Abstract
The double-stranded (ds) RNA-activated protein kinase, PKR, plays a key role in the innate immunity response to viral infection in higher eukaryotes. PKR contains an N-terminal dsRNA binding domain and C-terminal kinase domain. In the prevalent autoinhibition model for PKR activation, dsRNA binding induces a conformational change which leads to the release of the dsRNA binding domain from the kinase, thus relieving the inhibition of the latent enzyme. Structural and biophysical data now favor a model where dsRNA principally functions to induce dimerization of PKR via the kinase domain. This dimerization model has implications for other PKR regulatory mechanisms and represents a new structural paradigm for control of protein kinase activity.
Introduction
PKR is a soluble protein kinase that plays a key role in the innate immunity response to viral infection in higher eukaryotes and has also been implicated in several cellular signal transduction pathways [1–4]. PKR is induced by interferon in a latent state. Upon binding double stranded RNA (dsRNA), which is produced as a replication intermediate in virally-infected cells, PKR undergoes autophosphorylation reactions that activate the kinase leading it to phosphorylate the alpha subunit of the translational initiation factor eIF2, thus inhibiting protein synthesis in virally-infected cells. PKR is also activated by the cellular protein PACT [5], and is inhibited by viral proteins and RNAs [6]. PKR contains an N-terminal dsRNA binding domain (dsRBD), consisting of two dsRNA binding motifs, and C-terminal kinase domain (Figure 1). The NMR structure of the dsRBD revealed that each motif adopts a similar αβββα fold [7]. The X-ray structure of a complex of the PKR kinase domain with eIF2α was recently solved [8]. Like other eukaryotic protein kinases, the catalytic domain of PKR consists of N- and C-terminal lobes with the ATP binding site in the cleft between the two lobes. eIF2α binds to the larger C-lobe. Interestingly, the kinase crystallizes as a dimer with the interface formed by the N-terminal lobes (Figure 1).
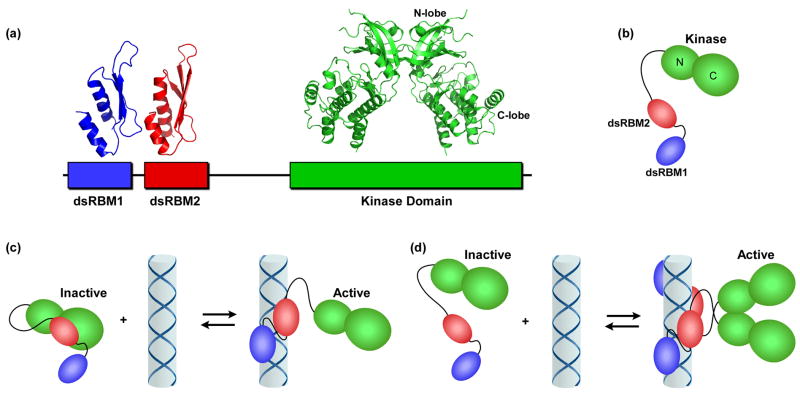
Figure 1.
PKR domains and their interactions. (a) Domain organization and structures. The N-terminal regulatory domain is comprised of two dsRNA binding motifs, dsRBM1 and dsRBM2 connected by an unstructured linker. Each of these motifs adopts the canonical αβββα fold in the NMR structure of dsRNA binding domain (1QU6). In the crystal structure of a complex of the PKR kinase domain and eIF2α (2A1A), the kinase domain has the typical bilobal structure observed in other eukaryotic protein kinases and dimerizes via the N-terminal lobe (note, the substrate eIF2α was omitted from the figure for clarity). (b) Schematic diagram of PKR. The color scheme corresponds to (a). (c) Autoinhibition model for PKR. The interaction of dsRBM2 with the kinase domain blocks activity of latent PKR. Binding to dsRNA activates PKR by removing dsRBM2 from kinase. (d) Dimerization model for PKR activation by dsRNA. Binding to dsRNA induces PKR dimerization via the kinase domain, resulting in activation.
