Abstract
Purpose
Recent data demonstrate that age may be a significant independent prognostic variable following treatment for renal cell carcinoma. We analyzed data from the SEER (Surveillance, Epidemiology and End Results) database to evaluate the relative survival of patients treated surgically for localized renal cell carcinoma as related to tumor size and patient age.
Materials and Methods
Patients in the SEER database with localized renal cell carcinoma were stratified into cohorts by age and tumor size. Three and 5-year relative survival, the ratio of observed survival in the cancer population to the expected survival of an age, sex and race matched cancer-free population, was calculated with SEER-Stat. Brown's method was used for hypothesis testing.
Results
A total of 8,578 patients with surgically treated, localized renal cell carcinoma were identified. While 3 and 5-year survival for patients with small (less than 4 cm) renal cell carcinoma was no different from that of matched cancer-free controls, patients treated for large (greater than 7 cm) localized renal cell carcinoma experienced decreased 5-year relative survival across all age groups. Therefore, age was not a significant predictor of relative survival for patients with small (less than 4 cm) or large (greater than 7 cm) tumors. However, a statistically significant trend toward lower relative survival with increasing age was demonstrated in patients with medium size tumors (4 to 7 cm). Hypothesis testing confirmed these findings.
Conclusions
These data suggest that relative survival is high in patients with tumors less than 4 cm and lower in patients with tumors larger than 7 cm regardless of age. However, increasing age may be related to worse outcomes in patients with tumors 4 to 7 cm. The cause of this observation warrants further investigation.
Keywords: kidney neoplasms, mortality
Like most malignancies RCC is a heterogeneous disease and its clinical course ranges from indolent to highly aggressive. Accurate risk stratification at diagnosis is imperative to determine individualized followup strategies and to identify appropriate candidates for adjuvant therapy trials. Although the TNM staging system developed by the International Union Against Cancer has been shown to stratify cancer related outcomes effectively, several nomograms incorporating other variables have shown greater prognostic value.1–3 The most widely accepted independent predictors of prognosis in RCC are stage, grade, histological type and performance status.4 Numerous studies have identified prognostic associations for other anatomical, histological, clinical and molecular factors. Continued investigation likely will lead to the refinement of algorithms to predict cancer progression and survival with greater accuracy.
Classically age has not been considered an independent predictor of survival in sporadic RCC. Recent studies have called this into question, particularly in young adults. Some investigators have identified an association between young age and negative prognostic features such as advanced stage or unfavorable histology.5,6 Other series suggest that young age confers a survival benefit in RCC.7–12 To our knowledge the largest published series evaluating age as a prognostic variable in young adults included 124 patients.9
This study uses patient data from the SEER cancer database to examine the relationship between age and outcome in a large cohort of patients with surgically treated localized RCC. To avoid the limitations of cause of death reporting we evaluated survival in terms of relative survival—the ratio of observed survival in the cancer population to expected survival in an age, sex and race matched cancer-free population.
Materials and Methods
We used data from the SEER 13 Public Use Registry to create a cohort of patients with kidney cancer diagnosed between 1988 and 1997.13 We selected this period to include patients who would have been diagnosed after the widespread introduction of computerized tomography and for whom appropriate followup data were available. Patients were included in the study if they were diagnosed at 30 to 79 years old and underwent definitive surgery for primary kidney tumors (C 64.9). Patients were excluded from analysis if the diagnosis was made on the basis of a death certificate or autopsy. Patients with positive regional lymph nodes or distant metastasis were also excluded from analysis.
Patients were classified as undergoing definitive surgery if they underwent partial or subtotal nephrectomy, complete/total nephrectomy with or without lymph node dissection, radical nephrectomy with or without lymph node dissection, or nephrectomy not otherwise specified. Patients who did not undergo surgery involving the kidney or did not have information regarding surgery were excluded from study.
Histology criteria were based on International Classification of Diseases for Oncology, Second Edition 8032, 8041, 8240, 8260, 8270, 8290, 8310, 8312, 8317, 8318, 8319, 8320, 8960, 8963 and 8966. We included the ICD codes clear cell (8310, 8312), granular (8320), chromophobe (8270), oncocytoma (8290), papillary (8050, 8340, 8260) or other.
