Abstract
Accommodation refers to the acquired resistance of a graft to immune-mediated injury. It is typically observed after antibodies that would cause rejection of a graft are removed from a recipient and then return. Besides being a condition so induced, accommodation can occur spontaneously, without the depleting of antibodies. Indeed, we postulate that spontaneous accommodation may be the most common outcome of clinical organ transplantation. This communication will review the current understanding of accommodation, emphasizing recent advances and important questions. Among the recent advances are the discoveries of potentially broader relevance of accommodation for biology and immunology and pathways by which accommodation may be achieved. To investigate these pathways and to understand how accommodation begins and how it evolves, clinical organ transplants might offer a useful and incisive model.
Keywords: accommodation, allotransplantation, xenotransplantation, humoral rejection, blood groups
Accommodation refers to acquired resistance of an organ or tissue to immune-mediated injury [1–3]. It is defined operationally as a condition in which an organ transplant functions normally despite the presence of antibodies in the recipient specific for the transplant.
We first observed accommodation in ABO-incompatible kidney transplants [4, 5]. Prior experience had suggested that these transplants would have a poor prospect for enduring success. Gleason and Murray [6] reported that nearly half of 24 ABO-incompatible kidney transplants suffered early failure; Wilbrandt et al. [7] found that nine of 12 ABO-incompatible kidney transplants suffered early failure. In contrast, we found that if antibodies directed against allogeneic blood groups are removed from the circulation temporarily, the transplants might function for months or years without evident injury after anti-blood group antibodies return to the circulation [4]. Alexandre et al. [8, 9] made similar observations.
Questioning why the kidney grafts might have survived the inevitable return of anti-blood group A or anti-blood group B antibodies, we showed that the donor blood group antigen persists in ABO-incompatible kidney transplants [5] and the antibody that returns is quite capable of binding to blood group antigen and activating complement [10]. Hence, the absence of injury could not be ascribed to lack of anti-blood group antibody or the antigen it recognizes and must reflect something else that mitigates the process of rejection [5]. After preliminary studies of pig-to-primate xenografts revealed a few instances in which heterotopic cardiac grafts in recipients depleted of xenoreactive antibodies survived without substantial injury for days after xenoreactive antibody returned to the circulation, we speculated that these xenografts, like ABO-incompatible allografts, might be protected from injury, and we named this condition accommodation [11, 12]. Here we consider how accommodation might apply more broadly in transplantation.
Accommodation in Clinical Transplantation
Accommodation is sometimes considered an unusual outcome of transplantation, if it is considered at all. For example, the attendees at a consensus conference of the National Institutes of Health on antibody-mediated rejection considered accommodation to be “a latent response” or “silent humoral rejection” [13]. In the report of the most recent Banff Conference on the pathology of organ transplants, accommodation is mentioned in the text but not listed in the table of outcomes of kidney transplantation [14]. Despite these considerations, we postulate that accommodation may well be the most prevalent outcome of clinical organ transplantation.
We think accommodation of clinical allografts may occur more often than generally appreciated because immune responses to these grafts is common, while graft damage is not. Pelletier et al. [15] found that 52% of renal transplant recipients have a detectable delayed-type hypersensitivity response to donor antigens; yet most recipients have normal or nearly normal graft function. This response was assayed using a “trans-vivo DTH assay” in which peripheral blood leukocytes of transplant recipients were combined with sonicated leukocytes from graft donors and injected in the pinnae or foot pads of mice and swelling measured 24 hours later; what fraction of responses might be missed by this assay is not clear. Presumably this DTH response represents a T cell effector response directed at the graft and a T cell helper response for generating alloreactive antibodies. Adeyi et al. [16] evaluated alloantibody specificities in the serum of 27 recipients of kidney transplants who underwent removal of their transplants. Before nephrectomy, 11% of renal allograft recipients had detectable antibodies specific for the donor; after nephrectomy 97% (26 of 27 subjects) had detectable levels of antibodies directed against donor HLA. Although the increase in alloantibodies may reflect surgical trauma associated with removal of the grafts, the presence of those antibodies indicates that sensitization had occurred at some time prior to removal of the graft yet the antibodies were not detected. The de novo appearance of alloantibodies after graft nephrectomy suggests that alloantibodies can be fully absorbed by the allograft and should arouse skepticism about the sensitivity of measurements of anti-graft antibodies in the blood of graft recipients. Moreover, since many recipients of organ allografts produce antibodies against their grafts, the majority of allografts, with normal or nearly normal function, may well have accommodation.
Explaining “the Paradox” of Frequent Accommodation and Low Prevalence of Anti-HLA Antibodies
Anti-HLA antibodies are generally considered detrimental for organ transplants, and the presence of these antibodies in the blood of a transplant recipient predicts rejection [17–20]. Consistent with this concept, anti-HLA antibodies are detected infrequently in those with normally functioning transplants. Hence, if the operational definition of accommodation (normal graft function in a recipient with antibodies specific for the graft) is applied, accommodation must be correspondingly rare. How could one conceive that accommodation is a frequent outcome of organ transplantation? The answer is suggested by work in experimental systems.