The regulation of PKR by dsRNA and other effectors has fascinated researchers for over 25 years. However, we lack a detailed molecular picture of how binding of dsRNA to the N-terminal dsRBD results in kinase activation. Evidence has accumulated in support of an autoinhibition model in which the latent form of PKR exists in a closed conformation where the dsRBD interacts with the kinase and blocks substrate binding [3]. In this model, dsRNA activates PKR by binding to the dsRBD, thereby releasing it from the kinase (Figure 1c). Other models stress the role of dimerization in PKR activation (Figure 1d). Here, we critically evaluate these models in light of recent studies of PKR activation. These data argue against a rigid closed conformation for the latent enzyme and favor a model where dsRNA principally functions to induce dimerization of PKR via the kinase domain. This model has implications for our understanding of the regulation of PKR by other activators and viral inhibitors and represents a new kinase regulatory mechanism.
The Autoinhibition Model
In many eukaryotic protein kinases, intramolecular interactions between the catalytic domain and a regulatory domain inhibit activity by directly blocking nucleotide or peptide substrate binding or by allosterically inducing an inactive conformation [9]. Four principle observations have been invoked in support of such an autoinhibition model for PKR. First, deletion of the dsRBD can produce a constitutively active kinase capable of autophosphorylation and phosphorylation of eIF2α in the absence of dsRNA [10]. Second, photoaffinity labeling of PKR with ATP derivatives requires either the presence of dsRNA [11, 12] or prior autophosphorylation [12], suggesting that enzymatic activation is associated with increased accessibility of the nucleotide binding site. Third, NMR studies of the dsRBD indicate that resonances associated with dsRBM2 are broadened or disappear upon addition of the kinase domain, suggesting that this domain contacts the catalytic domain [13]. This interaction would presumably occur in the context of the holenzyme and inhibit the kinase. Finally, affinity cleavage data support the importance of dsRBM2 in activation of PKR by RNA. In these measurements RNAs that activate PKR, such as the HIV TAR stem loop, appear to bind both dsRBMs whereas RNAs that fail to activate, such as adenovirus VAI, bind only dsRBM1 [14].
Recent results have led us to re-evaluate the autoinhibition model for PKR. Although several studies have confirmed that kinase domain can undergo constitutive autophosphorylation [15–17] and PKR is activated during apoptosis by caspase-mediated cleavage to release the kinase domain [18], these data do not necessarily indicate that the N-terminus constitutes an autoinhibitory domain. First, PKR constructs are often autophosphorylated when overexpressed [19] and the constitutive in vitro activity of kinase domain constructs may reflect enhanced autophosphorylation. To our knowledge, constitutive activity of an unphosphorylated PKR kinase domain construct has not been demonstrated. Second, fusion of a heterologous dimerization domain with the PKR kinase domain enhances autophosphorylation and phosphorylation of eIF2α [20, 21], suggesting that the constitutive activation of kinase domain constructs may be associated with dimerization. Indeed, expression of a fusion of PKR kinase with the dimeric GST affinity tag in E. coli yields an enzyme that is stoichiometrically phosphorylated at T446 in the activation loop [8], which is a conserved structural element in protein kinases that regulates activity and substrate binding. Third, the relative activities of N-terminal deletion constructs do not support a role for dsRBM2 in the repression of basal PKR activity. Deletion of dsRBM1 abolishes PKR activity, deletion of dsRBM2 only modestly activates whereas deletion of 25 residues N-terminal to dsRBM2 strongly activates [16]. These data do not support a specific role for the interaction of dsRBM2 with the kinase in suppressing PKR activity.
New biophysical and structural data also argue against direct blockage of the kinase active site by dsRBM2 in a rigid, closed conformation. Equilibrium and kinetic analysis of ATP binding to PKR reveals that ATP can indeed interact with the latent enzyme [22]. Furthermore, nucleotide binding affinities and kinetics are only weakly affected upon binding PKR to an activating dsRNA or by PKR autophosphorylation. Thus, the active site is freely accessible in both latent and activated PKR. Recent NMR shift perturbations measurements [23] support early work [13] indicating that dsRBM2 binds to the kinase domain. Consistent with open access of nucleotides, the binding site for dsRBM2 maps to a region in the kinase C-lobe distant from the catalytic cleft [23]. In contrast to this picture of a closed conformation mediated by intramolecular domain interactions, sedimentation velocity measurements demonstrate that latent PKR has a high frictional ratio of f/f0 = 1.62, consistent with an extended, asymmetric shape [24], and neutron scattering data indicate an elongated structure [25]. Atomic force microscopy images of latent PKR reveal monomers in extended conformations with clearly visible linker segments as well as compact molecules [22], suggesting that PKR can adopt a range of open and closed conformations. Consistent with this model, the interaction of dsRBM2 and kinase domains was reported to be weak [23].