Relative survival rates were calculated with SEER-Stat version 6.4.4, which compares the observed survival in the cancer population to the expected survival of a race, sex and age matched cancer-free population derived from life tables. STATA® 8.0 was used for chi-square tests to compare demographics across tumor sizes. We used Brown's method to compare the outcomes of older age cohorts to those of the 30 to 39-year-old group to examine the potential impact of age on RS.14
Results
Review of the SEER database identified 8,578 patients 30 to 79 years old who underwent definitive surgery for localized RCC between 1988 and 1997. Patient and tumor characteristics are summarized in the table. The incidence of RCC diagnosis increased during the years studied. A majority of patients were male (60.4%). Histology was predominantly clear cell or granular type (98.9%).
Patient and tumor characteristics
| No. Tumor Size (%) | ||||
|---|---|---|---|---|
| Less Than 4 cm | 4–7 cm | Greater Than 7 cm | p Value | |
| No. pts | 2,810 | 3,775 | 1,993 | |
| Race: | 0.053 | |||
| White | 2,337 (83.2) | 3,182 (84.3) | 1,676 (84.1) | |
| Black | 331 (11.8) | 369 (9.8) | 216 (10.8) | |
| Other | 142 (5.1) | 224 (5.9) | 101 (5.1) | |
| Gender: | 0.217 | |||
| Male | 1,696 (60.4) | 2,327 (61.6) | 1,252 (62.8) | |
| Female | 1,114 (39.6) | 1,448 (38.4) | 741 (37.2) | |
| Yr of diagnosis | <0.05 | |||
| 88–90 | 484 (17.2) | 824 (21.8) | 434 (21.8) | |
| 91–93 | 783 (27.9) | 1,149 (30.4) | 598 (30.0) | |
| 94–97 | 1,543 (54.9) | 1,802 (47.7) | 961 (48.2) | |
| Age at diagnosis | <0.05 | |||
| 30–39 | 166 (5.9) | 198 (5.2) | 126 (6.3) | |
| 40–49 | 419 (14.9) | 616 (16.3) | 402 (20.2) | |
| 50–59 | 654 (23.3) | 923 (24.5) | 530 (26.6) | |
| 60–69 | 853 (30.4) | 1,086 (28.8) | 524 (26.3) | |
| 70–79 | 718 (25.6) | 952 (25.2) | 411 (20.6) | |
| Extension: | <0.05 | |||
| Confined to parenchyma | 2,099 (74.7) | 2,564 (67.9) | 1,190 (59.7) | |
| Collecting system invasion | 410 (14.6) | 832 (22.0) | 610 (30.6) | |
| Localized not otherwise specified | 301 (10.7) | 379 (10.0) | 193 (9.7) | |
| Histology: | 0.68 | |||
| Clear cell + granular | 2,776 (98.8) | 3,733 (98.9) | 1,972 (98.9) | |
| Other | 34 (1.2) | 42 (1.1) | 21 (1.1) | |
| Grade: | <0.05 | |||
| Well | 561 (20.0) | 586 (15.5) | 228 (11.4) | |
| Moderate | 733 (26.1) | 969 (25.7) | 514 (25.8) | |
| Poor/anaplastic | 120 (4.3) | 290 (7.7) | 235 (11.8) | |
| Unknown | 1,396 (49.7) | 1,930 (51.1) | 1,016 (51.0) | |
RS was separately examined within 3 tumor size groups (less than 4 cm, 4 to 7 cm and greater than 7 cm) to identify tumor size specific differences in RS by decade of life (figs. 1 to 3). For small tumors (less than 4 cm) and large tumors (greater than 7 cm) there was no statistically significant difference in 3 or 5-year RS among age groups. Survival for small tumors was similar to cancer-free controls with 5-year RS rates greater than 94% across all ages. Survival for those with large tumors was worse than cancer-free controls across all age groups with 5-year RS ranging from 83.6% to 88.6%.
