Abstract
During early ontogeny heterogeneous rats are sensitive to ethanol’s stimulating effects. In adulthood locomotor activity in a novel environment is a valuable predictor of acute sensitivity to the activating effects of various drugs, including ethanol. Environmental novelty modulates response to ethanol and other drugs in adult rats. The present study analyzed the role of novelty in the acute locomotor response induced by ethanol earlier in development, during the preweanling period, a stage characterized by enhanced sensitivity to ethanol’s reinforcing effects. In Experiment 1 we evaluated the predictive value of baseline locomotor activity upon ethanol-induced locomotor effects in 12-day-old rats. In Experiment 2 we tested whether repeated familiarization with the testing environment would reduce the stimulating effects induced by ethanol on postnatal day 12. Individual differences in response to an inescapable novel environment significantly predicted the locomotor activating effects of ethanol, but not other acute effects of the drug, such as hypothermia, motor impairment or sedation. Behavioral activation induced by ethanol during the preweanling period was attenuated after familiarization with the testing environment, suggesting that environmental novelty is critical for activating effects of ethanol.
Keywords: Ethanol, preweanling rat, novelty, stimulation
Environmental novelty is an important factor for modulation of response to drugs of abuse (Caprioli, Celentano, Paolone, & Badiani, 2007). Acute or chronic locomotor-stimulating effects induced by a variety of drugs such as amphetamine or cocaine are, for example, more pronounced when subjects are tested in a novel environment (e.g. Badiani, Browman, & Robinson, 1995; Carey, DePalma, & Damianopoulos, 2005; Crombag, Badiani, & Robinson, 1996). Cocaine self-administration is also enhanced in novel environments (Caprioli et al., 2007). Furthermore, response to novelty is a valuable predictor of initial drug use and acute and chronic sensitivity to psychostimulant drugs (Cools & Gingras, 1998; Kabbaj, 2006; Piazza, Deminiere, Le Moal, & Simon, 1989). For example, sensitivity to acute and chronic effects of psychoactive drugs, including ethanol, is higher in subpopulations of rats that display higher levels of locomotion in a novel environment (referred to as “high responders”) compared to rats with lower levels of activity (“low responders”, Kabbaj, 2004, 2006; Piazza et al., 1989; Cools & Gingras, 1998; Gingras & Cools, 1996). In addition, enhanced locomotor activity in the open field or delayed habituation to a novel environment is also a valuable predictor of ethanol consumption and self-administration (e.g. Bisaga & Kostowski, 1993; Nadal, Armario, & Janak, 2002), although other studies have failed to find this association (e.g. Bienkowski, Koros, & Kostowski, 2001; Cools & Gingras, 1998; Koros, Piasecki, Kostowski, & Bienkowski, 1998). Overall, these studies indicate the relevance of individual differences in responsiveness to novel environments when analyzing locomotor and motivational effects of drugs of abuse.
In adult rats, novel environments induce a neurochemical and endocrine response characterized by an increase in catecholaminergic activity in the prefrontal cortex and nucleus accumbens as well as activation of the hypothalamic-pituitary-adrenal (HPA) axis (Rebec, Grabner, Johnson, Pierce, & Bardo, 1997). These neurochemical outcomes seem to underlie the enhanced response to psychostimulant drugs promoted by novel surroundings, and they have been interpreted as a stress response, since other stressors also increase the acute and chronic effects of drugs of abuse (Badiani, Morano, Akil, & Robinson, 1995). Preweanling rats avoid novel surroundings until postnatal day 19 (PD 19) and when younger infants are placed in an inescapable novel environment they show an increased locomotor activity pattern that seems to reflect distress or fear rather than exploration (Campbell & Raskin, 1978). Therefore, it is plausible that this state of arousal also affects responsiveness to drugs of abuse.
Ethanol induces stimulating effects in a variety of heterogeneous mouse strains (Dudek & Phillips, 1990; Dudek, Phillips, & Hahn, 1991; Randall, Carpenter, Lester, & Friedman, 1975), while adult heterogeneous rats typically show a dose-response suppression of locomotion after ethanol treatment (Chuck, McLaughlin, Arizzi-LaFrance, Salamone, & Correa, 2006). We recently reported, however, that preweanling heterogeneous rats are sensitive to ethanol’s activating effect. Relatively high ethanol doses (1.25 or 2.5 g/kg) increased locomotor activity when pups were tested during the initial phase of the blood ethanol curve in a novel environment (Arias, Mlewski, Molina, & Spear, in press; Arias, Mlewski, Molina, & Spear, in press; Arias, Molina, Mlewski, Pautassi, & Spear, 2008). Susceptibility to this stimulating effect of ethanol varies across the preweanling period: 8 and 12-day old rats are more sensitive than 15-day-old rats (Arias et al., in press). It is interesting to note that during early ontogeny preweanling heterogeneous rats seem to be particularly sensitive to ethanol’s reinforcing effects. Voluntary ethanol consumption is higher in 8- and 12-day-old infant rats than in later stages of development (Sanders & Spear, 2007; Truxell, Molina, & Spear, 2007). During the first and second postnatal weeks infants are highly sensitive to appetitive reinforcement by ethanol (Arias & Chotro, 2006; Cheslock et al., 2001; Chotro & Arias, 2007; Hunt, Spear, & Spear, 1991; Petrov, Varlinskaya, & Spear, 2003) and seem more resistant to the aversive consequences of the drug (Arias & Chotro, 2006; Hunt et al., 1991). Acute tolerance to ethanol motor impairment, for example, is more pronounced in preweanling than in adult heterogeneous rats (Arias et al., 2008; Silveri & Spear, 2001).
Considering these antecedents, we postulated two hypotheses: a) individual differences in response to an inescapable novel environment during the preweanling period will predict the locomotor activating effects of ethanol; and b) the behavioral activation induced by ethanol during the preweanling period will be modulated by novelty of the testing environment.
The present study was designed to evaluate these hypotheses. In Experiment 1, we evaluated the predictive value of baseline locomotor activity in a novel environment upon ethanol-induced locomotor effects. In Experiment 2, we analyzed whether experience with the testing environment reduced the locomotor stimulating effects induced by ethanol. Experiment 1 and 2 were both conducted with 12-day-old rats. Past animal research on individual differences in susceptibility to drugs of abuse has used adult rodents. If an association between response to novelty and susceptibility to drugs of abuse is also present during early ontogeny, the present study may contribute to research focused on early detection of traits that may help to predict differential responses to drugs of abuse.