The binding sites for dsRBM1 and dsRBM2 on viral activator and inhibitor RNAs have been recently characterized by NMR chemical shift mapping experiments [26, 27]. Regions within both dsRBM1 and dsRBM2 interact with the TAR RNA activator and the same interaction pattern occurs with viral inhibitors. In contrast to the earlier affinity cleavage data [14], the NMR data do not support a model where inhibitory RNAs interact exclusively with dsRBM1 and indicate that binding of dsRBM2 by RNA is not sufficient for activation.
The role of dimerization in PKR activation by dsRNA
It has long been recognized that PKR is capable of dimerizing and several lines of evidence support an important role for dimerization in the activation of PKR by dsRNA [3, 24, 28–31]. A defining feature of PKR is the “bell-shaped” curve for activation, where low concentrations of dsRNA activate but higher concentrations are inhibitory [32]. These data have generally been interpreted to indicate that low concentrations of dsRNA favor assembly of multiple proteins- possibly assembling as dimers- on a single dsRNA whereas higher dsRNA concentrations dilute PKR monomers onto separate molecules of dsRNA [33]. Although the optimal size of dsRNA for PKR activation is about 85 bp, activation can be detected on RNAs as short as 30 bp. Presumably, the minimal sized RNA must be long enough to accommodate binding of two PKR monomers and the RNA serves to enhance dimerization [3], as was observed for PKR binding to the HIV TAR stem loop RNA activator [28].
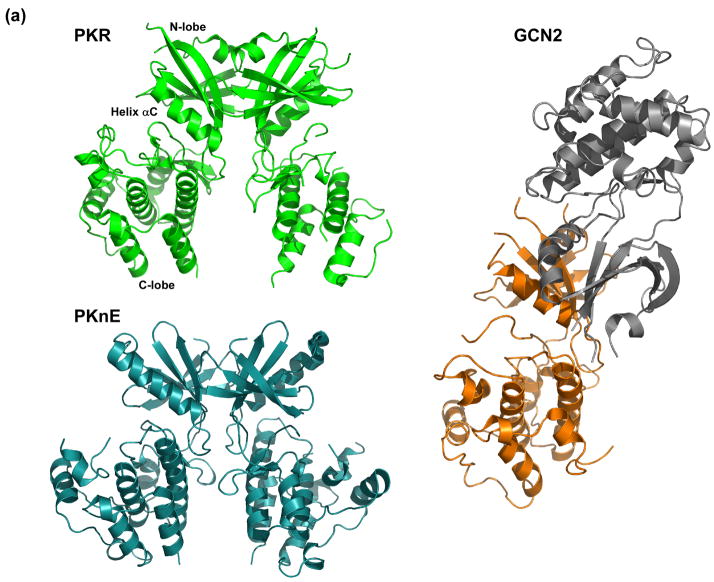
More generally, dimerization has been implicated in the activation of PKR even in the absence of RNA. Latent PKR exists predominantly as a monomer but can dimerize weakly, with Kd ~ 500 μM [24, 29]. Simply incubating PKR at relatively high concentrations (≥ 0.5 μM) in the presence of ATP induces autophosphorylation in the absence of dsRNA [24]. The kinetics and protein concentration dependence of this reaction are consistent with activation by dimerization. Activation of the isolated PKR kinase domain by fusion to dimerization motifs [20, 21] also supports the notion that dimerization is sufficient to activate PKR without the involvement of dsRNA. Although it has been reported that PKR dimerization is mediated by interactions involving the dsRBD [30], analytical ultracentrifugation [34], NMR [35] and gel filtration [36] measurements indicate that the dsRBD does not dimerize. Furthermore, the structure of the PKR kinase domain complexed with eIF2α revealed a dimerization interface localized on the N-terminal lobe (Figure 2) [8]. In the context of the holoenzyme, mutations designed to disrupt intermolecular interactions at this interface block PKR autophosphorylation [15]. Thus, it appears that a physiologically-relevant PKR dimerization interface is located on the N-lobe of the kinase. Despite low sequence homology to PKR, a strikingly similar dimer architecture occurs in the PknB [37, 38] and PknE Ser/Thr kinases [39] from Mycobacterium tuberculosis (Figure 2). Although a biochemical role for dimerization in these prokaryotic kinases has not been established, conservation of this oligomeric structure across diverse species suggests that it has biological relevance. In GCN2, an eIF2α kinase related to PKR, the catalytic domain also crystallized as a dimer via a similar interface on the N-lobe [40]. Thus, back-to-back dimerization may be a general feature of the eIF2α kinase family. However, in contrast to PKR and the M. tuberculosis kinases, the GCN2 monomers are antiparallel (Figure 2). The implications of this alternative dimer orientation are not yet clear.
