the TOD2AY SDE program will provide a much needed approach to diabetes education with new or recent onset type 2 diabetes. The majority of youth and families achieved SDE program mastery in 5 sessions, but time to mastery was longer for youth with diabetes of longer duration and when a translator was needed. Importantly, time required for mastery was not affected by primary language, gender, or site and group delivery. These materials will be available soon, but CDEs can use the program plans provided to educate their young patients with type 2 diabetes.
Acknowledgement
This work was completed with funding from NIDDK/NIH grant numbers U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, U01-DK61254, and from the National Center for Research Resources General Clinical Research Centers Program grant numbers M01-RR00036 (Washington University School of Medicine), M01-RR00043-45 (Childrens Hospital Los Angeles), M01-RR00069 (University of Colorado Health Sciences Center), M01-RR00084 (Children’s Hospital of Pittsburgh), M01-RR01066 (Massachusetts General Hospital), M01-RR00125 (Yale University), and M01-RR14467 (University of Oklahoma Health Sciences Center).
Appendix
The materials described were developed by the members Standard Diabetes Education Committee of the TOD2AY Study Group: Margaret Grey (chair), DrPH, FAAN, Ken Copeland, MD, Linda Delahanty, MS, RD, Sue McGirk, MSN, CDE, Suzanne Meyer, MSN, CDE, Guadalupe Rupert, MS, CDE, Barbara Schreiner, MN, RN, CDE, BC-ADM. Other contributors are C. Macha, C. Gualdalini, P. Rose, M. Larkin, S. Foster, N. Chang, B. Schwartzman.
The following individuals and institutions constitute the TODAY Study Group (* indicates principal investigator or director):
CLINICAL CENTERS: Baylor College of Medicine: M. Haymond*, B. Anderson, S. Gunn, H. Holden, M. Jones, K. Hwu, S. McGirk, S. McKay, B. Schreiner; Case Western Reserve University: L. Cuttler*, E. Abrams, T. Casey, W. Dahms, D. Drotar, S. Huestis, C. Levers-Landis, P. McGuigan, S. Sundararajan; Childrens Hospital Los Angeles: M. Geffner*, N. Chang, D. Dreimane, M. Halvorson, S. Hernandez, F. Kaufman (Study Chair), V. Mansilla, R. Ortiz, A. Ward, K. Wexler, P. Yasuda; Children’s Hospital of Philadelphia: L. Levitt Katz*, R. Berkowitz, S. Boyd, C. Carchidi, M. Cullen, J. Kaplan, C. Keating, S. Kneeshaw-Price, C. Lassiter, T. Lipman, B. Schwartzman, S. Suarez, S. Willi; Children’s Hospital of Pittsburgh: S. Arslanian*, F. Bacha, S. Foster, B. Galvin, T. Hannon, A. Kriska, I. Libman, M. Marcus, K. Porter, T. Songer, E. Venditti; Columbia University Medical Center: R. Goland*, R. Cain, I. Fennoy, D. Gallagher, P. Kringas, N. Leibel, R. Motaghedi, D. Ng, M. Ovalles, M. Pellizzari, R. Rapaport, K. Robbins, D. Seidman, L. Siegel-Czarkowski, P. Speiser; Joslin Diabetes Center: L. Laffel*, A. Goebel-Fabbri, L. Higgins, M. Malloy, K. Milaszewski, L. Orkin, A. Rodriguez-Ventura; Massachusetts General Hospital: D. Nathan*, L. Bissett, K. Blumenthal, L. Delahanty, V. Goldman, A. Goseco, M. Larkin, L. Levitsky, R. McEachern, K. Milaszewski, D. Norman, B. Nwosu, S. Park-Bennett, D. Richards, N. Sherry, B. Steiner; Saint Louis University: S. Tollefsen*, S. Carnes, D. Dempsher, D. Flomo, V. Kociela, T. Whelan, B. Wolff; State University of New York Upstate Medical University: R. Weinstock*, D. Bowerman, K. Duncan, R. Franklin, J. Hartsig, R. Izquierdo, J. Kanaley, J. Kearns, S. Meyer, R. Saletsky, P. Trief; University of Colorado Health Sciences Center: P. Zeitler* (Steering Committee Chair), A. Bradhurst, N. Celona-Jacobs, J. Glazner, J. Higgins, F. Hoe, G. Klingensmith, K. Nadeau, H. Strike, N. Walders, T. Witten; University of Oklahoma Health Sciences Center: