Abstract
Oxidative stress is a critical component of the injury response after hypoxia-ischemia (HI) in the neonatal brain, and this response is unique and at times paradoxical to that seen in the mature brain. Previously, we showed that copper-zinc superoxide-dismutase (SOD1) over-expression is not beneficial to the neonatal mouse brain with HI injury, unlike the adult brain with ischemic injury. However, glutathione peroxidase1 (GPx1) over-expression is protective to the neonatal mouse brain with HI injury. To further test the hypothesis that an adequate supply of GPx is critical to protection from HI injury, we crossed SOD1 over-expressing mice (hSOD-tg) with GPx1 over-expressing mice (hGPx-tg). Resulting litters contained wild-type (wt), hGPx-tg, hSOD-tg and hybrid hGPx-tg/hSOD-tg pups, which were subjected to HI at P7. Confirming previous results, the hGPx-tg mice had reduced injury compared to both Wt and hSOD-tg littermates. Neonatal mice over-expressing both GPx1 and SOD1 also had less injury compared to wt or hSOD-tg alone. A result of oxidative stress after neonatal HI is a decrease in the concentration of reduced (i.e. antioxidant-active) glutathione (GSH). In this study, we tested the effect of systemic administration of alpha-lipoic acid on levels of GSH in the cortex after HI. Although GSH levels were restored by 24h after HI, injury was not reduced compared to vehicle-treated mice. We also tested two other pharmacological approaches to reducing oxidative stress in hSOD-tg and wild-type littermates. Both the specific inhibitor of neuronal nitric oxide synthase, 7-nitroindazole (7NI), and the spin-trapping agent alpha-phenyl-tert-butyl-nitrone (PBN) did not reduce HI injury, however. Taken together, these results imply that H2O2 is a critical component of neonatal HI injury, and GPx1 plays an important role in the defense against this H2O2 and is thereby neuroprotective.
Keywords: oxidative stress, stroke, reactive oxygen species, nitric oxide, hydrogen peroxide, brain, mouse
1. Introduction
There is mounting evidence that oxidative stress contributes to the pathogenesis of brain injury in both the mature and immature nervous system. After perinatal asphyxia, it is believed that the newborn brain experiences a cascade of toxic events that begin with energy failure and involve glutamate release, activation of NMDA receptors, coupled to nitric oxide synthase (NOS), calcium flux, and release of nitric oxide (Ferriero, 2004) (Buonocore et al., 2007). These changes result in further mitochondrial dysfunction with superoxide leakage and formation of other reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), which attack lipids, proteins and other cell constituents, leading to cell injury or death (McLean et al., 2004).
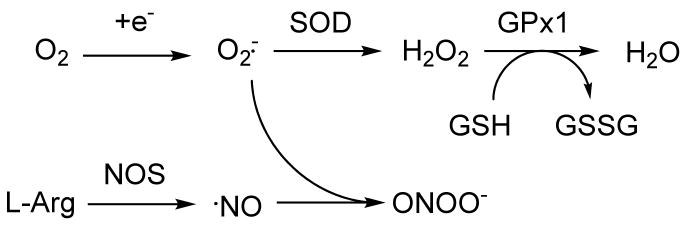
Under these conditions, the brain's endogenous antioxidant defense mechanisms, superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx), which, in the rat, display less activity in the immature brain than in the adult brain (Khan et al., 2003), may become overwhelmed (see Figure 1 for interconnection between the different ROS and how they are modulated by changing endogenous antioxidant enzyme levels). Cellular injury due to NOS activation is thought to be mediated by the reaction of NO with superoxide to form the more hazardous intermediate peroxynitrite (Huie et al., 1993). Thus, blocking the formation of peroxynitrite via inhibition of NO formation is another possible avenue for protection. In addition, the immature brain has more free iron than the adult. Consequently, the neonatal brain is more susceptible to hypoxic-ischemic (HI) injury than the mature brain (Ferriero, 2001) (Ferriero, 2004). Experimental studies aimed at reducing oxidative stress by manipulating levels of these enzymes have shown encouraging results for the mature brain. For example, in transgenic animals over-expressing the