Figure 2.
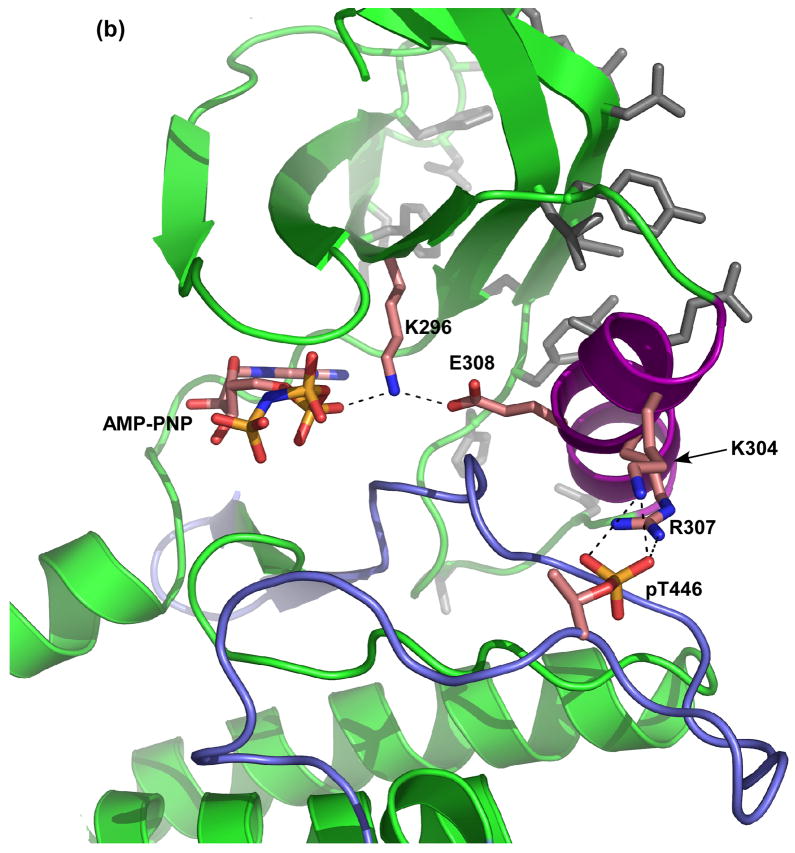
Structural analysis of PKR dimerization. (a) Comparison of the dimer structures of the kinase domains of PKR, PknE and GCN2. PKR (green) and M. tuberculosis PknE (aqua) dimerize in a similar parallel orientation using an interface on the back of the N-lobe. Although S. cerevisiae GCN2 (orange/grey) dimerizes via a similar interface, the monomers are anti-parallel. The two subunits of GCN2 are shown in different colors for clarity. (b) Helix αC links the dimerization interface to the nucleotide binding site and the activation loop. Helix αC is colored purple, residues comprising the dimer interface are colored grey and the activation loop is colored blue. Glu308 of helix αC interacts with Lys296, which orients the α and β phosphates of AMP-PNP. Two basic residues from helix αC, Lys304 and Arg307, form salt bridges with the negatively charged phosphate moiety of phospho-Thr446. The figure was generated using the following coordinates: PKR:AMP-PNP:eIF2α, 2A19; PknE, 2H34; GCN2, 1ZYC.