EXPERIMENT 1
The sensitivity of preweanling rats to the biphasic motor effects of ethanol has been established (Arias et al., in press; Arias et al., 2008). Relatively high ethanol doses increased locomotion when rats were tested during the rising phase of the blood ethanol curve (Arias et al., in press; Arias et al., in press; Arias et al., 2008). In contrast, when infants were tested at peak blood ethanol levels (30 minutes post-administration) infants showed a marked suppression in locomotor activity (Arias et al., 2008). In the present experiment, baseline locomotor activity was measured on PD 11. On PD 12 pups were tested in response to ethanol (0.0, 1.25 or 2.5 g/kg) either 5–10 minutes (Experiment 1a) or 25–30 min (Experiment 1b) after ethanol administration. The dependent variables analyzed on PD 12 were locomotion, rectal temperature and latency to complete the righting reflex.
In view of studies conducted with adult rats (Gingras & Cools, 1996; Hoshaw & Lewis, 2001), our working hypothesis is that baseline activity will predict the stimulating effect of ethanol, but not the sedative effects of the drug. Latency to complete the righting reflex and rectal temperature were registered immediately after the locomotor activity test to explore whether baseline activity predicts other disruptive effects of ethanol that may eventually modulate locomotor responses to the drug. In Experiment 1c, we studied a possible association between locomotor activity in a novel environment and ethanol pharmacokinetics that may account for the results obtained in Experiments 1a or 1b.
Material and Methods
Subjects
Forty-eight Sprague-Dawley pups (24 females and 24 males), representative of 8 litters, were utilized for each of the experiments 1a and 1b. Animals were born and reared at the vivarium of the Center for Development and Behavioral Neuroscience (Binghamton University, NY) under conditions of constant room temperature (22 ± 1.0 °C), on a 12-hour light 12-hour dark cycle. Births were examined daily and the day of parturition was considered postnatal day 0 (PD0). All litters were culled to 10 pups (5 females and 5 males, whenever possible) within 48 hours after birth. All procedures were in accordance with the guidelines for animal care and use established by the National Institute of Health (1986) and the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) as indicated by the Binghamton University institutional animal care and use committee.
Procedures
Phase 1: Baseline activity
On PD 11, pups from a given litter were separated from their mothers and placed in couples in a holding maternity cage (45 × 20 × 20 cm) partially filled with clean wood shavings. The floor of the cage was maintained at 33° C (± 1° C) through the use of a heating pad. One hour later, locomotor activity was evaluated in a Plexiglas container (10 × 10 × 12 cm). The floor of this environment was lined with a clean piece of absorbent paper for each subject. A circuit board (2-cm in width) surrounded the four sides of each chamber. This board had six infrared photo emitters and six infrared photoreceptors. The photo beams crossed the chamber generating a matrix of nine cells that allowed measurement of overall activity. Custom-made software developed by W. Kashinsky served to analyze the number of beams crossed by each subject every 10th of a second. Each activity test continued for 5 minutes. In a prior pilot study, this measure (number of beams broken) was highly and positively correlated with time spent walking and wall climbing during the preweanling period, holding experimental conditions constant (PD8: rxy = 0.89, n=15; PD12: rxy = 0.85, n=15, all ps < 0.0001; rxy represents Pearson’s product-moment correlation coefficient). For the second phase of the experiment, from each litter we selected the three pups with the highest and three pups with the lowest locomotor activity scores. This criterion was chosen to keep the highest variance in baseline activity, a procedure that can facilitate the detection of an association between baseline activity and ethanol’s effects.
Phase 2: Locomotor, thermal and motor impairment effects of ethanol
On PD 12, those six pups (with the highest and lowest baseline activity scores) from a given litter selected in the previous phase were separated from their mothers and placed in a holding maternity cage under the same conditions as Phase 1. From a given litter, those three subjects that showed highest baseline activity scores were quasi-randomly assigned to one specific ethanol condition (0.0, 1.25 or 2.5 g/kg). We explicitly avoided assigning more than one pup of the same sex and litter to the same ethanol condition. The same criterion of distribution was employed to assign pups that displayed lower activity levels during baseline. One hour later, pup’s body weights were individually recorded (± 0.01 g) and they immediately received an intragastric (i.g.) administration of ethanol (1.25 or 2.5 g/kg). The volume administered was equivalent to 0.015 ml per gram of body weight of a 10.5 % or 21 % v/v ethanol solution, respectively. An equivalent volume of water was administered to pups that were assigned to the vehicle control group. Intragastric administrations were performed using a 10-cm length of polyethylene tubing (PE-10 Clay Adams, Parsippany, New Jersey) attached to a 1 ml syringe with a 27 G × 1/2 needle. This tubing was gently introduced through the mouth and slowly guided into the stomach. The entire procedure took less than 20 seconds per pup.
Five (Experiment 1a) or 25 minutes (Experiment 1b) later, locomotor activity was assessed following the procedures described for the baseline activity. These post-administrations intervals were selected based on prior studies. Previously, in preweanling rats, we observed that 2.5 g/kg ethanol exerted locomotor stimulation in the first post-administration interval, and induced locomotor sedation in the later one (Arias et al., in press; Arias et al., 2008).
Rectal temperature and righting reflex assessment
Immediately after the locomotor activity test, pups were placed in a supine position over a smooth flat surface. Latency to complete the righting reflex was recorded as the time (in seconds) required for the animal to transition into the prone position. After this test, rectal temperature was recorded using a Physitemp Temperature Monitor (TH8 Model, Clifton, NJ) equipped with a rectal probe (RET-3, tip diameter: 0.065 in.). This probe was lubricated with mineral oil, kept at room temperature, and was then inserted 1 cm into the rectum. Temperature recordings were obtained 20 s following insertion of the probe.
Determination of blood ethanol concentration (BEC)
A different set of subjects (64 Sprague-Dawley pups, 32 females and 32 males, representative of 8 litters) was employed for the analysis of BECs. Baseline activity was registered on PD 11 and ethanol treatment was administered on PD 12 following procedures described in the previous section. Eight pups from each litter were selected for the present experiment. On PD 12, each of the four subjects from a given litter that showed the highest baseline activity scores was assigned to one of the four possible combinations of ethanol dose (1.25 or 2.5 g/kg) and post-administration interval (10 or 30 min). The same criterion of distribution was employed to assign pups that displayed the lowest activity levels during baseline. One hour later, body weights were individually recorded (± 0.01 g) and pups received an i.g. administration of 1.25 or 2.5 g/kg ethanol following the procedure described before.
Pups were sacrificed 10 or 30 minutes after receiving the corresponding ethanol dose, time points which coincided with the end of the testing intervals selected for Experiments 1a and 1b. Trunk blood was obtained following decapitation. Blood samples were collected using a heparinized capillary tube. The blood samples were immediately centrifuged (6.000 rpm; Micro-Haematocrit Centrifuge, Hawksley & Sons LTD, Sussex, England) and stored at −70 °C. BECs were determined using an AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA). Calculation of BECs was made by oxidating ethanol to acetaldehyde in the presence of ethanol oxidase. The apparatus measures the rate of oxygen required by this process, which is proportional to ethanol concentration. BECs were expressed as milligrams of ethanol per deciliter of body fluid (mg/dl = mg%).