copper-zinc superoxide-dismutase transgene (hSOD1-tg), Chan et al showed marked protection in adult mice (Chan et al., 1996), and rats (Chan et al., 1998), after focal and global ischemic insults. However, in the neonatal mouse expressing the same transgene, no protection was found (Ditelberg et al., 1996), possibly due to the imbalanced over-production of H2O2 in these animals (Fullerton et al., 1998), which is toxic to immature neurons in culture (Mischel et al., 1997). Recently, excess H2O2 was found to accumulate in neonatal mouse brain after HI, but not in adult brain after the same degree of injury (Lafemina et al., 2006). Consequently, GPx, which converts H2O2 to oxygen and water, is likely to be a critical factor in prevention of neonatal HI injury, and studies using mice that target the overproduction of H2O2, via GPx over-expression, have indeed shown decreased injury after neonatal HI (Sheldon et al., 2004). These hGPx-tg mice also accumulate less H2O2 after HI compared to Wt (Sheldon et al., 2007). Depletion of neuronal reduced glutathione (GSH), which is a required co-factor for the conversion of H2O2 to water by GPx, has been shown to exacerbate oxidative injury (Chen et al., 2003) (White et al., 2003). Recently, we showed that GSH is reduced in Wt brain after HI, but not in hGPx-tg (Sheldon et al., 2007).
Figure 1.
Schema of the interconnection of the pathways of reactive oxygen species (ROS) and reactive nitrogen species (RNS). In this study, the different species were targeted by genetic manipulation of the endogenous antioxidant enzymes, or by pharmacologic agents that inhibit the enzymes that generate them, or directly scavenge them.
To further explore the roles of SOD and GPx in neonatal HI, and the hypothesis that increasing GPx can ameliorate injury from excess H2O2 production, we combined SOD over-expression and GPx over-expression, by breeding heterozygotes of the two transgenic strains, hSOD-tg with hGPx-tg. The resulting offspring of this mating were subjected to HI at P7, and degree of brain injury, 5 days after the insult was compared. We also hypothesized that HI injury could be prevented if GSH levels were restored, and tested this with the systemic administration of α-lipoic acid. In addition, we targeted the NO generating pathway in hSOD-tg mice pharmacologically with 7-NI, and the downstream reactive nitrogen species (RNS), peroxynitrite, with the spin trapping and antioxidant agent PBN.
2. Experimental procedures
Animals
All animal research was approved by the Institutional Animal Care and Use Committee at the University of California San Francisco and performed with the highest standards of humane care as set forth in the “Guide for the Care and Use of Laboratory Animals”, U.S. Dept. of Health and Human services, 85-23, 1985.
Wild-type litters were all CD1 (Charles River) and hSOD1-tg and hGPx1-tg both have background strain CD1. For the SOD1/GPx1 hybrids, an hSOD1-tg heterozygous male was bred with an hGPx1-tg heterozygous female. Genotyping of litters was carried out by as previously described by PAGE for hSOD-tg (Sheldon et al., 2002) or by PCR on tail DNA for hGPx-tg (Sheldon et al., 2004).
Hypoxic-ischemic injury
At postnatal day 7 (P7), all animals underwent a permanent ligation of the right common carotid artery under isoflurane anesthesia (3% in oxygen), with an intervening 90-minute recovery and feeding period with the dam prior to the institution of systemic hypoxia. For the hypoxia, mice were then placed in interconnected chambers through which flowed 8% oxygen, 92% nitrogen for 30 minutes. This duration was chosen based on previous studies that showed that variation in brain injury due to background strain of the mouse can be controlled for by using durations of hypoxia tailored to strain (Sheldon et al., 1998). The chamber apparatus floated in a 37 degree C water bath, and temperature of one mouse was monitored by probe.