The PKR crystal structure suggests a possible mechanism linking the dimer interface to enzymatic activation (Figure 2) [8]. Helix αC in the N-lobe comprises part of the dimer interface. Conformational changes in this helix often regulates protein kinase activity [9]. A conserved glutamate in helix αC (Glu308) forms an ion pair with Lys296 that coordinates the α and β phosphates of ATP in a geometry that promotes the phosphoryl transfer reaction. Thus, PKR dimerization may alter the conformation of helix αC to enhance catalytic activity.. As well as interacting with Lys296, helix αC is also connected to the activation loop through the interaction of Lys304 and Arg307 with the phosphate moiety of Thr446. This interaction may mediate the enhancement in dimerization affinity of PKR [24, 29] or the isolated kinase domain [15] observed upon autophosphorylation. Conversely, dimerization may allosterically modulate enzymatic activity via conformational changes of the activation loop.
Concluding remarks
On close examination, the case for latent PKR being locked in a closed, auto-inhibited state by interaction of dRBM2 with the kinase is not well supported by the evidence. Instead, recent biophysical data support a model where PKR exists in an open conformation, or an equilibrium between open and closed states where dsRBM2 transiently interacts with the kinase at a location on the C-lobe far from the active site. In either case, nucleotides bind readily to PKR in the absence of dsRNA in contrast to the predictions of the autoinhibition model. It is possible that interaction of dsRBM2 with the kinase allosterically regulates PKR activity [23] or selectively interferes with binding of the peptide substrate. However, it appears more plausible that PKR is simply inactive as a monomer due to misalignment of critical catalytic residues. Because dsRBM2 binds at the eIF2α docking site, the intramolecular interaction may function to prevent binding of eIF2α before PKR has been activated by autophosphorylation. In the context of the isolated kinase domain, it has been reported that phosphorylation in the activation loop is required for binding of eIF2α [15]. Possibly, additional phosphorylation sites in the dsRBD regulates association with the kinase [23] and thus controls eIF2α binding.
Structural and biophysical data suggest that dimerization represents the key step in the activation of PKR by dsRNA. In this model (Figure 3), PKR exists in a weak monomer-dimer equilibrium. ATP is capable of binding to both monomeric and dimeric enzyme. However, the rate of the phosphoryl transfer reaction is enhanced by dimerization. Although dimerization of PKR can induce autophosphorylation in the absence of dsRNA, this reaction is unlikely to occur under physiological conditions. Instead, dsRNA binding enhances functional dimerization of the kinase and subsequent autophosphorylation. Phosphorylation of PKR enhances dimer stability [24, 29] and reduces dsRNA binding affinity [41]. Thus the phospho-PKR dimer readily dissociates from dsRNA and this dimer likely represents the active enzyme form that phosphorylates eIF2α. The biological significance of PKR dimerization is underscored by several viral inhibitors which act by blocking dimerization [6].
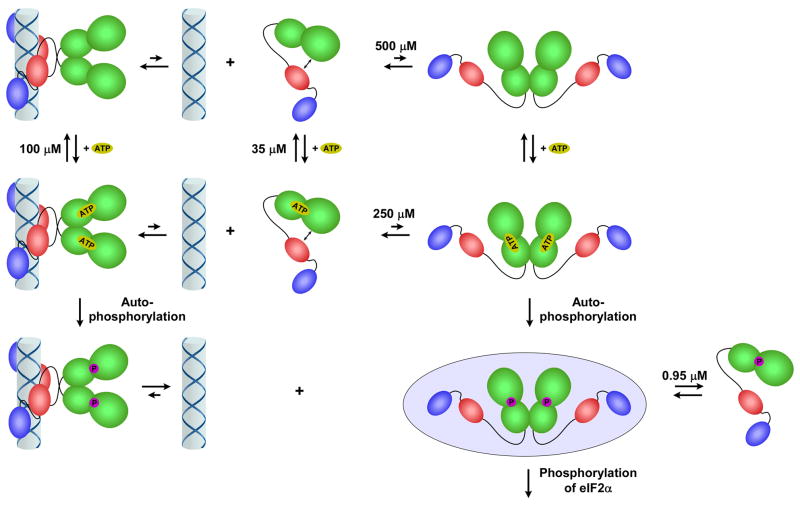
Figure 3.
Model for PKR activation. PKR exists in an open conformation (i) in a weak monomer-dimer equilibrium. Dimerization is induced by binding to dsRNA (ii) or at high protein concentration (iii), producing an enzyme form competent to undergo autophosphorylation. ATP is able to bind readily to both monomeric and dimeric PKR. The autophosphorylated dimer dissociates readily from dsRNA (iv). The PKR dimer is stabilized by phosphorylation (v) and represents the enzyme form competent to phosphorylate eIF2α. The PKR dimerization dissociation constants were obtained from reference [24] and the ATP dissociation constants were obtained from reference [22].