Data analysis
As mentioned, subjects from Experiments 1a, 1b and 1c came from independent litters. Baseline activity from these samples was employed to correlate different outcomes in each experiment (motor effects, motor impairment and rectal temperature in different post-administration times in Experiment 1a and 1b, and blood ethanol levels in Experiment 1c). Hence, we wanted to corroborate that baseline activity data were normally distributed and that means and variances did not differ across experiments. Descriptive statistics for baseline locomotor activity in Experiments 1a, 1b and 1c are presented in Table 1. Baseline data from each experiment were normally distributed (evaluated through the Kolmogorov-Smirnov test: Experiment 1a: d=0.07; Experiment 1b: d=0.08; Experiment 1c: d=0.08, all ps > 0.20). Levene’s test revealed that variances from samples in each experiment were homogeneous [F(2,157) = 1.40, p=0.25]. An ANOVA also revealed that baseline activity means from subjects in each experiment did not differ [F (2,157) = 0.18, p=0.83].
Table 1.
Descriptive statistics and sample size corresponding to baseline locomotor activity from subjects included in Experiment 1a, 1b and 1c.
| EXPERIMENT 1a | EXPERIMENT 1b | EXPERIMENT 1c | |
|---|---|---|---|
| Mean | 147.06 | 146.69 | 141.88 |
| Standard Deviation | 45.96 | 47.90 | 57.53 |
| Minimum | 57 | 59 | 41 |
| Maximum | 246 | 237 | 270 |
| Median | 146.5 | 145.0 | 138.5 |
| N | 48 | 48 | 64 |
Data from Experiment 1a, 1b and 1c were statistically analyzed using an analysis of variance (ANOVA) as well as correlational tests. Locomotor activity data (Experiments 1a and 1b) were analyzed with a 3 (ethanol treatment: 0, 1.25, or 2.5 g/kg) × 2 (day of assessment: P11 and P12) mixed ANOVA. Rectal temperature and latency to complete the righting reflex (Experiments 1a and 1b) were analyzed using one-way between-factor ANOVAs with ethanol treatment as the only independent variable. Blood ethanol levels (Experiment 1c) were analyzed by means of a 2 (ethanol treatment: 1.25 or 2.5 g/kg) × 2 (post-administration time: 10 or 30 min) between-factor ANOVA. Significant main effects or interactions indicated by the ANOVAs were further analyzed through post-hoc tests (Newman Keuls post-hoc test with a Type I error set at 0.05).
Since the main goal of the present experiment was to test whether baseline locomotor activity predicts the stimulant effect of ethanol, data were also analyzed by means of a correlational approach. Pearson’s product-moment correlation coefficients were calculated to examine the strength of the association existing between individual baseline locomotor activity scores (PD 11) and ethanol-induced locomotor activity, latency to complete the righting reflex and rectal temperature measured on PD 12.
Results
Experiment 1a
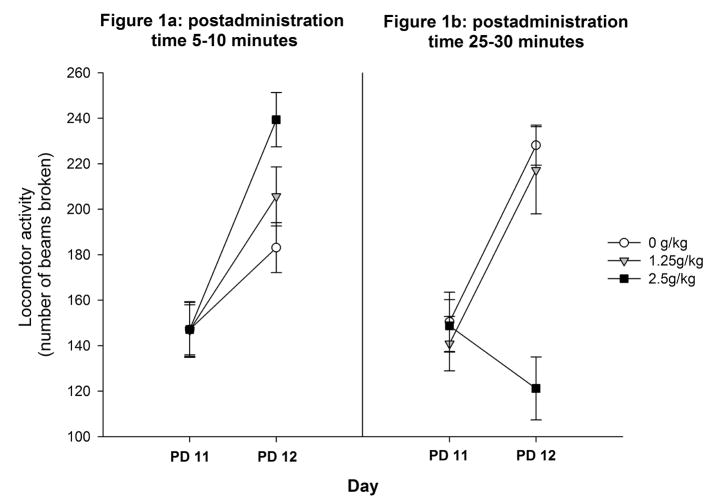
Figure 1a depicts locomotor activity scores on PD11 (baseline) and PD12 as a function of ethanol dose (0.0, 1.25 or 2.5 g/kg) in pups tested 5–10 minutes after ethanol administration. The ANOVA indicated a significant main effect of day [F(1,45) = 70.94, p < 0.0001], as well as an interaction between ethanol treatment and day [F(2,45) = 4.88, p < 0.05]. To determine the loci of this interaction, one-way between-factor ANOVAs were performed for each day. There were no significant differences on PD 11 (baseline). In contrast, activity scores on PD 12 differed significantly as a function of ethanol dose [F(2,45) = 5.59, p < 0.01]. Subsequent analysis revealed that, on PD 12, pups given 2.5 g/kg ethanol had higher locomotor activity scores than those given water. Pups that received 1.25 g/kg did not significantly differ from the 0 or 2.5 g/kg groups.
Figure 1.
Locomotor activity scores on PD11 (baseline) and PD12 as a function of ethanol dose (0.0, 1.25 or 2.5 g/kg) in pups tested 5–10 (Figure 1a) or 25–30 (Figure 1b) minutes after ethanol administration. Vertical lines illustrate standard errors of the means.
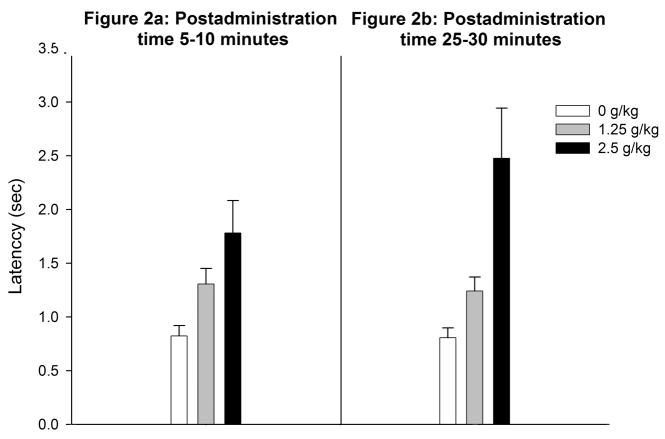
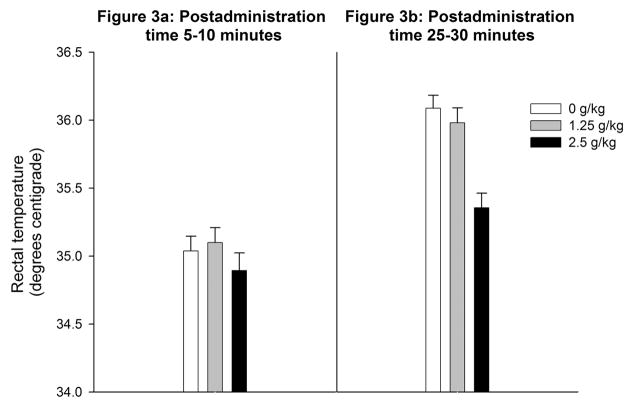
Figure 2a depicts latency to right as a function of ethanol treatment. The ANOVA revealed a significant main effect of ethanol dose [F(2,45) = 5.72, p < 0.01]. Further analysis revealed that pups given the highest ethanol dose (2.5 g/kg) had longer latencies to right than water-treated controls. Latencies from pups given 1.25 g/kg did not differ significantly from the remaining groups. There was no significant effect of ethanol dose on rectal temperatures (see Figure 3a).