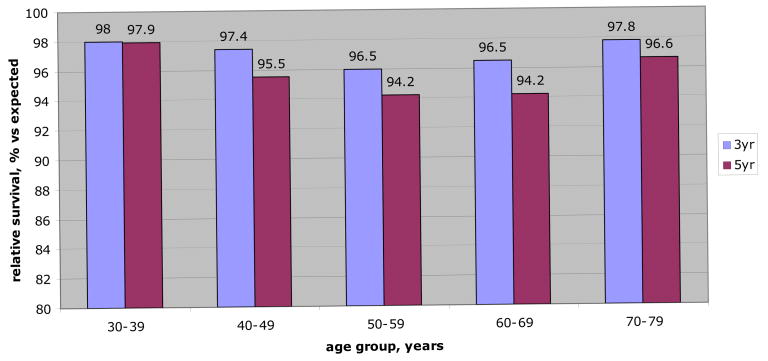
Figure 1.
Relative survival (3 and 5-year) for localized RCC less than 4 cm by age group. RS rates represent comparison of observed survival in patients with small (less than 4 cm) tumors to expected survival for age, sex and race matched cancer-free population. Therefore, likelihood of 5-year survival for 38-year-old patient treated surgically for small (less than 4 cm) localized RCC is nearly identical to that of age, sex and race matched control without cancer (97.9%). Similarly elderly patients with small treated localized RCC have nearly identical survival compared to peers without cancer. Moreover, comparisons of RS rates among age cohorts revealed no statistically significant differences.
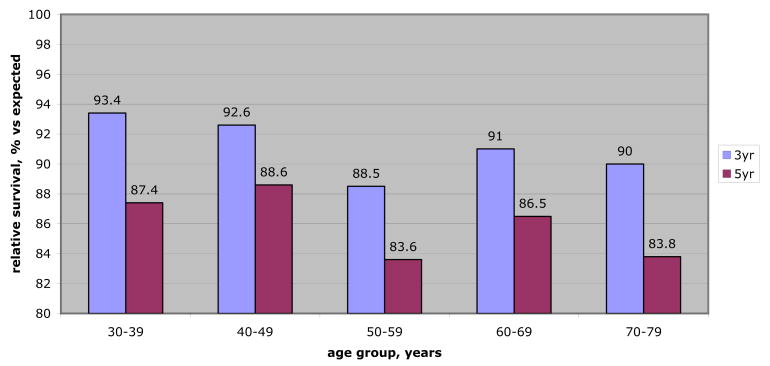
Figure 3.
Relative survival (3 and 5-year) for localized RCC greater than 7 cm by age group. RS rates represent comparison of observed survival in patients with large (greater than 7 cm) tumors to expected survival for age, sex and race matched cancer-free population. Therefore, likelihood of 5-year survival for 38-year-old patient treated surgically for large localized RCC is less than that of age, sex and race matched control without cancer (87.4%). Similarly elderly patients with large, surgically treated, localized RCC demonstrate reduced overall survival compared to peers without cancer. Comparisons of RS rates among age cohorts revealed no statistically significant differences, suggesting that large tumor size decreases relative survival equally across all age groups.
Among patients with medium size tumors (4 to 7 cm) 5-year RS was significantly less in the 50 to 59, 60 to 69 and 70 to 79-year-old groups compared to the 30 to 39-year-old group (p <0.05, p <0.05 and p = 0.01, respectively). Moreover, 3-year RS was significantly worse in the 60 to 69 and 70 to 79-year-old groups compared to the 30 to 39-year-old group (p <0.05 and p <0.01, respectively). There was no significant 3 or 5-year RS difference between the 30 to 39-year-old group and the 40 to 49-year-old group.