K. Copeland* (Steering Committee Vice-Chair), R. Brown, J. Chadwick, L. Chalmers, C. Macha, A. Nordyke, T. Poulsen, L. Pratt, J. Preske, J. Schanuel, J. Smith, S. Sternlof, R. Swisher; University of Texas Health Science Center at San Antonio: J. Lynch*, N. Amodei, R. Barajas, C. Cody, D. Hale, J. Hernandez, J. Lynch, E. Morales, S. Rivera, G. Rupert, A. Wauters; Washington University School of Medicine: N. White*, A. Arbeláez, J. Jones, T. Jones, M. Sadler, M. Tanner, R. Welch; Yale University: S. Caprio*, M. Grey, C. Guandalini, S. Lavietes, P. Rose, A. Syme, W. Tamborlane
COORDINATING CENTER: George Washington University Biostatistics Center: K. Hirst*, L. Coombs, S. Edelstein, N. Grover, C. Long, L. Pyle
PROJECT OFFICE: National Institute of Diabetes and Digestive and Kidney Diseases: B. Linder*
CENTRAL UNITS: Central Blood Laboratory (Northwest Lipid Research Laboratories, University of Washington): S. Marcovina*, J. Chmielewski, M. Ramirez, G. Strylewicz; DEXA Reading Center (University of California at San Francisco): J. Shepherd*, B. Fan, L. Marquez, M. Sherman, J. Wang; Diet Assessment Center (University of South Carolina): E. Mayer-Davis*, Y. Liu, M. Nichols; Lifestyle Program Core (Washington University): D. Wilfley*, D. Aldrich-Rasche, K. Franklin, C. Massmann, D. O’Brien, J. Patterson, T. Tibbs, D. Van Buren
OTHER Centers for Disease Control: P. Zhang; Hospital for Sick Children, Toronto: M. Palmert; State University of New York at Buffalo: L. Epstein; University of Florida: J. Silverstein
References
- 1.Pihoker C, Scott CR, Lensing SY, Cradock MM, Smith J. Non-insulin dependent diabetes mellitus in African-American youth of Arkansas. Clin Pediatr. 1998;37:97–102. doi: 10.1177/000992289803700206. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–354. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23:381–389. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 4.Hampson SE, Skinner TC, Hart J, et al. Behavioral interventions for adolescents with type 1 diabetes: how effective are they? Diabetes Care. 2000;23:1416–1422. doi: 10.2337/diacare.23.9.1416. [DOI] [PubMed] [Google Scholar]
- 5.Brown SA. Quality of reporting in diabetes patient education research: 1954-1986. Res Nurs Health. 1990;13:52–62. doi: 10.1002/nur.4770130109. [DOI] [PubMed] [Google Scholar]
- 6.Brown SA, Grimes DE. A meta-analysis of process of care, clinical outcomes, and cost-effectiveness of nurses in primary care roles: Nurse practitioners and nurse midwives. American Nurses Association; Washington, DC: 1993. [Google Scholar]
- 7.Padgett D, Mumford E, Hynes M, Carter R. Meta-analysis of the effects of educational and psychosocial interventions on management of diabetes mellitus. J Clin Epidemiol. 1988;41:1007–1030. doi: 10.1016/0895-4356(88)90040-6. [DOI] [PubMed] [Google Scholar]
- 8.The TOD2AY Study Group Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagot-Campagna A, Pettit DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136:664–672. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- 10.Grey M, Davidson M, Berry D, Melkus GD, Peckham L, Gustafson E. Preventing type 2 diabetes in high risk youth: Results of a pilot study. Diabetes. 2002;64:A66. [Google Scholar]
- 11.Deakin T, McShane C, Cade JE, Williams RDR. Group based training for self-management strategies in people with type 2 diabetes mellitus. The Cochrane Library. 2006;(4) doi: 10.1002/14651858.CD003417.pub2. CD003417. [DOI] [PubMed] [Google Scholar]
- 12.Rickheim PL, Weaver TW, Flader JL, Kendall DM. Assessment of group versus individual diabetes education: a randomized study. Diabetes Care. 2002;25:269–274. doi: 10.2337/diacare.25.2.269. [DOI] [PubMed] [Google Scholar]