It should be appreciated that the dimer model for activation of PKR by dsRNA does not necessarily imply intermolecular autophosphorylation within the dimer. In the back-to-back PKR dimer structure, the active sites are pointed outward, which would seem to preclude intermolecular autophosphorylation within the dimer [8]. Thus, if this geometry is maintained, the reaction must be intramolecular (cis) or the dimer must phosphorylate other PKR dimers or monomers. This issue is also complicated by the fact that PKR contains multiple phosphorylation sites and it is possible that PKR undergoes both cis and trans autophosphorylation upon activation by dsRNA. Clearly, the molecular mechanism of PKR activation will continue to challenge biochemists and structural biologists for years to come (Box 1).
Box 1. Outstanding questions
What is the structure of the PKR holenzyme?
Although structures are available for the dsRBD and the kinase domain, we do not know the relative orientation of these domains in the holoenzyme. The structure of the region lying between these domains is not known. Also, the kinase domain used for structure determination lacked a conserved loop within the N-lobe that may be important for function. Structures of full-length PKR in both the free and dsRNA-bound forms will be required to define how RNA binding is linked to productive dimerization and activation.
What features distinguish RNA activators of PKR from inhibitors?
PKR is activated by dsRNA sequence 30 bp or longer. The HIV TAR RNA consists of a bulged stem loop containing only ~22 bp, yet it activates PKR. Does this short, structured RNA activate PKR by the same mechanism as a homogeneous dsRNA? Other highly structured viral RNAs bind to PKR, but fail to activate and serve as in vivo inhibitors of PKR activation. What is the nature of this nonproductive binding mode?
How does PACT activate PKR?
The cellular activator of PKR, PACT, contains two dsRNA binding domains and a C-terminal activation domain [5]. The activation domain binds to the N-lobe of the PKR kinase [36] to induce autophosphorylation. Does this domain induce PKR dimerization or induce conformational changes that activate the PKR monomer?
How does Heparin activate PKR?
Like dsRNA, heparin induces PKR autophosphorylation. Unlike dsRNA, heparin binds to the kinase domain and mediates cis but not trans autophosphorylation [42, 43], suggesting that the activation mechanism may be different. Does heparin activation involve PKR dimerization? Why is trans-autophosphorylation not observed?
Acknowledgments
I thank Andrei Alexandrescu, Ray Brown, Peter Lemaire and Victoria Robinson for critical reading of this manuscript. This work was supported by grant number AI-53615 from the NIH.
References
- 1.Clemens MJ, Elia A. The double-stranded RNA-dependent protein kinase PKR: structure and function. J Interferon Cytokine Res. 1997;17:503–524. doi: 10.1089/jir.1997.17.503. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman RJ. The double stranded RNA-activated protein kinase PKR. In: Sonenberg N, et al., editors. Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press; 2000. pp. 503–528. [Google Scholar]
- 3.Robertson HD, Mathews MB. The regulation of the protein kinase PKR by RNA. Biochimie. 1996;78:909–914. doi: 10.1016/s0300-9084(97)86712-0. [DOI] [PubMed] [Google Scholar]
- 4.Williams BR. Signal integration via PKR. Sci STKE. 2001:RE2. doi: 10.1126/stke.2001.89.re2. [DOI] [PubMed] [Google Scholar]