Discussion
Traditionally age has not been considered a prognostic factor in RCC. None of the 3 major prognostic nomograms for RCC uses age to predict outcome. The University of California-Los Angeles Integrated Staging System is based on a multivariate analysis that found performance status rather than age to be predictive of survival in localized RCC (p = 0.001 vs p = 0.122).3 In the Mayo Clinic series used to design the SSIGN (stage, size, grade and necrosis) score age was not significantly associated with death from clear cell carcinoma and performance status lost statistical significance on multivariate analysis.2 The Kattan nomogram from the Memorial Sloan-Kettering Cancer Center, which predicts recurrence rather than survival, was developed without consideration for age or performance status.1
Other recent large series have differed on the importance of age in prognosis. A multicenter European study of 1,138 patients treated surgically for localized disease found young age to be independently predictive of cancer specific survival.15 Likewise a nationwide cohort of patients in Iceland with all stages of RCC demonstrated improved disease specific survival with younger age.16 Unfortunately neither study incorporated performance status data in the multivariate analysis. An international collaboration that included more than 4,000 patients with all stages of RCC also found young age to be associated with increased 5-year overall survival on univariate analysis. However, when multivariate analysis was performed Eastern Cooperative Oncology Group performance status was retained as a predictor of overall survival but age lost statistical significance.17 These results suggest that age may serve as a loose surrogate for performance status in predicting prognosis in RCC.
A recent analysis by Hollingsworth et al established a correlation between advanced age and decreased overall survival but not cancer specific survival in patients with surgically treated renal tumors of various sizes.18 Using cause of death data from the SEER database they attributed the decrease in overall survival to competing cause mortality. For each tumor size group patients generally had stable cancer specific mortality across age groups while competing cause mortality increased with increasing age. The authors reasonably concluded from these data that active surveillance of renal masses in older patients warrants further investigation.
By calculating survival in terms of RS we examined the impact of cancer diagnosis on all cause mortality. This large cohort study demonstrated that RS was largely unaffected by age in surgically treated, localized RCC. However, in a subset of patients with medium size tumors there was a statistically significant RS benefit to younger age. A possible explanation for these findings is that the natural history of localized renal masses smaller than 4 cm may be so indolent that age does not affect RS, while the worse biology of tumors greater than 7 cm uniformly decreases RS across all age groups. Between these 2 size cutoffs there may exist a spectrum of tumor biology that results in increased aggressiveness with advancing age.
Moreover, the observed benefit of young age for tumors between 4 and 7 cm may explain the disparities in the various published series regarding the relationship between age and survival. In our review of the literature we noted that where size information was published, recent series with a greater proportion of medium size tumors tended to identify an independent association between patient age and survival,15,16 whereas the studies with a smaller proportion of medium size tumors did not.2,3
Additionally, there has been recent debate surrounding the survival of young adults diagnosed with RCC. In 2004 Sanchez-Ortiz et al reported the M. D. Anderson experience with 106 patients 14 to 40 years old with nonhereditary RCC.6 Compared to older patients young adults more often had unfavorable histological features such as sarcomatoid differentiation or collecting duct type (24% vs 12%) and were more likely to have node positive disease (25% vs 12%). However, they did not find an association between young age and distant metastasis. Moreover, they observed significantly improved 5-year disease specific survival in younger adults (66% vs 52%), recurrence-free survival (time to recurrence 32.4 vs 23.5) and likelihood of advanced local pathological stage (T3a or greater 31% vs 46%). On the contrary Gillett et al studied 124 patients 18 to 40 years old with RCC at the Mayo Clinic and found no trend toward worse histological features or positive lymph nodes.9 In fact, they identified an increased incidence of positive histological features such as cystic disease and chromophobe histological type. Their younger group demonstrated a trend toward improved cancer specific survival that approached statistical significance (p = 0.127). Two other groups recently studied cohorts of young patients with RCC and identified a significant improvement in cancer specific survival with young age on multivariate analysis, although neither study included performance status data.8,10 The present study of localized RCC demonstrated improved RS for 30 to 39-year-olds only in the subset with medium size tumors and in no size subset did it identify a worse prognosis for young patients.
A strength of this study is that the calculation of survival in terms of RS eliminates the biases of cause of death reporting. Mortality data in the cancer literature are derived mostly from cause of death reporting, usually extracted from death certificates. This type of reporting is limited not only because it relies on a medical professional's subjective attribution of a patient's underlying cause of death, but also because it fails to account for the multicausal nature of most deaths.19 Indeed it has been shown that many cancer related deaths are not reported as such on death certificates.20 As a statistical method relative survival avoids the limitations of cause of death reporting. By comparing the overall survival of patients with cancer to the expected survival of an age, sex and race matched cancer-free population, RS effectively calculates the percentage decrease in survival attributable to the cancer diagnosis.