Figure 2.
Latency (seconds) to complete the righting reflex as a function of post-administration time (Figure 2a: 5–10 or Figure 2b: 25–30 min) and ethanol treatment (0.0, 1.25 or 2.5 g/kg ethanol). Vertical lines illustrate standard errors of the means.
Figure 3.
Rectal temperature in preweanling rats as a function ethanol treatment (0.0, 1.25 or 2.5 g/kg ethanol) and post-administration time (Figure 3a: 5–10 or Figure 3b: 15–20 min). Vertical lines illustrate standard errors of the means.
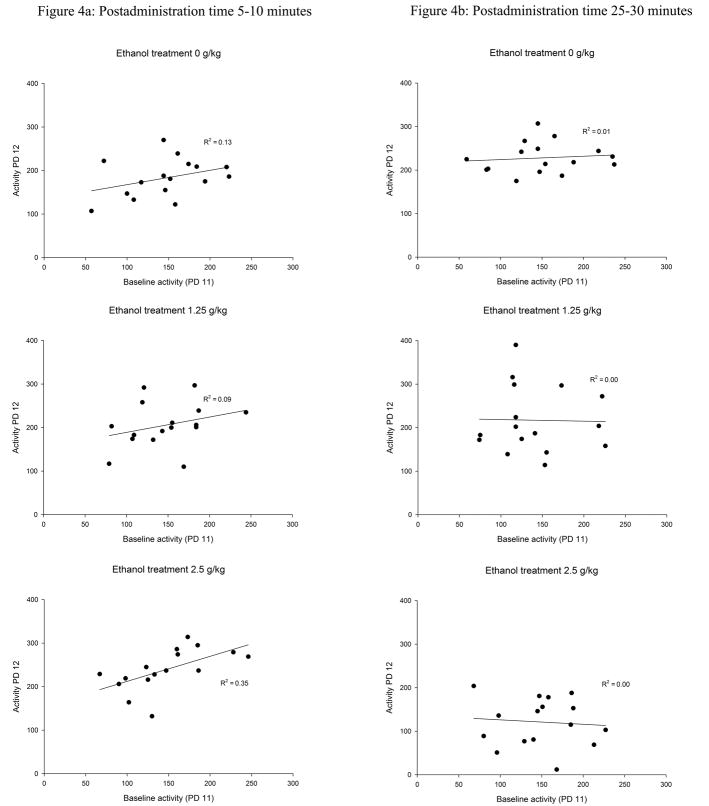
Correlations comprising baseline activity and the dependent variables considered in the present study are presented in Figure 4a (locomotor activity on P12) and in Table 2 (latency to complete the righting reflex and rectal temperature). Baseline activity correlated positively and significantly with locomotor activity at testing in the case of pups given 2.5 g/kg ethanol (rxy = 0.59, p<0.05), but in the remaining groups (groups 0.0 and 1.25 g/kg) baseline activity was not associated with locomotor activity at testing. Baseline activity did not significantly correlate with the remaining variables considered in the analysis (i.e. rectal temperature or motor impairment; see Table 2).
Figure 4.
Correlations and regression lines comprising baseline activity and locomotor activity on P12 when preweanling rats received the ethanol treatment (0, 1.25 or 2.5 g/kg) 5–10 (Figure 4a) or 25–30 (Figure 4b) after drug treatment. “R2” represents the determination coefficients. Baseline activity correlated positively and significantly with locomotor activity at testing only in the case of pups given 2.5 g/kg ethanol and tested 5–10 minutes postadministration time.
Table 2.
Pearson’s product-moment correlation coefficients (rxy) were calculated for Experiments 1a (5–10 min post-administration time) and 1b (25–30 min post-administration time) to examine the strength of the association existing between individual baseline locomotor activity scores and latency to complete the righting reflex or rectal temperature measured on PD 12. Correlation coefficients were calculated independently for each ethanol dose (0.0, 1.25 or 2.5 g/kg). “n” represents the number of subjects included in each condition.
| Postadministration interval |
||||
|---|---|---|---|---|
| Ethanol Dose | 5–10 min (Exp 1a) | 25–30 min (Exp 1b) | N | |
| Latency to perform the righting reflex | 0.0 g/kg | rxy=−0.29, p=0.27 | rxy=0.14, p=0.60 | 16 |
| 1.25 g/kg | rxy=−0.13, p=0.61 | rxy=0.17, p=0.52 | 16 | |
| 2.5 g/kg | rxy=−0.17, p=0.53 | rxy=0.23, p=0.37 | 16 | |
|
| ||||
| Rectal temperature | 0.0 g/kg | rxy=−0.05, p=0.85 | rxy=−0.05, p=0.86 | 16 |
| 1.25 g/kg | rxy=−0.38, p=0.14 | rxy=−0.11, p=0.70 | 16 | |
| 2.5 g/kg | rxy=−0.07, p=0.79 | rxy=−0.31, p=0.23 | 16 | |
p<0.05
Experiment 1b
As can be observed in Figure 1b, 25–30 minutes after ethanol administration, the 2.5 g/kg ethanol dose suppressed motor activity. The ANOVA revealed significant main effects of ethanol treatment [F(2,45) = 9.38, p < 0.0005], and day [F(1,45) = 14.63, p < 0.0005], as well as their interaction [F(2,45) = 9.99, p < 0.0005]. Follow-up one-way ANOVAs were conducted with activity scores from each day separately. These analyses detected no significant effects of ethanol during baseline (P11). Yet, on PD12, ethanol treatment exerted a significant effect [F(2,45) = 16.37, p < 0.0001]. Further post-hoc comparisons revealed that pups given 2.5 g/kg ethanol exhibited significantly lower locomotor activity than the remaining groups (0 or 1.25 g/kg ethanol).
Ethanol treatment also significantly affected latency to complete the righting reflex [F(2,45) = 9.28, p < 0.0005] and rectal temperature [F(2,45) = 14.48, p < 0.0001]. Post-hoc comparisons revealed that pups given 2.5 g/kg showed significantly higher latencies and lower rectal temperature than those pups given water or 1.25 g/kg ethanol (see Figures 2b and 3b).