Accommodation in Experimental Models
We explored accommodation in experimental organ transplants for nearly two decades. Generally, the model systems used involve the transplantation of organs between disparate species, and the recipients of these transplants had natural and acquired antibodies specific for the grafts [11, 21, 22]. In these model systems we found that depletion of all immunoglobulin or species-specific immunoglobulin could allow survival of the organ graft and accommodation to ensue (Figure 1) [23, 24]. Figure 1 shows the levels of xenoreactive antibodies in the blood of a xenograft recipient from which antibody was depleted from the time of transplantation. Xenoreactive antibodies are not detectable in the blood until rejection occurs on day 12. In separate studies in which an organ xenograft was not placed but antibodies were depleted, we found that xenoreactive antibodies return immediately to the circulation after depletion, despite treatment with immunosuppression [25, 26]. The results of a typical experiment are shown in Figure 1. Note in the figure that the antibodies studied (anti-Galα1-3Gal antibodies) return immediately to the circulation after specific depletion; but, are not detected in the circulation until approximately 10 days after transplantation of a xenogeneic heart. Analysis of serial biopsies during this 10-day period reveal that antibodies are bound to the heart and rejection is initiated before the antibodies are detected in the circulation. Thus, the results shown in Figure 1 suggest that while antibodies clearly cause rejection, the process of rejection precedes rather than follows the increase of antibodies in the blood. Moreover, when rejection was averted by expressing human complement regulatory proteins in the xenogeneic source, rather than by depleting antibodies, removal of a functioning transplant led to immediate increase of the level of xenoreactive antibodies in the blood [27]. These experimental observations led us to suggest that graft-specific antibodies might be produced in large amounts but might evade detection in the blood because those antibodies bound to the graft; and, as a corollary, the presence of antibodies may indicate that damage or decrease in blood flow has occurred [28].
Figure 1.
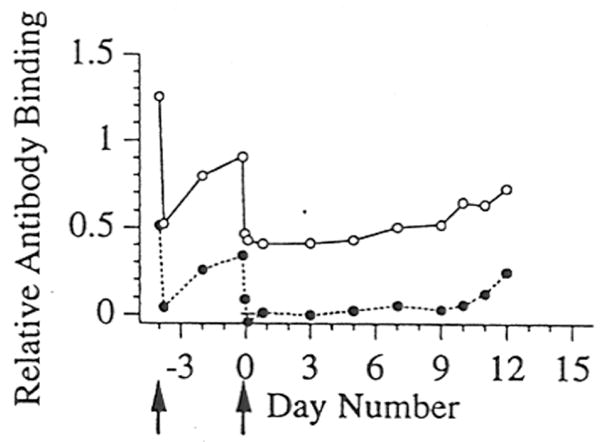
Levels of xenoreactive IgM and anti-Galα1-3Gal IgM in baboons following depletion of anti-Galα1-3Gal antibodies using affinity columns and hetertopic cardiac xenotransplantation. The relative levels of xenoreactive IgM (open circles) and anti-Galα1-3Gal IgM (filled circles) were determined by measuring the binding to cultured porcine endothelial cells. The arrows below the abscissa indicate the time at which a treatment with the Galα1-3Gal affinity columns was performed. Day “0” refers to the day of xenotransplantation. The standard error for each value is shown. (Adapted from Lin et al. Transplant Immunol 5:212, 1997.)
Estimating the Prevalence of Accommodation in Clinical Organ Transplantation
Given the experimental results described above, we would suggest several provisional concepts. First, the operational definition of accommodation (normal graft function in those with anti-donor antibodies in the circulation) probably misses accommodation in many cases. Second, accommodation might also occur but escape detection because anti-donor antibodies are cleared from the circulation and from graft cells. To address this limitation we have tried to use the presence of C4d bound to a graft in the absence of anti-donor antibodies as a way to identify a broader group of those with accommodation. However, for reasons discussed below, this definitions may also fail to identify many examples.
In any case, although increased levels of anti-graft antibodies detected at the time of rejection is often interpreted to suggest that the increase caused rejection, our studies suggest the opposite—the onset of rejection slows the flow of blood to the graft, and as a result less antibody is absorbed [28]. Given the high frequency of immune response to the graft and the relative infrequency of acute cellular or humoral rejection, one can conclude that what appear to be normal transplants on surveillance biopsies may well be accommodation.
The Fate of Bound Antibody and Mechanisms of Accommodation
Understanding how accommodation occurs and by what mechanism it is manifest is of obvious importance in clinical transplantation, transplantation biology and more broadly in immunology [29, 30]. We pursued this question using experimental model systems.
To explore the fate of antibody bound to grafts we used cultured endothelial cells as a model system. Cultured endothelial cells are recognized by antibodies specific for the source of a graft [11, 31]. Antibodies bound to metabolically active cultured endothelial cells are rapidly removed from the cell surface and may be metabolized [32]. Hence, one must “fix” endothelial cells to study the interaction of antibodies with cellular antigens [33, 34].
Assuming that endothelial cells in a graft can take up and metabolize large amounts of antibody directed against the graft, does this process account for accommodation? We suspect this process is only one of a range of changes in the graft that allow survival and function in the face of a humoral immune response.
We originally conceived that accommodation might reflect a change in antibodies, a change in the antigen or an acquired resistance of the graft to injury by antibodies and complement [11]. All three changes have been observed. Yu et al. [35] found that human IgG2 specific for Galα1-3Gal blocks binding of IgM and inhibits complement activation on target cells expressing Galα1-3Gal. Mohiuddin et al. [36] reported that accommodation in rodents might be associated with production of IgG subclass that inefficiently activates complement. Yu et al. [37] showed that new heavy chain variable region families are also utilized following a xenogeneic stimulus. However, the extent to which these changes, or other factors, limit injury in accommodation is unclear. And, since IgG2 antibodies are mainly observed in response to carbohydrate antigens, this mechanism can hardly explain accommodation of grafts in which recipients produce antibodies specific for MHC or other antigenic proteins in the graft.