A few limitations to our study should be noted. Most importantly although RCC was clearly associated with decreased RS, an assumption of independence regarding other causes of death must be made to reason that the decreased relative survival in patients with an RCC diagnosis was caused by the cancer. Moreover, SEER does not collect performance status data, which would facilitate a multivariate analysis to confirm that the age specific differences in RS in medium tumors are not due to comorbid conditions. Additionally, SEER does not distinguish sporadic RCC from familial cases such as Birt-Hogg-Dubé and von Hippel-Lindau syndromes. Patients younger than 30 years were excluded from analysis to avoid familial cases. Finally this study was limited to 3 and 5-year survival. It is possible that during a 10 or 15-year time frame other significant age related differences in relative survival might emerge, particularly in the less than 4 cm tumors in which the 5-year relative survival rates were high.
Conclusions
Although RS decreased with increasing tumor size in this population based study of patients with localized RCC, RS was largely unaffected by age. Patients of all ages with small tumors (less than 4 cm) experienced survival rates similar to those of matched controls, whereas RS for large tumors (greater than 7 cm) was lower in all age groups. In a subset of patients with medium (4 to 7 cm) tumors a statistically significant RS benefit associated with younger age was identified. This observation may explain the conflicting findings in the RCC literature with regard to age and prognosis, and warrants further investigation. These data may yield insight into the growth kinetics and biology of localized RCC, suggesting that between 4 and 7 cm there may exist a spectrum of tumor biology which results in increased aggressiveness with advancing age.
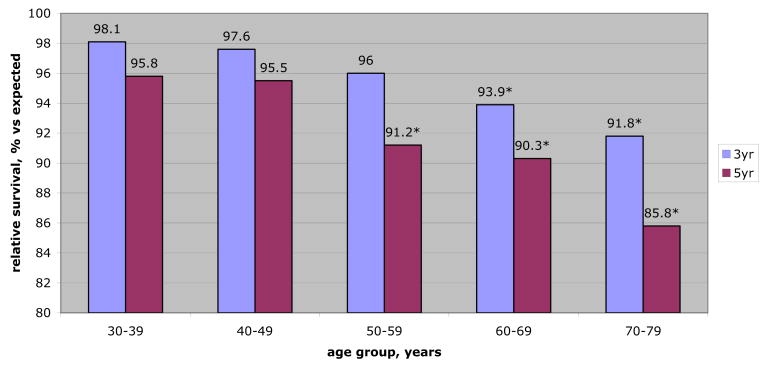
Figure 2.
Relative survival (3 and 5-year) for localized RCC 4 to 7 cm by age group. RS rates represent comparison of observed survival in patients with medium (4 to 7 cm) tumors to expected survival for age, sex and race matched cancer-free population. Statistically significant differences (*) were identified in 3 and 5-year RS rates with increasing age. Therefore, 38-year-old treated surgically for medium size RCC has likelihood of 5-year survival more similar to that of cancer-free peers than does elderly patient (95.8% vs 85.8%).
Acknowledgments
Supported by Grant P30 CA006927 from the National Cancer Institute, and by Fox Chase Cancer Center via institutional support of the Kidney Keystone Program.