The correlational analyses did not reveal significant associations between baseline activity on PD 11 and locomotor activity on PD 12 in any of the ethanol conditions (Figure 4b). Baseline activity and the remaining dependent variables (latency to complete the righting reflex and rectal temperature) were also not significantly correlated (see Table 2).
Experiment 1c
The ANOVA conducted with BECs revealed significant main effects of ethanol treatment [F(1,60) = 1061.56, p < 0.0001] and post-administration time [F(1,60) = 138.174, p < 0.0001]. As could be expected, at both post-administration times, the 2.5 g/kg ethanol dose resulted in significantly higher BECs than those obtained with 1.25 g/kg ethanol. In addition, BECs varied as a function time, with those detected at 10 min being significantly lower than those seen at 30 min. BECs as a function of ethanol treatment and time were as follows: (1.25 g/kg at 10 min: 88.81+/−4.21 mg%; 1.25 g/kg at 30 min: 101.56+/−7.87 mg%; 2.5 g/kg at 10 min: 167.33+/−14.94 mg%; 2.5 g/kg at 30 min: 228.86+/−18.33 mg%; values represent mean +/− standard errors). Baseline activity (PD11) was not significantly correlated with BECs generated by either of the ethanol doses (all ‘r’ values are less than .18 and all ‘ps’ are higher than 0.5).
In summary, a relatively high ethanol dose (2.5 g/kg) significantly increased locomotor activity 5–10 minutes after ethanol administration (Experiment 1a), and suppressed locomotion 25–30 minutes post-administration (Experiment 1b). At both post-administration times ethanol induced motor impairment, operationalized through increased latency to complete the righting reflex. In contrast, ethanol-mediated hypothermia was detected only with the highest dose (2.5 g/kg) 30 minutes after ethanol administration. According to the present experiments, locomotor activity in a novel environment is a valuable predictor of ethanol’s activating effects, but not of other ethanol effects, such us hypothermia, motor impairment or motor suppressive effects of the drug. There was no association between baseline activity and BECs.
EXPERIMENT 2
In Experiment 1 we observed that the level of response to a novel environment significantly predicted ethanol’s activating effects. Considering this result, it is also plausible that the stimulating effect of ethanol is modulated by novelty of the testing environment during the preweanling period. Adult high responder rats are more sensitive to ethanol’s activating effects than low responders but only when they are tested in a novel environment (Cools & Gingras, 1998; Gingras & Cools, 1996). In adulthood, familiarity with the testing environment attenuates the stimulant effect of a variety of psychostimulant drugs (Caprioli et al., 2007). In Experiment 2 we tested whether locomotor stimulating effects of ethanol are modulated by familiarization with the testing environment. Since in Experiment 1 one prior exposure to the testing environment was not sufficient to eliminate ethanol’s activating effects, we selected a longer preexposure treatment. Pups were exposed to the testing environment for three days on PDs 9, 10 and 11. On PD 12 pups were evaluated in terms of locomotor activity 5–10 minutes after receiving ethanol (0.0 or 2.5 g/kg).
Material and Methods
Subjects
Ninety-six preweanling Sprague-Dawley pups (47 females and 49 males), representative of 10 litters were utilized for Experiment 2. Animals were born and reared at the vivarium of the Center for Developmentand Behavioral Neuroscience (Binghamton University, NY). Housing conditions were identical to those described in Experiment 1.
Procedures
Phase 1: Preexposure
Two factors were considered during this phase: the intragastric intubation (ig or no-ig), which can act as a stressor during the preweanling period (Arias et al., 2008; Pautassi, Nizhnikov, Molina, Boehm, & Spear, 2007) and preexposure to the environmental context (context or no-context). For three consecutive days (PDs 9, 10 and 11) pups were exposed exclusively to the testing environment (group no-ig/context), or to the intragastric intubation procedure (group ig/no context), to both conditions (group ig/context), or remained undisturbed (group no-ig/no-context). Pups assigned to the ig condition received 0.015 ml per gram of body weight in each intragastric intubation. During these days pups were separated from their mothers and maintained under the same conditions described for Experiment 1. One hour after maternal separation, body weights were recorded (± 0.01 g) and some pups received an intragastric administration of water (vol equivalent to 0.015 ml per gram of body weight; groups ig/context and ig/no-context). The remaining groups (groups no-ig/context and no-ig/no-context) were left undisturbed. Five minutes later, pups from the groups ig/context and no-ig/context were placed in the testing chamber for five minutes.
Phase 2: Test
On PD 12 pups were separated from their mothers and placed in a holding maternity cage. One hour later, pups received an intragastric administration of 0.0 or 2/5 g/kg, following the procedure described in Experiment 1. Pups were quasirandomly assigned to each specific experimental condition to avoid litter and sex overrepresentation in any given group. No more than one pup from a given litter was assigned to a specific group. Five minutes later, locomotor activity was measured in the testing environment.
Data analysis
The factorial design of the present experiment was defined by the following variables: Preexposure to the testing chamber (context or no-context), preexposure to the intubation (ig or no-ig) and ethanol treatment (0.0 or 2.5 g/kg). Activity data from the preexposure phase was analyzed using a 2 (Preexposure to the intubation) × 3 (Days) mixed ANOVA. Activity scores at testing were analyzed by means of a 2 (Preexposure to the testing chamber) × 2 (Preexposure to the intubation) × 2 (Ethanol treatment) between-factor ANOVA. Significant main effects or interactions indicated by the ANOVAs were further analyzed through post-hoc tests (Newman-Keuls test with a Type I error set at 0.05).
Results
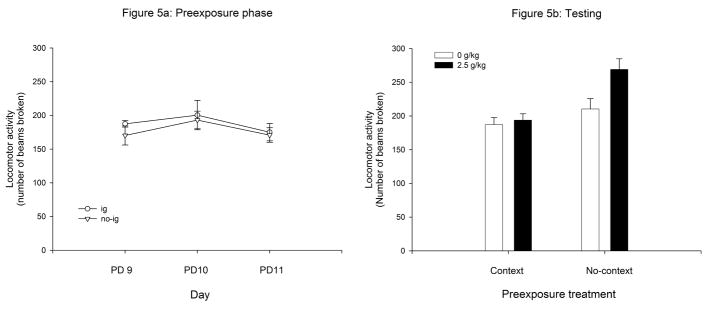
Figure 5 depicts locomotor activity during the pre-exposure phase (PDs 9, 10 and 11) and testing day (PD 12). During the preexposure phase the ANOVA did not reveal any significant effects. Preexposure to the ig procedure did not exert a significant effect nor interact with the remaining variables included in the analysis during the pre-exposure or testing phase (Figure 5a). Hence, for the visual representation of the test data, activity scores at testing were collapsed across this factor. On the testing day, prior experience with the testing environment attenuated ethanol’s activating effects (Figure 5b). This observation was confirmed by inferential analysis of the data. The ANOVA revealed a significant effect of ethanol treatment, F(1,88) = 14.76, p < 0.0005. More important for the goals of the present study was the significant effect generated by preexposure to the context, [F(1,88) = 5.93, p < 0.05], which interacted with ethanol treatment, F(1,88) = 4.28, p < 0.05. Post-hoc analyses revealed that pups treated with ethanol but not pre-exposed to the context before testing, showed higher activity scores than water-treated controls as well as pups given ethanol after context pre-exposure.