Drug treatments
Alpha-lipoic acid (25 mg/kg i.p.) was dissolved in Tris (0.1 M, pH 7.4) and administered once (s.c.) at 0h or 4 times, at 0h, 4h, 20h, and 24h after the end of the HI procedure (n = 55 for GSH assay; n = 20 for determination of injury score). For most experiments racemic (±)-α-lipoic acid (Sigma-Aldrich) was used. In some experiments, the natural enantiomer R-(+)-α-lipoic acid was used (ASTA Medica, Frankfurt, Germany) and yielded comparable results. 7NI (MTM Research Chemicals, Windham, NH; 10 mg/kg, 25 mg/kg or 50 mg/kg i.p.) was dissolved by sonication in peanut oil and given three times at 0h, 4h, and 24h after HI. PBN (Sigma-Aldrich, St. Louis, MO) was dissolved in normal saline and given three times, at 0h, 4h and 24h after HI. All control animals received the same volume of vehicle with the same dosing paradigm as treated animals.
Histology
Mice were anesthetized with pentobarbital (100 mg/kg) and perfused through the left ventricle with cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4), five days after the HI procedure. The brains were removed and post-fixed in the same fixative for four hours, then transferred to 30% sucrose in 0.1 M phosphate buffer. Coronal sections were cut through the forebrain at 50 μm intervals on a Vibratome (Ted Pella, Inc). Alternate sections were stained with cresyl violet and with Perl's iron stain, mounted onto gelatinized slides, dehydrated and coverslipped with Permount.
Brains were scored, in a masked fashion, for degree of injury using both cresyl violet and Perl's stains. All sections were examined, and eight regions of the brain were scored: the anterior, middle and posterior cortex, CA1, CA2, CA3 and dentate gyrus of the hippocampus, and caudate putamen, with the contralateral side serving as a reference for uninjured tissue. Each region was given a score of 0-3 such that 0 = no detectable neuronal loss, 1 = small focal areas of neuronal loss, 2 = columnar damage in the cortex involving predominately layers II-IV and/or moderate cell loss in the hippocampus, and 3 = cystic infarction and gliosis. The score for each region was then added for a final score ranging from 0 – 24.
Glutathione levels
At either 2h or 24h after HI, Wt pups were anesthetized with pentobarbital (100 mg/kg) and perfused intracardiac with ice-cold deoxygenated 0.1 M sodium phosphate buffered saline (pH 7.2). Brains were removed, cortices dissected free on a cold pack, weighed, and 9 volumes of ice-cold perchloric acid (0.6 M) with L-methionine (0.5 mM) were added to tissue and rapidly frozen on dry ice. Total glutathione (which is >99.5% GSH) was measured by a modified Tietze assay using the Bioxytech GSH/GSSG-412 kit from Oxis Health Products (Portland, OR).
Statistical measures
Injury scores for two groups (Figure 3B) were analyzed by Mann-Whitney test. Injury scores for multiple groups were analyzed by Kruskal-Wallis. Mean values were analyzed by ANOVA followed by Bonferroni post-hoc testing for multiple comparisons for continuous data, and are presented as mean ± SD. All analysis was performed with Prism version 4 (Graphpad Software Inc, San Diego, CA).