Another potential mechanism of accommodation involves a change in the antigen targeted by humoral response after transplantation. Although specific changes in antigen have not been described in xenotransplantation, Ulfvin et al. [38] described changes in glycolipids following allotransplantation of kidneys. Yuzawa et al. [39] observed that the Forssmann antigen is internalized and/or shed with antibody binding and thus becomes a less abundant target of humoral responses. Adres et al. [40] described similar changes. However, the antigen expressed on endothelial cells of organ transplants do not appear to change in such a way that would explain accommodation [5, 32, 34, 41, 42].
The most compelling mechanism of accommodation involves changes in the graft that confer protection against injury caused by bound antibody and activation of complement. Several mechanisms of “protection” have been proposed. One potential type of protection may be conferred by exposure to sub-lethal amounts of a toxin or to an injurious but non-fatal process. For example, Nath et al. [43] showed that induction of heme oxygenase-1 protects against renal injury in rhabdomyolysis. Bach et al. [44] found that rodent xenografts with accommodation express genes that protect cells against apoptosis. Expression of these genes, such as heme oxygenase-1, has been linked to survival of grafts in the face of antibody-mediated rejection [45]. Delikouris et al. [46] found that binding of human IgG to porcine endothelial cells induces expression of cytoprotective genes. Jindra et al. [47] showed that binding of antibodies to MHC class I molecules can promote expression of proteins that protect cells against toxic challenges. But, whether products of these genes per se mediate accommodation remains to be tested. We found that cytoprotective genes are expressed not only in accommodation but also in rejection, at least prior to manifest necrosis [48, 49]. Others have found that these genes are not expressed at an increased level in accommodation of ABO-incompatible allografts [50]. Thus, expression of cytoprotective genes may be essential for graft survival, as the organs from mice with targeted disruption of the genes are rapidly destroyed after transplantation, but may or may not represent a part of the process of accommodation.
Grafts might also resist injury by manifesting heightened resistance to complement. Dalmasso et al. [51–53] found that xenoreactive antibodies induce expression of CD59, which controls the membrane attack complex of complement, and this change heightens resistance to complement-mediated lysis. Dorling et al. [54, 55] also found that xenoreactive IgG acting on porcine endothelial cells induces resistance to lysis. However, xenografts expressing CD59 as the product of a transgene do not avoid rejection [56]. In fact, the expression complement inhibitory proteins separately and together have thus far failed by itself to engender prolonged survival of xenografts unless xenoreactive antibodies or the antigen they recognize were also manipulated [57–60]. Consistent with the possibility that accommodation may reflect something other than improved control of complement, Grehan et al. [61] and Black et al. [62] found that IL-4 and IL-13 induce changes in endothelial cells that include heightened resistance to lysis, apoptosis, both evidently via the AKT/PI3 kinase pathways and independent of complement control. We found that hepatocytes naturally resist complement-mediated lysis owing to heightened control of complement independent of the level of expression of complement regulatory proteins but also requiring function of the AKT/PI3 kinase pathways [63].
Toward a Broader Concept of Accommodation
Acquired resistance to injury, as such, can be found in many biological systems [63–66]. Which if any of these systems represent pathways or mechanisms by which grafts acquire resistance to injury in accommodation is unknown. For example, treatment of drosophila with DDT induces production of cytochrome P450 and possibly other genes that confer resistance to further exposure to this toxic substance [67], although other genes must be involved [68]. Exposure of organs to ischemia “preconditions” organs to resist injury by subsequent episodes of ischemia. Treatment with lipopolysaccharide induces resistance to subsequent exposure to lipopolysaccharide. Treatment of cells with complement induces resistance to complement-mediated injury [69, 70]. A pathway that may be common to many types of acquired resistance to injury is the AKT/PI3 kinase pathway [61–63, 71].
Since accommodation is induced and not constitutive, we would assume that some dysphysiological impact must exist [66]. While accommodation can be thought to allow grafts to survive long enough to acquire chronic changes [28, 72], we have speculated that accommodation might allow chronic rejection to occur or that the process of accommodation might even cause chronic injury [2, 28, 66].
Toward a New Definition of Accommodation
Identifying and understanding accommodation depend of the development of a more compressive and sensitive definition of that condition. Clearly the operative definition mentioned above (presence of anti-donor antibodies in a subject with normal graft function) may fail to detect many instances of accommodation, and in fact this definition may identify the exceptional cases when antibody is not effectively cleared by the graft. A rigorous working definition of accommodation in experimental models might be: the continued function and/or survival of an engrafted organ when a freshly engrafted organ from the same source is rejected and perhaps the resistance of an “accommodated” organ to injury when engrafted in a new host that would otherwise reject an organ from that source. But, this definition cannot be used to study clinical grafts or grafts for which re-transplantation would pose a technical challenge. Perhaps we are best to allow the definition of accommodation to remain unsettled until we gain a more complete understanding of the mechanisms.