- 5.Patel RC, Sen GC. PACT, a protein activator of the interferon -induced protein kinase PKR. EMBO J. 1998;17:4379–4390. doi: 10.1093/emboj/17.15.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langland JO, et al. Inhibition of PKR by RNA and DNA viruses. Virus Res. 2006;119:100–110. doi: 10.1016/j.virusres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Nanduri S, et al. Structure of the double-stranded RNA binding domain of the protein kinase PKR reveals the molecular basis of its dsRNA-mediated activation. EMBO J. 1998;17:5458–5465. doi: 10.1093/emboj/17.18.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dar AC, et al. Higher-order substrate recognition of eIF2alpha by the RNA-dependent protein kinase PKR. Cell. 2005;122:887–900. doi: 10.1016/j.cell.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Huse M, Kuriyan J. The conformational plasticity of protein kinases. Cell. 2002;109:275–282. doi: 10.1016/s0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- 10.Wu S, Kaufman RJ. A model for the double-stranded RNA (dsRNA)-dependent dimerization and activation of the dsRNA-activated protein kinase PKR. J Biol Chem. 1997;272:1291–1296. doi: 10.1074/jbc.272.2.1291. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff JR, Samuel CE. Mechanism of interferon action. The interferon-induced phosphoprotein P1 possesses a double-stranded RNA-dependent ATP-binding site. J Biol Chem. 1985;260:8237–8239. [PubMed] [Google Scholar]
- 12.Galabru J, Hovanessian A. Autophosphorylation of the protein kinase dependent on double-stranded RNA. J Biol Chem. 1987;262:15538–15544. [PubMed] [Google Scholar]
- 13.Nanduri S, et al. A dynamically tuned double-stranded RNA binding mechanism for the activation of antiviral kinase PKR. EMBO J. 2000;19:5567–5574. doi: 10.1093/emboj/19.20.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spanggord RJ, et al. Identification of binding sites for both dsRBMs of PKR on kinase- activating and kinase-inhibiting RNA ligands. Biochemistry. 2002;41:4511–4520. doi: 10.1021/bi0120594. [DOI] [PubMed] [Google Scholar]
- 15.Dey M, et al. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2alpha substrate recognition. Cell. 2005;122:901–913. doi: 10.1016/j.cell.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 16.Vattem KM, et al. Inhibitory sequences in the N-terminus of the double-stranded-RNA-dependent protein kinase, PKR, are important for regulating phosphorylation of eukaryotic initiation factor 2alpha (eIF2alpha) Eur J Biochem. 2001;268:1143–1153. doi: 10.1046/j.1432-1327.2001.01979.x. [DOI] [PubMed] [Google Scholar]
- 17.Wu S, Kaufman RJ. trans-Autophosphorylation by the isolated kinase domain is not sufficient for dimerization or activation of the dsRNA-activated protein kinase PKR. Biochemistry. 2004;43:11027–11034. doi: 10.1021/bi0360105. [DOI] [PubMed] [Google Scholar]
- 18.Saelens X, et al. Translation inhibition in apoptosis: caspase-dependent PKR activation and eIF2-alpha phosphorylation. J Biol Chem. 2001;276:41620–41628. doi: 10.1074/jbc.M103674200. [DOI] [PubMed] [Google Scholar]
- 19.Barber GN, et al. Functional expression and characterization of the interferon-induced double-stranded RNA activated P68 protein kinase from Escherichia coli. Biochemistry. 1991;30:10356–10361. doi: 10.1021/bi00106a038. [DOI] [PubMed] [Google Scholar]
- 20.Ung TL, et al. Heterologous dimerization domains functionally substitute for the double-stranded RNA binding domains of the kinase PKR. EMBO J. 2001;20:3728–3737. doi: 10.1093/emboj/20.14.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vattem KM, et al. Mechanism of activation of the double-stranded-RNA-dependent protein kinase, PKR: role of dimerization and cellular localization in the stimulation of PKR phosphorylation of eukaryotic initiation factor-2 (eIF2) Eur J Biochem. 2001;268:3674–3684. doi: 10.1046/j.1432-1327.2001.02273.x. [DOI] [PubMed] [Google Scholar]
- 22.Lemaire PA, et al. Unactivated PKR exists in an open conformation capable of binding nucleotides. Biochemistry. 2006;45:9074–9084. doi: 10.1021/bi060567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelev V, et al. Mapping of the Auto-inhibitory Interactions of Protein Kinase R by Nuclear Magnetic Resonance. J Mol Biol. 2006 doi: 10.1016/j.jmb.2006.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemaire PA, et al. Mechanism of PKR activation: dimerization and kinase activation in the absence of double-stranded RNA. J Mol Biol. 2005;345:81–90. doi: 10.1016/j.jmb.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Gabel F, et al. Dynamic Flexibility of Double-stranded RNA Activated PKR in Solution. J Mol Biol. 2006;359:610–623. doi: 10.1016/j.jmb.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 26.Kim I, et al. Specific recognition of HIV TAR RNA by the dsRNA binding domains (dsRBD1-dsRBD2) of PKR. J Mol Biol. 2006;358:430–442. doi: 10.1016/j.jmb.2006.01.099. [DOI] [PubMed] [Google Scholar]