Figure 5.
Figure 5a depicts locomotor activity during the pre-exposure phase (PDs 9, 10 and 11) as a function of the preexposure treatment (ig or no-ig). Figure 5b represents locomotor activity at testing as a function of the prior experience with the testing environment (context or no-context) and ethanol treatment (0 or 2.5 g/kg). Vertical lines illustrate standard errors of the means.
Guided by results obtained in Experiment 1 and by the working hypothesis of the present study, we ran an additional correlation analysis aimed at analyzing whether ethanol’s effects were associated with baseline activity levels. Obviously, for this analysis we only included pups that were preexposed to the testing environment. Specifically, we explored possible associations between locomotor activity scores during the preexposure phase (PD 9, 10 and 11) with locomotor activity at testing. A separate analysis was conducted for pups given ethanol or water at testing. Locomotor activity at test in pups given water on P12 was not related to activity scores from the preexposure phase. However, in pups given ethanol on P12 there was a significant and positive correlation between activity scores on the first day of preexposure (PD 9) and locomotor activity at testing (see Table 3).
Table 3.
Pearson’s product-moment correlation coefficients (rxy) were calculated for Experiment 2 to examine the strength of the association between locomotor activity during context preexposure (PDs 9, 10 and 11) and locomotor activity induced by ethanol (0.0 or 2.5 g/kg) on PD12. n represents the number of subjects included in each condition.
| Ethanol Dose | PD9 | PD10 | PD11 | n |
|---|---|---|---|---|
| 0.0 g/kg | rxy=0.08, p=0.72 | rxy=0.16, p=0.46 | rxy=0.24, p=0.26 | 24 |
| 2.5 g/kg | rxy=0.46, p=0.02* | rxy=0.36, p=0.11 | rxy=0.19, p=0.37 | 24 |
p<0.05
Discussion
The present study was designed to test whether novelty modulates ethanol’s motor effects during the preweanling period. The highest ethanol dose employed (2.5 g/kg) exerted clear biphasic locomotor effects. Five to ten minutes after drug treatment, this ethanol dose increased locomotor response (Experiment 1a), while it suppressed activity when infants were tested 25–30 minutes post-administration (Experiment 1b). This biphasic locomotor effect of ethanol during the preweanling period replicates previous findings from our laboratory (Arias et al., 2008). The stimulating effect of ethanol was markedly attenuated when infants had sufficient experience with the testing environment. Three exposures prior to testing significantly attenuated locomotor activating effects induced by ethanol (Experiment 2). In addition, according to the present data, sensitivity to ethanol’s activating effects was significantly predicted by the locomotor response displayed in a novel environment (Experiments 1a and 2). Novelty of the testing environment modulated the stimulating effect of ethanol during the preweanling period, as suggested also for adult rats (Cools & Gingras, 1998; Hoshaw & Lewis, 2001). Furthermore, the present data suggest that the locomotor response in a novel environment may represent a valuable predictor of response to ethanol’s effects during the preweanling period.
In our study, the highest ethanol dose clearly induced motor impairment, hypothermia and suppressed locomotion, effects that were particularly observed 30 minutes after ethanol administration. Ethanol-mediated hypothermic and motor impairment effects have been previously observed during the preweanling period when employing similar ethanol treatments (Arias et al., in press; Hunt, Molina, Rajachandran, Spear, & Spear, 1993). Relatively high ethanol doses also suppressed locomotion when preweanling rats were tested at post-administration intervals similar to the one employed here (Arias et al., 2008). The present data indicate that baseline activity is not associated with these suppressive effects of ethanol. Nevertheless, in our study the sedative, hypothermic and motor impairment effects of ethanol were only measured at one time point and thus the time course of these disruptive effects of ethanol were not analyzed. The time course of such effects may be especially important, however, since duration of loss of righting reflex seems to be an accurate indicator of the hypnotic effect of ethanol and other drugs (e.g., Cha, Li, Wilson, Swartzwelder, 2006; Little, Kuhn, Wilson, Swartzwelder, 1996). It is plausible that different indices of these ethanol effects may be correlated with baseline activity levels.
From a descriptive point of view, rectal temperature of water-treated controls seemed to be lower in Experiment 1a than in Experiment 1b. Inferential statistical comparisons across experimentwere not performed since these experiments were conducted at different times and with subjects provided by different dams. Nevertheless, this difference in rectal temperature may be explained by the delay between the ig administration and the evaluation. Unpublished data from our laboratory indicate that the ig procedure significantly decreases rectal temperature. Hence, pups tested 5 minutes after the ig showed lower rectal temperature than those pups tested 30 minutes after the ig administration, which also had more time to warm up in the heated holding tubs than pups from Experiment 1a. This effect, inherent in ig administration, may have interfered with the observation of a decrease in the body temperature of rats from Experiment 1a.
With these caveats in mind, results of Experiment 1 are congruent with studies conducted with adult rats, in which high and low responders to novelty respond similarly to the sedative effects of ethanol (Gingras & Cools, 1996). The fact that these subpopulations of rats may specifically differ in their sensitivity to ethanol’s activating effects supports the hypothesis that sedative and stimulating effects of the drug are mediated by different mechanisms. Similar to what has been observed in adult rodents (e.g., Boehm, Piercy, Bergstrom, & Phillips, 2002; Pastor, Miquel, & Aragon, 2005), ethanol’s activating effects in preweanling rats seems to be associated with the dopaminergic mesocorticolimbic pathway. Opioid and dopamine antagonists as well as GABA B agonists reduced ethanol’s activating effect during the preweanling period (Arias et al., in press; Arias, Mlewski, Molina, & Spear, submitted). In addition, acute administration of 2.5 g/kg ethanol increased synthesis of dopamine in the dorsal striatum during the preweanling period at the same post-administration interval wherein locomotor stimulating effects were observed (Mlewski, Arias, Hansen, Haymal, Molina, Paglini, & Spear, 2007; Arias et al., submitted). However, motor impairment induced by ethanol seems to be mediated by GABA A receptors from the cerebellar granule cells (Carta, Mameli, & Valenzuela, 2004; Hanchar, Dodson, Olsen, Otis, & Wallner, 2005), while motor suppressive effects of the drug have been associated with peripheral metabolism of ethanol, specifically with the accumulation of acetate and acetate-derived adenosine (Arizzi, Correa, Betz, Wisniecki, & Salamone, 2003; Correa, Arizzi, Betz, Mingote, & Salamone, 2003).