How then might accommodation be studied? Analysis of outcome of grafts using mice with targeted disruptions of the genes for one or another protective protein may provide clues about which proteins are essential for organ survival but probably cannot prove that a given protein is singularly important for accommodation. Identifying genes, the heightened or lowered expression of which is associated with accommodation, may also fail because the expression of many genes changes in the course of tissue injury, owing in part to influx of inflammatory or reparative cells rather than changes in expression per se, identifying the relevant genes (if any) would be a daunting exercise. Ultimately, the answer may emerge from methods to test whether the deliberate expression of one or a few molecules in a graft will protect that graft from humoral or other forms of toxic injury.
Conclusions
Accommodation should be pursued in those settings in which it is thought to occur naturally. For example, Segev et al. [73] recently reported on the transplantation of ABO-incompatible kidneys in four subjects with relatively high levels of isohemagglutinins specific for the graft. The subjects all had detectable isohemagglutinins although at low titers after transplantation, and perhaps accommodation. How these kidneys change over time might provide clues about what sustains accommodation and whether it has physiologic complications. Stussi et al. [74, 75] have studied ABO-incompatible bone marrow and stem cell transplants. These transplants are potentially impaired by the humoral barrier but only transiently. The authors suggest cogently that understanding how the grafts and the recipients accommodate to the transplants could provide clues to mechanisms of accommodation. Importantly, since these grafts are hematopoietic, the study of the grafts is rather easier than the study of allografted or xenografted organs. In a recent review of ABO-incompatible organ transplantation, Warner et al. [76] postulate that survival of grafts by accommodation may be a multi-step process, initially facilitated by cytoprotective factors and later by decreases in TNF-α, TGFβ and SMAD5; and increases in Muc1 and GFRA1. This concept of accommodation changing over time is quite compatible with the observations of Lin et al. [77] and Ogawa et al. [78] in rodents and West et al. [79] in human subjects that what begins as accommodation can become some different process, perhaps including tolerance.
We think accommodation should also be pursued by exploring the broad range of conditions in which immunological responses “protect” against infections and toxins. We have postulated that accommodation could provide an essential limb of the immune response, making tissues less susceptible to injury as foreign organisms are controlled and destroyed [2]. For example, the control of viral replication by cellular immunity may, in part, require the development of accommodation to prevent T cell-mediated cytotoxicity. This concept would place responsibility for host defense on the tissues to be defended as much as it does on the immune system. Of course, accommodation may have a biological cost (otherwise it would not have to be induced). This cost may include defects in function of accommodated cells and tissues, as the cellular machinery is diverted to opposing toxicity, and it may account for chronic changes seen in various types of chronic rejection [28]. Studying accommodation in this broader range of settings, rather than in those few instances in which recipients have normally functioning grafts with antibodies and circulating antibodies specific for those grafts, may allow greater and more rapid progress on this subject to be made.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL079067, HL52297 and GM96922).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cascalho M. Accommodation. Am J Transplant. In Press. [Google Scholar]
- 2.Koch CA, Khalpey ZI, Platt JL. Accommodation: preventing injury in transplantation and disease. J Immunol. 2004;172(9):5143. doi: 10.4049/jimmunol.172.9.5143. [DOI] [PubMed] [Google Scholar]
- 3.King KE, Warren DS, Samaniego-Picota M, Campbell-Lee S, Montgomery RA, Baldwin WM., 3rd Antibody, complement and accommodation in ABO-incompatible transplants. Curr Opin Immunol. 2004;16(5):545. doi: 10.1016/j.coi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Bannett AD, McAlack RF, Raja R, Baquero A, Morris M. Experiences with known ABO-mismatched renal transplants. Transplant Proc. 1987;19:4543. [PubMed] [Google Scholar]
- 5.Bannett AD, McAlack RF, Morris M, Chopek M, Platt JL. ABO incompatible renal transplantation: a qualitative analysis of native endothelial tissue ABO antigens after transplant. Transplant Proc. 1989;21:783. [PubMed] [Google Scholar]