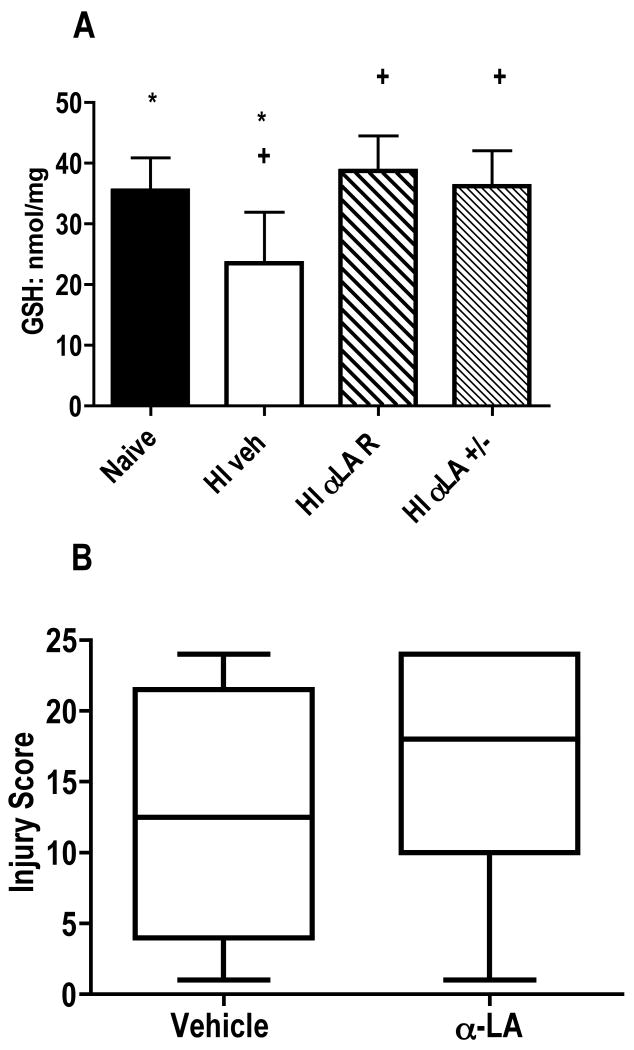
Figure 3.
Alpha-lipoic acid treatment following neonatal hypoxia-ischemia. A) GSH is depleted 24h after HI in vehicle-treated compared to naïve mice (*p<0.05). Treatment with α-lipoic acid either prevents this depletion, or restores GSH to naïve levels. Mice treated with either form of α-lipoic acid after HI had higher GSH levels than vehicle-treated (+p<0.05 for both the R and± enantiomers). Analysis by ANOVA, GSH (nmol/mg protein) is expressed as mean ± SD. B) Brain injury scores for mice treated with racemic α-lipoic acid were not different than vehicle-treated (p>0.30). The horizontal line is the median value. Analysis by Mann-Whitney.
3. Results
hSOD-tg x hGPx-tg hybrid mice
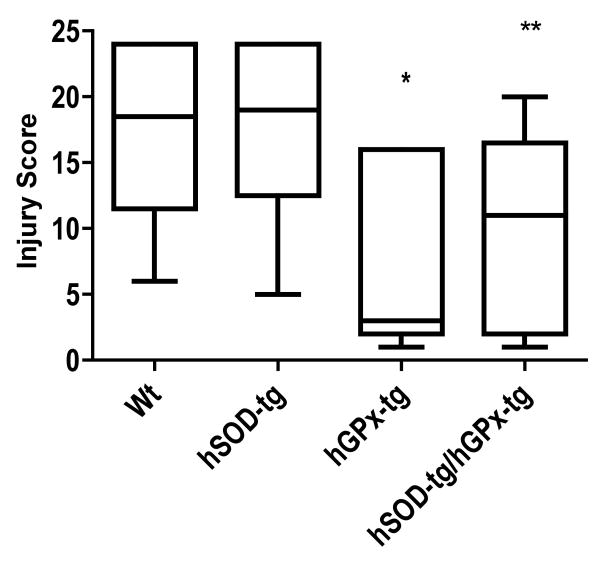
Confirming previous studies, the hGPx-tg mice had less injury than their Wt littermates (hGPx-tg median score = 3, range 1-16; Wt median score = 18.5, range 6-24, p<0.02). The hGPx-tg also had less injury than their hSOD-tg littermates (hSOD-tg median score = 19, range 5-24, p<0.04). Injury scores for the hybrid mice, hSOD-tg x hGPx-tg, (median score = 11, range 1-20) were lower than either Wt (p<0.04) or hSOD-tg (p<0.05) (Figure 2). There was no effect on injury scores due to gender of the mice.