In adult rats, novelty is an important factor that modulates locomotor responses produced by a variety of drugs, including ethanol (Caprioli et al., 2007; Carey et al., 2005). Prior experience with the testing environment reduces the acute stimulatory effect of various drugs (Caprioli et al., 2007; Cools & Gingras, 1998) as well as the development of sensitization to their stimulating effects (e.g., Badiani, Anagnostaras, & Robinson, 1995). The mechanism by which novelty modulates the drug-induced acute psychomotor response is not completely understood, although several promising hypotheses have been proposed on the basis of the effects of novelty on dopamine function and activation of the stress response (Cools & Gingras, 1998; Badiani et al., 1998; Caprioli et al., 2007). The potentiation effect of novelty upon the acute stimulating effects of different drugs may be associated with the stress-response induced by novelty or possibly more direct effects of novel environments upon dopaminergic activity (Caprioli et al., 2007). Further research will be required to analyze the role of these mechanisms in response to acute ethanol or other psychoactive drugs during early ontogeny. It will also be interesting for future experiments to analyze whether baseline activity levels during infancy are predictive of response to the stimulatory effects of ethanol in adolescence.
Finally, data from the present study suggest that early in development individual differences in sensitivity to acute stimulatory effects of ethanol can be detected. It will be interesting to investigate whether this differential susceptibility is associated with genetic or developmental mechanisms or perhaps both. Individual differences in locomotor activity in an open field can be associated with differences in maternal care. Specifically, low-responder dams exhibit more attentive behaviors to their pups than high responders during the first two postnatal weeks (Clinton et al., 2007). Quality of maternal care has also been associated with offspring displaying differential sensitivity to stress. Pups derived from mothers that display lower quality of maternal care show greater reactivity to stress (Huot, Thrivikraman, Meaney, & Plotsky, 2001; Meaney, 2001). In addition, genetic or heritable factors can also be implicated in the development and expression of behavioral phenotypes that differ in response to stress and drugs (Stead et al., 2006).
In summary, two main conclusions are derived from the present study. First, novelty modulates ethanol’s activating effects during the preweanling period. Second, response to novelty predicts ethanol’s activating effects but not other effects of the drug, such as motor sedation, motor impairment or hypothermia.
Acknowledgments
This work was supported by grants from NIAAA (AA11960, AA013098, AA015992) and NIMH (MH035219) to NES and from NIAAA (F31AA017339) to SSM, Agencia Nacional de Promocion Cientifica y Tecnologica (PICT 05-14024) to JCM, Postdoctoral fellowship from Ministerio de Educacion y Ciencia from Spain to CA, as well as Fundacion Antorchas, Argentina, CONICET (PIP 6485) and FONCyT (PICT 05-38084) to E.C.M (this study has been conducted during the period in which E.C.M. was subscripted in the Doctorate Program in Biological Sciences, Cordoba University). The authors wish to express their gratitude to Teri Tanenhaus and Heather Murphy for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arias C, Chotro MG. Ethanol-induced preferences or aversions as a function of age in preweanling rats. Behav Neurosci. 2006;120(3) doi: 10.1037/0735-7044.120.3.710. [DOI] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Naloxone and Baclofen attenuate ethanol’s locomotor-activating effects in preweanling Sprague-Dawley rats. Behavl Neurosci. doi: 10.1037/a0014049. (in press) [DOI] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Dopamine receptors modulate ethanol’s locomotor-activating effects in preweanling rats. Behav Pharmacol. doi: 10.1002/dev.20407. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Ethanol induces locomotor activating effects in preweanling Sprague-Dawley rats. Alcohol. doi: 10.1016/j.alcohol.2008.09.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Molina JC, Mlewski EC, Pautassi RM, Spear N. Acute sensitivity and acute tolerance to ethanol in preweanling rats with or without prenatal experience with the drug. Pharmacol Biochem Behav. 2008;89(4):608–622. doi: 10.1016/j.pbb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arizzi MN, Correa M, Betz AJ, Wisniecki A, Salamone JD. Behavioral effects of intraventricular injections of low doses of ethanol, acetaldehyde, and acetate in rats: studies with low and high rate operant schedules. Behav Brain Res. 2003;147(1–2):203–210. doi: 10.1016/s0166-4328(03)00158-x. [DOI] [PubMed] [Google Scholar]
- Badiani A, Anagnostaras SG, Robinson TE. The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopharmacology (Berl) 1995;117(4):443–452. doi: 10.1007/BF02246217. [DOI] [PubMed] [Google Scholar]
- Badiani A, Browman KE, Robinson TE. Influence of novel versus home environments on sensitization to the psychomotor stimulant effects of cocaine and amphetamine. Brain Res. 1995;674(2):291–298. doi: 10.1016/0006-8993(95)00028-o. [DOI] [PubMed] [Google Scholar]
- Badiani A, Morano MI, Akil H, Robinson TE. Circulating adrenal hormones are not necessary for the development of sensitization to the psychomotor activating effects of amphetamine. Brain Res. 1995;673(1):13–24. doi: 10.1016/0006-8993(94)01365-o. [DOI] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. J Neurosci. 1998;18(24):10579–10593. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Koros E, Kostowski W. Novelty-seeking behaviour and operant oral ethanol self-administration in Wistar rats. Alcohol Alcohol. 2001;36(6):525–528. doi: 10.1093/alcalc/36.6.525. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Kostowski W. Individual behavioral differences and ethanol consumption in Wistar rats. Physiol Behav. 1993;54(6):1125–1131. doi: 10.1016/0031-9384(93)90336-e. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Piercy MM, Bergstrom HC, Phillips TJ. Ventral tegmental area region governs GABA(B) receptor modulation of ethanol-stimulated activity in mice. Neuroscience. 2002;115(1):185–200. doi: 10.1016/s0306-4522(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Campbell RA, Raskin LA. Ontogeny of behavioral arousal: the role of environmental stimuli. J Comp Physiol Psychol. 1978;92(1):176–184. doi: 10.1037/h0077423. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Celentano M, Paolone G, Badiani A. Modeling the role of environment in addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(8):1639–1653. doi: 10.1016/j.pnpbp.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E. Acute and chronic cocaine behavioral effects in novel versus familiar environments: open-field familiarity differentiates cocaine locomotor stimulant effects from cocaine emotional behavioral effects. Behav Brain Res. 2005;158(2):321–330. doi: 10.1016/j.bbr.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24(15):3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YM, Li Q, Wilson WA, Swartzwelder HS. Sedative and GABAergic effects of ethanol on male and female rats. Alcohol Clin Exp Res. 2006;30(1):113–8. doi: 10.1111/j.1530-0277.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Cheslock SJ, Varlinskaya EI, Petrov ES, Silveri MM, Spear LP, Spear NE. Ethanol as a reinforcer in the newborn’s first suckling experience. Alcohol Clin Exp Res. 2001;25(3):391–402. [PubMed] [Google Scholar]