Abbreviations and Acronyms
- RCC
renal cell carcinoma
- RS
relative survival
- SEER
Surveillance, Epidemiology and End Results
References
- 1.Kattan MW, Reuter V, Motzer RJ, Katz J, Russo P. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166:63. [PubMed] [Google Scholar]
- 2.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 3.Zisman A, Pantuck AJ, Dorey F, Said JW, Shvarts O, Quintana D, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19:1649. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 4.Campbell SC, Novick AC, Bukowski R. Renal tumors. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 9th. Vol. 2. Philadelphia: WB Saunders; 2007. pp. 1567–1637. chapt 47. [Google Scholar]
- 5.Jun C, Zhishun X, Xianzhou J, Qiang F, Jin W. Association between age and clinical characteristics of renal cell carcinoma in adult patients. Int J Urol. 2006;13:515. doi: 10.1111/j.1442-2042.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Ortiz RF, Rosser CJ, Madsen LT, Swanson DA, Wood CG. Young age is an independent prognostic factor for survival of sporadic renal cell carcinoma. J Urol. 2004;171:2160. doi: 10.1097/01.ju.0000125487.96469.2e. [DOI] [PubMed] [Google Scholar]
- 7.Eggener SE, Rubenstein JR, Smith ND, Nadler RB, Kontak J, Flanigan RC, et al. Renal tumors in young adults. J Urol. 2004;171:106. doi: 10.1097/01.ju.0000099028.95679.52. [DOI] [PubMed] [Google Scholar]
- 8.Denzinger S, Otto W, Burger M, Hammerschmied C, Junker K, Hartmann A, et al. Sporadic renal cell carcinoma in young and elderly patients: are there different clinicopathological features and disease specific survival rates? World J Surg Oncol. 2007;5:16. doi: 10.1186/1477-7819-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillett MD, Cheville JC, Karnes RJ, Lohse CM, Kwon ED, Leibovich BC, et al. Comparison of presentation and outcome for patients 18 to 40 and 60 to 70 years old with solid renal masses. J Urol. 2005;173:1893. doi: 10.1097/01.ju.0000158157.57981.80. [DOI] [PubMed] [Google Scholar]
- 10.Taccoen X, Valeri A, Descotes JL, Morin V, Stindel E, Doucet L, et al. Renal cell carcinoma in adults 40 years old or less: young age is an independent prognostic factor for cancer-specific survival. Eur Urol. 2007;51:980. doi: 10.1016/j.eururo.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Goetzl MA, Desai M, Mansukhani M, Goluboff ET, Katz AE, Sawczuk IS, et al. Natural history and clinical outcome of sporadic renal cortical tumors diagnosed in the young adult. Urology. 2004;63:41. doi: 10.1016/j.urology.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Abou El Fettouh HI, Cherullo EE, El-Jack M, Al Maslamani Y, Novick AC. Sporadic renal cell carcinoma in young adults: presentation, treatment, and outcome. Urology. 2002;60:806. doi: 10.1016/s0090-4295(02)01884-8. [DOI] [PubMed] [Google Scholar]
- 13.Surveillance, Epidemiology, and End Results (SEER) program (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER 13 Regs Public-Use, Nov 2004 Sub (1973–2002 varying), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2005, based on the November 2004 submission.
- 14.Brown CC. The statistical comparison of relative survival rates. Biometrics. 1983;39:941. [PubMed] [Google Scholar]
- 15.Ficarra V, Guille F, Schips L, de la Taille A, Prayer Galetti T, Tostain J, et al. Proposal for revision of the TNM classification system for renal cell carcinoma. Cancer. 2005;104:2116. doi: 10.1002/cncr.21465. [DOI] [PubMed] [Google Scholar]
- 16.Gudbjartsson T, Hardarson S, Petursdottir V, Thoroddsen A, Magnusson J, Einarsson GV. Histological subtyping and nuclear grading of renal cell carcinoma and their implications for survival: a retrospective nation-wide study of 629 patients. Eur Urol. 2005;48:593. doi: 10.1016/j.eururo.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23:2763. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 18.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer. 2007;109:1763. doi: 10.1002/cncr.22600. [DOI] [PubMed] [Google Scholar]
- 19.Begg CB, Schrag D. Attribution of deaths following cancer treatment. J Natl Cancer Inst. 2002;94:1044. doi: 10.1093/jnci/94.14.1044. [DOI] [PubMed] [Google Scholar]
- 20.Pritt BS, Hardin NJ, Richmond JA, Shapiro SL. Death certification errors at an academic institution. Arch Pathol Lab Med. 2005;129:1476. doi: 10.5858/2005-129-1476-DCEAAA. [DOI] [PubMed] [Google Scholar]