Figure 2.
HI brain injury in offspring of heterozygous hGPx-tg mated with heterozygous hSOD-tg. The resulting hGPx-tg neonatal mice had lower injury scores than both Wt (*p<0.02) and hSOD-tg (*p<0.04) littermates. Injury scores for the hybrid overexpressors (hSOD-tg x hGPx-tg) were lower than either Wt (**p<0.04) or hSOD-tg (**p<0.05). Compared to hGPx-tg, the median injury score of the hybrid mice is higher, but not significantly (p>0.13). The horizontal line is the median value. Analysis by Kruskal-Wallis.
Treatment with α-lipoic acid
GSH is diminished in the mouse cortex 24h after HI compared to naïve (Figure 3A; p<0.05), but not at 2h (data not shown). α-lipoic acid treatment using either the natural R enantiomer or the racemic (±) form at the time of reoxygenation prevented the loss of GSH at 24h after HI (Figure 3A). The dosing paradigm used was chosen based on toxicity tests that showed a single dose of 100 mg/kg to be toxic to the P7 CD1 mice, with 44% mortality. These mice became lethargic and died within 3 h, with no further mortality in this group. Mice also died with 50 mg/kg or 75 mg/kg, but several days after treatment. However, 25 mg/kg was well tolerated (Table 1). Despite mortality with higher doses, all treatment groups gained weight equally over the course of two weeks (Table 2). Repletion of GSH (by the racemic enantiomer of α-lipoic acid) was not sufficient to protect the brain from histological injury, however, when measured 5 days after HI. Injury scores for vehicle-treated mice (median score = 12.5, range 1-24) were not significantly different than α-lipoic acid-treated mice (median score =18, range 1-24 (Figure 3B; p = 0.30). There was no effect due to gender on injury outcome.
Table 1.
Mortality due to α-lipoic acid
| Vehicle | 25 mg/kg | 50 mg/kg | 75 mg/kg | 100 mg/kg | |
|---|---|---|---|---|---|
| P7 | 0* | 0 | 0 | 0 | 0 |
| P8 | 0 | 0 | 0 | 0 | 44 |
| P9 | 0 | 0 | 0 | 0 | 44 |
| P12-14 | 0 | 0 | 0 | 12 | 44 |
| P21-22 | 0 | 0 | 30 | 25 | 44 |
Percent of mice that died (or missing and presumed dead) from each treatment group.
Table 2.
Weight change over treatment course
| Vehicle | 25 mg/kg | 50 mg/kg | 75 mg/kg | 100 mg/kg | |
|---|---|---|---|---|---|
| P7 | 4.4±1.1 | 5.4±0.05 | 4.7±0.6 | 4.3±0.7 | 4.4±0.4* |
| P8 | 5.1±0.9 | 6.0±0.03 | 5.5±0.5 | 5.0±0.6 | 4.8±0.4 |
| P9 | 6.3±0.5 | 6.6±0.1 | 6.5±0.7 | 6.0±0.3 | 5.7±0.05 |
| P12-14 | 7.0±0.1 | 7.0±0.6 | 7.5±0.6 | 6.9±0.8 | 6.9±0.6 |
| P21-22 | 14.5±0.5 | 12.9±0.7 | 14.7±1.5 | 13.1±2.4 | 12.9±4.4 |
Mean weight (g) ± SD.