- Chotro MG, Arias C. Ontogenetic difference in ethanol reinforcing properties: the role of the opioid system. Behavioural Pharmachology. 2007;18(7):661–666. doi: 10.1097/FBP.0b013e3282f00754. [DOI] [PubMed] [Google Scholar]
- Chuck TL, McLaughlin PJ, Arizzi-LaFrance MN, Salamone JD, Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: sedation, ataxia, and bradykinesia. Life Sci. 2006;79(2):154–161. doi: 10.1016/j.lfs.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Vazquez DM, Kabbaj M, Kabbaj MH, Watson SJ, Akil H. Individual differences in novelty-seeking and emotional reactivity correlate with variation in maternal behavior. Horm Behav. 2007;51(5):655–664. doi: 10.1016/j.yhbeh.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools AR, Gingras MA. Nijmegen high and low responders to novelty: a new tool in the search after the neurobiology of drug abuse liability. Pharmacol Biochem Behav. 1998;60(1):151–159. doi: 10.1016/s0091-3057(97)00586-8. [DOI] [PubMed] [Google Scholar]
- Correa M, Arizzi MN, Betz A, Mingote S, Salamone JD. Open field locomotor effects in rats after intraventricular injections of ethanol and the ethanol metabolites acetaldehyde and acetate. Brain Res Bull. 2003;62(3):197–202. doi: 10.1016/j.brainresbull.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Robinson TE. Signalled versus unsignalled intravenous amphetamine: large differences in the acute psychomotor response and sensitization. Brain Res. 1996;722(1–2):227–231. doi: 10.1016/0006-8993(96)00066-2. [DOI] [PubMed] [Google Scholar]
- Dudek BC, Phillips TJ. Distinctions among sedative, disinhibitory, and ataxic properties of ethanol in inbred and selectively bred mice. Psychopharmacology (Berl) 1990;101(1):93–99. doi: 10.1007/BF02253724. [DOI] [PubMed] [Google Scholar]
- Dudek BC, Phillips TJ, Hahn ME. Genetic analyses of the biphasic nature of the alcohol dose-response curve. Alcohol Clin Exp Res. 1991;15(2):262–269. doi: 10.1111/j.1530-0277.1991.tb01867.x. [DOI] [PubMed] [Google Scholar]
- Gingras MA, Cools AR. Analysis of the biphasic locomotor response to ethanol in high and low responders to novelty: a study in Nijmegen Wistar rats. Psychopharmacology (Berl) 1996;125(3):258–264. doi: 10.1007/BF02247337. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci. 2005;8(3):339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshaw BA, Lewis MJ. Behavioral sensitization to ethanol in rats: evidence from the Sprague-Dawley strain. Pharmacol Biochem Behav. 2001;68(4):685–690. doi: 10.1016/s0091-3057(01)00489-0. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Molina JC, Rajachandran L, Spear LP, Spear NE. Chronic administration of alcohol in the developing rat: expression of functional tolerance and alcohol olfactory aversions. Behav Neural Biol. 1993;59(2):87–99. doi: 10.1016/0163-1047(93)90795-j. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Spear LP, Spear NE. An ontogenetic comparison of ethanol-mediated taste aversion learning and ethanol-induced hypothermia in preweanling rats. Behav Neurosci. 1991;105(6):971–983. doi: 10.1037//0735-7044.105.6.971. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158(4):366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, C. O. L. S. National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academic Press; 1996. [Google Scholar]
- Kabbaj M. Neurobiological bases of individual differences in emotional and stress responsiveness: high responders-low responders model. Arch Neurol. 2004;61(7):1009–1012. doi: 10.1001/archneur.61.7.1009. [DOI] [PubMed] [Google Scholar]
- Kabbaj M. Individual differences in vulnerability to drug abuse: the high responders/low responders model. CNS Neurol Disord Drug Targets. 2006;5(5):513–520. doi: 10.2174/187152706778559318. [DOI] [PubMed] [Google Scholar]
- Koros E, Piasecki J, Kostowski W, Bienkowski P. Saccharin drinking rather than open field behaviour predicts initial ethanol acceptance in Wistar rats. Alcohol Alcohol. 1998;33(2):131–140. doi: 10.1093/oxfordjournals.alcalc.a008369. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20(8):1346–51. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Mlewski EC, Arias C, Hansen C, Haymal B, Molina JC, Paglini MG, Spear NE. Ethanol-induced locomotor stimulation is modulated by the opioid system in preweanling rats (El efecto estimulante del alcohol en la cría de rata se encuentra modulado por el sistema opiáceo); Paper presented at the Sociedad Argentina de Neurociencias; Los Cocos, Córdoba. 2007. [Google Scholar]
- Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162(3):333–338. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals (DHEW Publication No. 86-23) Washington, DC: U.S. Government Printing Office; 1986. [Google Scholar]
- Pastor R, Miquel M, Aragon CM. Habituation to test procedure modulates the involvement of dopamine D2- but not D1-receptors in ethanol-induced locomotor stimulation in mice. Psychopharmacology (Berl) 2005;182(3):436–446. doi: 10.1007/s00213-005-0115-3. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov M, Molina JC, Boehm SL, 2nd, Spear N. Differential effects of ethanol and midazolam upon the devaluation of an aversive memory in infant rats. Alcohol. 2007;41(6):421–431. doi: 10.1016/j.alcohol.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Spear NE. Reinforcement from pharmacological effects of ethanol in newborn rats. Alcohol Clin Exp Res. 2003;27(10):1583–1591. doi: 10.1097/01.ALC.0000089960.62640.58. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245(4925):1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Randall CL, Carpenter JA, Lester D, Friedman HJ. Ethanol-induced mouse strain differences in locomotor activity. Pharmacol Biochem Behav. 1975;3(3):533–535. doi: 10.1016/0091-3057(75)90069-6. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. 1997;76(3):707–714. doi: 10.1016/s0306-4522(96)00382-x. [DOI] [PubMed] [Google Scholar]
- Sanders S, Spear NE. Ethanol acceptance is high during early infancy and becomes still higher after previous ethanol ingestion. Alcohol Clin Exp Res. 2007;31(7):1148–1158. doi: 10.1111/j.1530-0277.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25(9):1301–1308. [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, et al. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36(5):697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31(5):755–765. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]