- 6.Gleason RE, Murray JE. Report from kidney transplant registry: analysis of variables in the function of human kidney transplants. Transplantation. 1967;5:343. [Google Scholar]
- 7.Wilbrandt R, Tung KSK, Deodhar SD, Nakamoto S, Kolff WJ. ABO blood group incompatibility in human renal homotransplantation. Am J Clin Pathol. 1969;51:15. doi: 10.1093/ajcp/51.1.15. [DOI] [PubMed] [Google Scholar]
- 8.Alexandre GPJ, Squifflet JP, De Bruyere M, Latinne D, Moriau M, Ikabu N, Carlier M, Pirson Y. Splenectomy as a prerequisite for successful human ABO-incompatible renal transplantation. Transplant Proc. 1985;17:138. [Google Scholar]
- 9.Alexandre GPJ, Squifflet JP, De Bruyere M, Latinne D, Reding R, Gianello P, Carlier M, Pirson V. Present experiences in a series of 26 ABO-incompatible living donor renal allografts. Transplant Proc. 1987;19:4538. [PubMed] [Google Scholar]
- 10.Parker W, Lundberg-Swanson K, Holzknecht ZE, Lateef J, Washburn SA, Braedehoeft SJ, Platt JL. Isohemagglutinins and xenoreactive antibodies are members of a distinct family of natural antibodies. Hum Immunol. 1996;45:94. doi: 10.1016/0198-8859(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 11.Platt JL, Vercellotti GM, Dalmasso AP, Matas AJ, Bolman RM, Najarian JS, Bach FH. Transplantation of discordant xenografts: a review of progress. Immunol Today. 1990;11:450. doi: 10.1016/0167-5699(90)90174-8. [DOI] [PubMed] [Google Scholar]
- 12.Platt JL, Fischel RJ, Matas AJ, Reif SA, Bolman RM, Bach FH. Immunopathology of hyperacute xenograft rejection in a swine-to-primate model. Transplantation. 1991;52:214. doi: 10.1097/00007890-199108000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, Kupiec-Weglinski J, Matas A, Montgomery RA, Nickerson P, Platt JL, Rabb H, Thistlethwaite R, Tyan D, Delmonico FL. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4(7):1033. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 14.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madrigal L, Salomon DR, Seron D, Sheaff M, Weening JJ. Banff ‘05 meeting report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’) Am J Transplant. 2007;7:518. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 15.Pelletier RP, Hennessy PK, Adams PW, Orosz CG. High incidence of donor-reactive delayed-type hypersensitivity reactivity in transplant patients. Am J Transplant. 2002;2(10):926. doi: 10.1034/j.1600-6143.2002.21008.x. [DOI] [PubMed] [Google Scholar]
- 16.Adeyi OA, Girnita AL, Howe J, Marrari M, Awadalla Y, Askar M, Martell J, Zeevi A, Shapiro R, Nalesnik M, Randhawa P, Demetris AJ, Duquesnoy RJ. Serum analysis after transplant nephrectomy reveals restricted antibody specificity patterns against structurally defined HLA class I mismatches. Transpl Immunol. 2005;14(1):53. doi: 10.1016/j.trim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Liang LW, Gjertson DW, Lassman C, Wilkinson AH, Kendrick E, Pham PT, Danovitch GM, Gritsch HA, Reed EF. Development of posttransplant antidonor HLA antibodies is associated with acute humoral rejection and early graft dysfunction. Transplantation. 2005;79(5):591. doi: 10.1097/01.tp.0000155246.52249.ac. [DOI] [PubMed] [Google Scholar]
- 18.Mizutani K, Terasaki P, Rosen A, Esquenazi V, Miller J, Shih RN, Pei R, Ozawa M, Lee J. Serial ten-year follow-up of HLA and MICA antibody production prior to kidney graft failure. Am J Transplant. 2005;5(9):2265. doi: 10.1111/j.1600-6143.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- 19.Vasilescu ER, Ho EK, Colovai AI, Vlad G, Foca-Rodi A, Markowitz GS, D’Agati V, Hardy MA, Ratner LE, Suciu-Foca N. Alloantibodies and the outcome of cadaver kidney allografts. Hum Immunol. 2006;67(8):597. doi: 10.1016/j.humimm.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Lefaucheur C, Nochy D, Hill GS, Suberbielle-Boissel C, Antoine C, Charron D, Glotz D. Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am J Transplant. 2007;7(4):832. doi: 10.1111/j.1600-6143.2006.01686.x. [DOI] [PubMed] [Google Scholar]
- 21.Parker W, Bruno D, Platt JL. Xenoreactive natural antibodies in the world of natural antibodies: typical or unique? Transpl Immunol. 1995;3:181. doi: 10.1016/0966-3274(95)80024-7. [DOI] [PubMed] [Google Scholar]
- 22.Cascalho M, Platt JL. Xenotransplantation and other means of organ replacement. Nat Rev Immunol. 2001;1:154. doi: 10.1038/35100578. [DOI] [PubMed] [Google Scholar]
- 23.Lin SS, Weidner BC, Byrne GW, Diamond LE, Lawson JH, Hoopes CW, Daniels LJ, Daggett CW, Parker W, Harland RC, Davis RD, Bollinger RR, Logan JS, Platt JL. The role of antibodies in acute vascular rejection of pig-to-baboon cardiac transplants. J Clin Invest. 1998;101:1745. doi: 10.1172/JCI2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin SS, Hanaway MJ, Gonzalez-Stawinski GV, Lau CL, Parker W, Davis RD, Byrne GW, Diamond LE, Logan JS, Platt JL. The role of anti-Galα1-3Gal antibodies in acute vascular rejection and accommodation of xenografts. Transplantation (Rapid Communication) 2000;70:1667. doi: 10.1097/00007890-200012270-00002. [DOI] [PubMed] [Google Scholar]