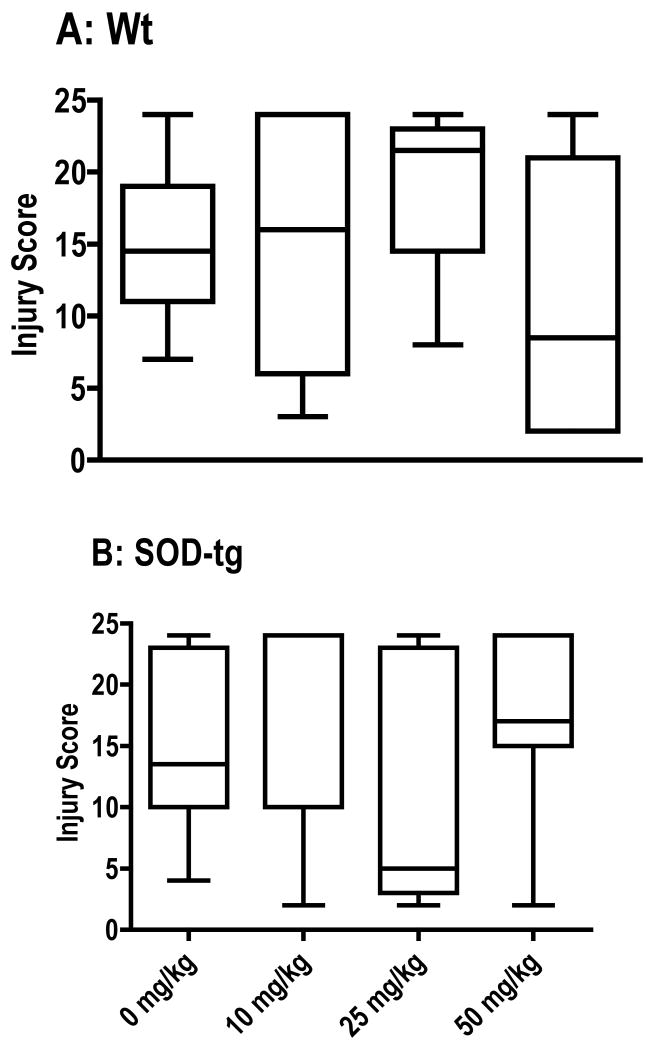
Treatment with 7NI
The specific inhibitor of the neuronal NOS isoform, 7NI (Mayer et al., 1994), had no effect on injury score in either hSOD-tg or Wt littermates (Figure 4).
Figure 4.
7-Nitroindazole-treatment following neonatal hypoxia-ischemia in A) Wt and B) hSOD-tg. Brain injury scores were not different than vehicle-treated. The horizontal line is the median value. Analysis by Kruskal-Wallis.
Treatment with PBN
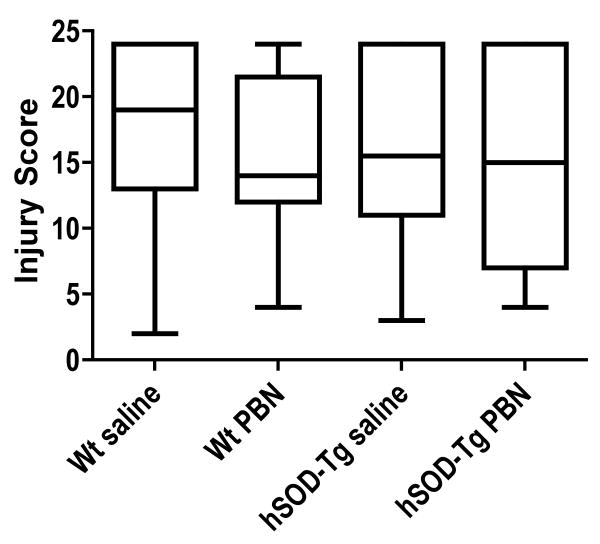
The spin trapping and antioxidant agent PBN, administered in both pre-treatment and post-treatment paradigms, had no effect on injury score in either hSOD-tg or wt littermates (Figure 5).
Figure 5.