- 27.McKenna SA, et al. Uncoupling of RNA binding and PKR kinase activation by viral inhibitor RNAs. J Mol Biol. 2006;358:1270–1285. doi: 10.1016/j.jmb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Carpick BW, et al. Characterization of the solution complex between the interferon-induced double-stranded RNA-activated protein kinase and HIV-I trans-activating region RNA. J Biol Chem. 1997;272:9510–9516. doi: 10.1074/jbc.272.14.9510. [DOI] [PubMed] [Google Scholar]
- 29.Langland JO, Jacobs BL. Cytosolic double-stranded RNA-dependent protein kinase is likely a dimer of partially phosphorylated Mr=66,000 subunits. J Biol Chem. 1992;267:10729–10736. [PubMed] [Google Scholar]
- 30.Patel RC, et al. The interferon-inducible double-stranded RNA-activated protein kinase self-associates in vitro and in vivo. Proc Natl Acad Sci USA. 1995;92:8283–8287. doi: 10.1073/pnas.92.18.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian B, Mathews MB. Functional characterization of and cooperation between double-stranded RNA binding motifs of the protein kinase PKR. J Biol Chem. 2001;276:9936–9944. doi: 10.1074/jbc.M007328200. [DOI] [PubMed] [Google Scholar]
- 32.Hunter T, et al. The characteristics of inhibition of protein synthesis by double-stranded ribonucleic acid in reticulocyte lysates. J Biol Chem. 1975;250:409–417. [PubMed] [Google Scholar]
- 33.Kostura M, Mathews MB. Purification and activation of the double-stranded RNA-dependent eIF-2 kinase DAI. Mol Cell Biol. 1989;9:1576–1586. doi: 10.1128/mcb.9.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ucci JW, et al. Mechanism of assembly of PKR on double stranded RNA. Biochemistry. 2006 in press. [Google Scholar]
- 35.Nanduri S, et al. 1H, 13C, 15N resonance assignments of the 20 kDa double stranded RNA binding domain of PKR. J Biomol NMR. 1998;12:349–351. doi: 10.1023/a:1008259729432. [DOI] [PubMed] [Google Scholar]
- 36.Li S, et al. Molecular basis for PKR activation by PACT or dsRNA. Proc Natl Acad Sci U S A. 2006;103:10005–10010. doi: 10.1073/pnas.0602317103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortiz-Lombardia M, et al. Crystal structure of the catalytic domain of the PknB serine/threonine kinase from Mycobacterium tuberculosis. J Biol Chem. 2003;278:13094–13100. doi: 10.1074/jbc.M300660200. [DOI] [PubMed] [Google Scholar]
- 38.Young TA, et al. Structure of Mycobacterium tuberculosis PknB supports a universal activation mechanism for Ser/Thr protein kinases. Nat Struct Biol. 2003;10:168–174. doi: 10.1038/nsb897. [DOI] [PubMed] [Google Scholar]
- 39.Gay LM, et al. A conserved dimer and global conformational changes in the structure of apo-PknE Ser/Thr protein kinase from Mycobacterium tuberculosis. J Mol Biol. 2006;360:409–420. doi: 10.1016/j.jmb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Padyana AK, et al. Structural basis for autoinhibition and mutational activation of eukaryotic initiation factor 2alpha protein kinase GCN2. J Biol Chem. 2005;280:29289–29299. doi: 10.1074/jbc.M504096200. [DOI] [PubMed] [Google Scholar]
- 41.Jammi NV, Beal PA. Phosphorylation of the RNA-dependent protein kinase regulates its RNA-binding activity. Nucleic Acids Res. 2001;29:3020–3029. doi: 10.1093/nar/29.14.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.George CX, et al. Characterization of the heparin-mediated activation of PKR, the interferon-inducible RNA-dependent protein kinase. Virology. 1996;221:180–188. doi: 10.1006/viro.1996.0364. [DOI] [PubMed] [Google Scholar]
- 43.Fasciano S, et al. Identification of the heparin-binding domains of the interferon-induced protein kinase, PKR. FEBS J. 2005;272:1425–1439. doi: 10.1111/j.1742-4658.2005.04575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]