- 25.Lin SS, Kooyman DL, Daniels LJ, Daggett CW, Parker W, Lawson JH, Hoopes CW, Gullotto C, Li L, Birch P, Davis RD, Diamond LE, Logan JS, Platt JL. The role of natural anti-Galα1-3Gal antibodies in hyperacute rejection of pig-to-baboon cardiac xenotransplants. Transpl Immunol. 1997;5:212. doi: 10.1016/s0966-3274(97)80040-8. [DOI] [PubMed] [Google Scholar]
- 26.Lin SS, Platt JL. The role of immunoabsorption in xenotransplantation. In: Brunkhorst R, Koch KM, Koll R, editors. Klinische immunadsorption. Stuttgart: Wissenschaftliche Verlagsgesellschaft mbH; 2000. p. 133. [Google Scholar]
- 27.McCurry KR, Parker W, Cotterell AH, Weidner BC, Lin SS, Daniels LJ, Holzknecht ZE, Byrne GW, Diamond LE, Logan JS, Platt JL. Humoral responses in pig-to-baboon cardiac transplantation: implications for the pathogenesis and treatment of acute vascular rejection and for accommodation. Hum Immunol. 1997;58:91. doi: 10.1016/s0198-8859(97)00229-2. [DOI] [PubMed] [Google Scholar]
- 28.Platt JL. C4d and the fate of organ allografts. J Am Soc Nephrol. 2002;13(9):2417. doi: 10.1097/01.asn.0000030140.74450.0b. [DOI] [PubMed] [Google Scholar]
- 29.Cozzi E, Bosio E, Seveso M, Vadori M, Ancona E. Xenotransplantation-current status and future perspectives. Br Med Bull. 2005:75–76. doi: 10.1093/bmb/ldh061. [DOI] [PubMed] [Google Scholar]
- 30.Koch CA, Jordan CE, Platt JL. Complement-dependent control of teratoma formation by embryonic stem cells. J Immunol. 2006;177:4803. doi: 10.4049/jimmunol.177.7.4803. [DOI] [PubMed] [Google Scholar]
- 31.Dalmasso AP, Vercellotti GM, Fischel RJ, Bolman RM, Bach FH, Platt JL. Mechanism of complement activation in the hyperacute rejection of porcine organs transplanted into primate recipients. Am J Pathol. 1992;140:1157. [PMC free article] [PubMed] [Google Scholar]
- 32.Parker W, Holzknecht ZE, Song A, Blocher BA, Bustos M, Reissner KJ, Everett ML, Platt JL. Fate of antigen in xenotransplantation: implications for acute vascular rejection and accommodation. Am J Pathol. 1998;152:829. [PMC free article] [PubMed] [Google Scholar]
- 33.Lin SS, Parker W, Everett ML, Platt JL. Differential recognition by proteins of α-galactosyl residues on endothelial cell surfaces. Glycobiology. 1998;8:433. doi: 10.1093/glycob/8.5.433. [DOI] [PubMed] [Google Scholar]
- 34.Parker W, Stitzenberg KB, Yu PB, Pratt VSW, Nakamura YC, Farel LS, Ward CM, Everett ML, Lin SS, Platt JL. Biophysical characteristics of anti-Galα1-3Gal IgM binding to cell surfaces: implications for xenotransplantation. Transplantation. 2001;71:440. doi: 10.1097/00007890-200102150-00018. [DOI] [PubMed] [Google Scholar]
- 35.Yu PB, Holzknecht ZE, Bruno D, Parker W, Platt JL. Modulation of natural IgM binding and complement activation by natural IgG antibodies. J Immunol. 1996;157:5163. [PubMed] [Google Scholar]
- 36.Mohiuddin MM, Ogawa H, Yin DP, Shen J, Galili U. Antibody-mediated accommodation of heart grafts expressing an incompatible carbohydrate antigen. Transplantation. 2003;75(3):258. doi: 10.1097/01.TP.0000053616.61907.D5. [DOI] [PubMed] [Google Scholar]
- 37.Yu PB, Parker W, Nayak JV, Platt JL. Sensitization with xenogeneic tissues alters the heavy chain repertoire of human anti-Galalpha1-3Gal antibodies. Transplantation. 2005;80(1):102. doi: 10.1097/01.tp.0000162976.07023.6d. [DOI] [PubMed] [Google Scholar]
- 38.Ulfvin A, Backer AE, Clausen H, Hakomori S, Rydberg L, Samuelsson BE, Breimer ME. Expression of glycolipid blood group antigens in single human kidneys: change in antigen expression of rejected ABO incompatible kidney grafts. Kidney Int. 1993;44:1289. doi: 10.1038/ki.1993.381. [DOI] [PubMed] [Google Scholar]
- 39.Yuzawa Y, Brentjens JR, Brett J, Caldwell PRB, Esposito C, Fukatsu A, Godman G, Stern D, Andres G. Antibody-mediated redistribution and shedding of endothelial antigens in the rabbit. J Immunol. 1993;150:5633. [PubMed] [Google Scholar]
- 40.Andres G, Yamaguchi N, Brett J, Caldwell PRB, Godman G, Stern D. Cellular mechanisms of adaptation of grafts to antibody. Transpl Immunol. 1996;4:1. doi: 10.1016/s0966-3274(96)80027-x. [DOI] [PubMed] [Google Scholar]
- 41.Parker W, Lin SS, Platt JL. Antigen expression in xenotransplantation: how low must it go? Transplantation. 2001;71:313. doi: 10.1097/00007890-200101270-00025. [DOI] [PubMed] [Google Scholar]
- 42.Everett ML, Lin SS, Worrell SS, Platt J, Parker W. The footprint of antibody bound to pig cells: evidence of complex surface topology. Biochem Biophys Res Commun. 2003;301:751. doi: 10.1016/s0006-291x(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 43.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bach FH, Ferran C, Hechenleitner P, Mark W, Koyamada N, Miyatake T, Winkler H, Badrichani A, Cardinas D, Hancock WH. Accommodation of vascularized xenografts: Expression of “protective genes” by donor endothelial cells in a host Th2 cytokine environment. Nat Med. 1997;3:196. doi: 10.1038/nm0297-196. [DOI] [PubMed] [Google Scholar]