PBN treatment following neonatal hypoxia-ischemia in Wt and hSOD1-tg. Brain injury scores were not different than vehicle-treated, for either Wt or hSOD-tg. The horizontal line is the median value. Analysis by Kruskal-Wallis.
4. Discussion
This study supports the idea that manipulation of the brain's antioxidant defense mechanisms is critical to protection after HI injury. By combining SOD over-expression with GPx over-expression, we show that increased GPx protects against the deleterious effect of unbalanced SOD over-expression. However, simply maintaining levels of GSH, a required cofactor of GPx for the conversion of H2O2 to water, by α-lipoic acid treatment does not prevent injury. Attempts to ameliorate injury in the hSOD-tg mice with NOS inhibition was ineffective, as was PBN administration.
We have previously shown that hSOD-tg pups were more injured than their Wt littermates after HI (Ditelberg et al., 1996), and that more H2O2 accumulated in the hSOD-tg mice (Fullerton et al., 1998). Here, with less severe HI insult, both the Wt and hSOD-tg groups have comparable injury, with reduced mortality. The fact that SOD and GPx over-expression combined produces a moderate injury that is less than Wt or SOD over-expression alone, but not significantly different than GPx over-expression alone, indicates that increasing GPx in a setting of over-production of H2O2 reduces injury. This is supported by in vitro experiments. In hippocampal cultures, over-expression of SOD1 led to increased cell death, lipid peroxidation and H2O2 accumulation after exposure to kainic acid (Zemlyak et al., 2006). For example, neurons cultured from GPx-tg brains demonstrated protection from exogenously applied H2O2 (McLean et al., 2005). Additionally, in both hippocampal and cortical neuronal cultures over-expressing GPx, there was decreased cell death after exposure to kainic acid, along with decreased lipid peroxidation and H2O2 accumulation (Wang et al., 2003). If adequate GPx activity is indeed a key to injury prevention, it follows that GSH levels should also be maintained (Dringen et al., 2005). Since GSH was maintained at naïve levels by α-lipoic acid administration, when measured at 24h after HI, but injury is not prevented when measured histologically five days after HI, it is likely that injury mechanisms are activated throughout this period. It also appears that dose, timing and route of administration of α-lipoic acid are important, and administration of α-lipoic acid before the HI injury or for longer periods after injury may be necessary to prevent damage. For example, in an adult stroke model, pretreatment with both enantiomers of α-lipoic acid reduced brain injury but only when given at specific times, and only when given subcutaneously (Wolz et al., 1996). These adult rats and mice also tolerated a dose of 100 mg/kg, unlike our neonatal mice. Others have shown efficacy with long-term (30 days) prophylactic treatment with a combination of α-lipoic acid and vitamin E in adult stroke (Garcia-Estrada et al., 2003). The reduction in GSH we see at 24h after HI presumably occurs as injury develops over the course of 24h. While cells undergoing delayed death might benefit from continued administration of α-lipoic acid, the dosing paradigm used was chosen to limit mortality, and it is unclear what the mechanism of death is with the higher doses of α-lipoic acid in our toxicity studies. Weight loss does not seem to be a factor, since there is no difference in mean weights between treatment groups.