- 45.Hancock WW, Buelow R, Sayegh MH, Turka LA. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med. 1998;4(12):1392. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 46.Delikouras A, Hayes M, Malde P, Lechler RI, Dorling A. Nitric oxide-mediated expression of Bcl-2 and Bcl-xl and protection from TNFα-mediated apoptosis in porcine endothelial cells after exposure to low concentrations of xenoreactive natural antibody. Transplantation. 2001;71(5):599. doi: 10.1097/00007890-200103150-00004. [DOI] [PubMed] [Google Scholar]
- 47.Jindra PT, Zhang X, Mulder A, Claas F, Veale J, Jin YP, Reed EF. Anti-HLA antibodies can induce endothelial cell survival or proliferation depending on their concentration. Transplantation. 2006;82(1 Suppl):S33. doi: 10.1097/01.tp.0000231447.34240.3c. [DOI] [PubMed] [Google Scholar]
- 48.Holzknecht ZE, Kuypers KL, Plummer TB, Williams J, Bustos M, Gores GJ, Brunn GJ, Platt JL. Apoptosis and cellular activation in the pathogenesis of acute vascular rejection. Circ Res. 2002;91(12):1135. doi: 10.1161/01.res.0000046236.20251.fa. [DOI] [PubMed] [Google Scholar]
- 49.Saadi S, Takahashi T, Holzknecht RA, Platt JL. Pathways to acute humoral rejection. Am J Pathol. 2004;164(3):1073. doi: 10.1016/S0002-9440(10)63194-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park W, Grande JPDN, Nath KA, Platt JL, Gloor JM, Stegall MD. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant. 2003;3(8):952. doi: 10.1034/j.1600-6143.2003.00179.x. [DOI] [PubMed] [Google Scholar]
- 51.Dalmasso AP, He T, Benson BA. Human IgM xenoreactive antibodies can induce resistance of porcine endothelial cells to complement-mediated injury. Xenotransplantation. 1996;3:54. [Google Scholar]
- 52.Dalmasso AP, Benson BA, Johnson JS, Lancto C, Abrahamsen MS. Resistance against the membrane attack complex of complement induced in porcine endothelial cells with a Gal alpha(1-3)Gal binding lectin: up-regulation of CD59 expression. J Immunol. 2000;164(7):3764. doi: 10.4049/jimmunol.164.7.3764. [DOI] [PubMed] [Google Scholar]
- 53.Grubbs BC, Benson BA, Dalmasso AP. Characteristics of CD59 up-regulation induced in porcine endothelial cells by alphaGal ligation and its association with protection from complement. Xenotransplantation. 2003;10(5):387. doi: 10.1034/j.1399-3089.2003.02088.x. [DOI] [PubMed] [Google Scholar]
- 54.Dorling A, Stocker C, Tsao T, Haskard DO, Lechler RI. In vitro accommodation of immortalized porcine endothelial cells: resistance to complement mediated lysis and down-regulation of VCAM expression induced by low concentrations of polyclonal human IgG antipig antibodies. Transplantation. 1996;62(8):1127. doi: 10.1097/00007890-199610270-00018. [DOI] [PubMed] [Google Scholar]
- 55.Dorling A, Delikouras A, Nohadani M, Polak J, Lechler RI. In vitro accommodation of porcine endothelial cells by low dose human anti-pig antibody: reduced binding of human lymphocytes by accommodated cells associated with increased nitric oxide production. Xenotransplantation. 1998;5(1):84. doi: 10.1111/j.1399-3089.1998.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 56.Diamond LE, McCurry KR, Oldham ER, McClellan SB, Martin MJ, Platt JL, Logan JS. Characterization of transgenic pigs expressing functionally active human CD59 on cardiac endothelium. Transplantation. 1996;61:1241. doi: 10.1097/00007890-199604270-00021. [DOI] [PubMed] [Google Scholar]
- 57.Cozzi E, Bhatti F, Schmoeckel M, Chavez G, Smith KG, Zaidi A, Bradley JR, Thiru S, Goddard M, Vial C, Ostlie D, Wallwork J, White DJ, Friend PJ. Long-term survival of nonhuman primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation. 2000;70(1):15. [PubMed] [Google Scholar]
- 58.Diamond LE, Quinn CM, Martin MJ, Lawson J, Platt JL, Logan JS. A human CD46 transgenic pig model system for the study of discordant xenotransplantation. Transplantation. 2001;71:132. doi: 10.1097/00007890-200101150-00021. [DOI] [PubMed] [Google Scholar]
- 59.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, O’Malley P, Nobori S, Vagefi PA, Patience C, Fishman J, Cooper DK, Hawley RJ, Greenstein J, Schuurman HJ, Awwad M, Sykes M, Sachs DH. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 60.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K, Hisashi Y, Mueller N, Yamada K, Greenstein JL, Hawley RJ, Patience C, Awwad M, Fishman JA, Robson SC, Schuurman HJ, Sachs DH, Cooper DK. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11(1):29. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 61.Grehan JF, Levay-Young BK, Fogelson JL, Francois-Bongarcon V, Benson BA, Dalmasso AP. IL-4 and IL-13 induce protection of porcine endothelial cells from killing by human complement and from apoptosis through activation of a phosphatidylinositide 3-kinase/Akt pathway. J Immunol. 2005;175(3):1903. doi: 10.4049/jimmunol.175.3.1903. [DOI] [PubMed] [Google Scholar]
- 62.Black SM, Grehan JF, Rivard AL, Benson BA, Wahner AE, Koch AE, Levay-Young BK, Dalmasso AP. Porcine endothelial cells and iliac arteries transduced with AdenoIL-4 are intrinsically protected, through Akt activation, against immediate injury caused by human complement. J Immunol. 2006;177(10):7355. doi: 10.4049/jimmunol.177.10.7355. [DOI] [PubMed] [Google Scholar]
- 63.Koch CA, Kanazawa A, Nishitai R, Knudsen BE, Ogata K, Plummer TB, Butters K, Platt JL. Intrinsic resistance of hepatocytes to complement-mediated injury. J Immunol. 2005;174:7302. doi: 10.4049/jimmunol.174.11.7302. [DOI] [PubMed] [Google Scholar]
- 64.Holzknecht ZE, Platt JL. The fine cytokine line between graft acceptance and rejection. Nat Med. 2000;6(5):497. doi: 10.1038/74963. [DOI] [PubMed] [Google Scholar]
- 65.Holzknecht ZE, Platt JL. Accommodation and the reversibility of biological systems. Transplantation. 2001;71:594. doi: 10.1097/00007890-200103150-00002. [DOI] [PubMed] [Google Scholar]
- 66.Platt JL, Nath KA. Heme oxygenase: protective gene or Trojan horse. Nat Med. 1998;4(12):1364. doi: 10.1038/3947. [DOI] [PubMed] [Google Scholar]
- 67.Daborn PJ, Yen JL, Bogwitz MR, Le Goff G, Feil E, Jeffers S, Tijet N, Perry T, Heckel D, Batterham P, Feyereisen R, Wilson TG, ffrench-Constant RH. A single p450 allele associated with insecticide resistance in Drosophila. Science. 2002;297(5590):2253. doi: 10.1126/science.1074170. [DOI] [PubMed] [Google Scholar]
- 68.Kuruganti S, Lam V, Zhou X, Bennett G, Pittendrigh BR, Ganguly R. High expression of Cyp6g1, a cytochrome P450 gene, does not necessarily confer DDT resistance in Drosophila melanogaster. Gene. 2007;388(1–2):43. doi: 10.1016/j.gene.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 69.Carney DF, Lang TJ, Shin ML. Multiple signal messengers generated by terminal complement complexes and their role in terminal complement complex elimination. J Immunol. 1990;145:623. [PubMed] [Google Scholar]
- 70.Marchbank KJ, Van Den Berg CW, Morgan BP. Mechanisms of complement resistance induced by non-lethal complement attack and by growth arrest. Immunology. 1997;90:647. doi: 10.1046/j.1365-2567.1997.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Narayanan K, Jendrisak MD, Phelan DL, Mohanakumar T. HLA class I antibody mediated accommodation of endothelial cells via the activation of PI3K/cAMP dependent PKA pathway. Transpl Immunol. 2006;15(3):187. doi: 10.1016/j.trim.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Smith RN, Kawai T, Boskovic S, Nadazdin O, Sachs DH, Cosimi AB, Colvin RB. Chronic antibody mediated rejection of renal allografts: pathological, serological and immunologic features in nonhuman primates. Am J Transplant. 2006;6(8):1790. doi: 10.1111/j.1600-6143.2006.01351.x. [DOI] [PubMed] [Google Scholar]
- 73.Segev DL, Simpkins CE, Warren DS, King KE, Shirey RS, Maley WR, Melancon JK, Cooper M, Kozlowski T, Montgomery RA. ABO incompatible high-titer renal transplantation without splenectomy or anti-CD20 treatment. Am J Transplant. 2005;5(10):2570. doi: 10.1111/j.1600-6143.2005.01031.x. [DOI] [PubMed] [Google Scholar]
- 74.Stussi G, Halter J, Schanz U, Seebach JD. ABO-histo blood group incompatibility in hematopoietic stem cell and solid organ transplantation. Transfus Apher Sci. 2006;35(1):59. doi: 10.1016/j.transci.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 75.Stussi G, West L, Cooper DK, Seebach JD. ABO-incompatible allotransplantation as a basis for clinical xenotransplantation. Xenotransplantation. 2006;13(5):390. doi: 10.1111/j.1399-3089.2006.00324.x. [DOI] [PubMed] [Google Scholar]
- 76.Warner PR, Nester TA. ABO-incompatible solid-organ transplantation. Am J Clin Pathol. 2006;125 (Suppl):S87. doi: 10.1309/8W4X9H6F8FTLCGYX. [DOI] [PubMed] [Google Scholar]
- 77.Lin Y, Vandeputte M, Waer M. Accommodation and T-independent B cell tolerance in rats with long term surviving hamster heart xenografts. J Immunol. 1998;160(1):369. [PubMed] [Google Scholar]
- 78.Ogawa H, Mohiuddin MM, Yin DP, Shen J, Chong AS, Galili U. Mouse-heart grafts expressing an incompatible carbohydrate antigen. II. Transition from accommodation to tolerance. Transplantation. 2004;77(3):366. doi: 10.1097/01.TP.0000109276.57772.6D. [DOI] [PubMed] [Google Scholar]
- 79.Fan X, Ang A, Pollock-BarZiv SM, Dipchand AI, Ruiz P, Wilson G, Platt JL, West LJ. Donor-specific B-cell tolerance after ABO-incompatible infant heart transplantation. Nat Med. 2004;10(11):1227. doi: 10.1038/nm1126. [DOI] [PubMed] [Google Scholar]