While transgenic animals have provided a wealth of information on the mechanisms of HI injury, clinical relevance lies in finding an appropriate pharmacologic agent; perhaps an agent that mimics the effect of beneficial genetic manipulation. Since perinatal asphyxia initiates a cascade of neurotoxic events, there are several possibilities for intervention. For example, limiting excitotoxicity by reducing nitric oxide or peroxynitrite production. We have previously shown (in a different mouse background strain – Sv129xBl/6) that targeted disruption of the neuronal NOS gene via genetic deletion is protective (Ferriero et al., 1996), as is elimination of neurons containing NOS with direct application of quisqualate to the brain (Ferriero et al., 1995). However, pharmacologic inhibition of the neuronal NOS enzyme with cysteamine was less effective in protecting the neonatal brain from HI (Spanggord et al., 1996). We have also administered a non-specific inhibitor of the NOS enzyme, N-nitro-L-arginine methyl ester (L-NAME), since studies have shown that low-dose L-NAME reduces infarct volume in rat models of adult (Ashwal et al., 1993) and neonatal (Ashwal et al., 1995) stroke. However, post-treatment with either 3 or 30 mg/kg L-NAME did not alter the degree of injury in either wt or nNOS knockout mice (unpublished observations), perhaps because L-NAME must be given before the hypoxic insult to be effective in HI (Hamada et al., 1994). NOS1 knockout mice were also used to determine whether L-NAME therapy might actually exacerbate injury, since this has been reported in adult NOS1 knockout mice given an inhibitor of endothelial NOS activity after MCAO (Huang et al., 1994). This exacerbation may be expected due to the potential inhibition of the endothelial form of NOS. The lack of protection with the neuronal NOS inhibitor 7NI in hSOD-tg mice seen here confirms our previous work in CD1 wt mice (Muramatsu et al., 2000), and also shows that pharmacological inhibition of NOS activity does not protect the neonatal brain, especially in the presence of increased SOD activity and H2O2. The potential anti-oxidative properties of the vehicle (peanut oil) could possibly affect degree of injury and thus the significance of 7NI in this study. However, volumes used were minimal (0.05 mL/mouse).
The free radical scavenger PBN has been shown to penetrate the central nervous system and provide protection from ischemia (Cao et al., 1994; Zhao et al., 1994) (Yue et al., 1992). It was our assumption that since the hSOD-tg produced more H2O2, there might be an effect from scavenging therapy in these neonatal mice, since other reactive oxygen species might also be produced in these animals. Other avenues of reducing oxidative injury include blocking the Fenton reaction, for example, with the iron chelator desferioxamine. Desferioxamine has been shown to protect the neonatal brain when given after HI (Palmer et al., 1994) (Sarco et al., 2000). Also, both desferioxamine (pretreatment) and TPEN have been shown to protect neurons from injury mediated by high doses of H2O2 in vitro (Almli et al., 2001). Desferioxamine was also protective against NMDA exposure, but TPEN was not. The sum of these results on studies targeting the excitotoxic pathway by us, and others, has led us to believe that therapeutic targets, at least in the neonate, lie with preventing, or ameliorating, accumulation of H2O2 over other ROS or RNS (cf. Figure 1).
A conundrum of stroke research is the fact that a number of potential therapies that have reduced brain injury in animal models have not proven effective in the human. Finding effective therapies for the developing brain is even more challenging, since the developing brain has unique properties that make comparisons with the adult data of limited value (Ferriero, 2004) (McQuillen et al., 2004). In addition, timing of therapy may be crucial. Experimental therapeutic agents that are administered before the completion of the HI insult, such as before the onset of hypoxia, are of limited clinical value. However, the neonatal brain may be more amenable to treatments that increase neurogenesis and improve remodeling after injury. We have used both transgenic animals and pharmacological agents to expand our understanding of the mechanisms of HI injury.
Acknowledgments
We would like to thank Dr. Bruce N. Ames, Children's Hospital Oakland Research Institute, for his kind gift of R-lipoic acid. We also thank David Glidden, PhD for statistical advice and John Lee and Joel Jacobsen MD, for technical assistance.
Supported by National Institutes of Health Grant NS33997
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almli LM, Hamrick SEG, Koshy AA, Täuber MG, Ferriero DM. Multiple pathways of neuroprotection against oxidative stress and excitotoxic injury in immature primary hippocampal neurons. Brain Research. Developmental Brain Research. 2001;132:121–129. doi: 10.1016/s0165-3806(01)00302-9. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Cole DJ, Osborne S, Osborne TN, Pearce WJ. L-NAME reduces infarct volume in a filament model of transient middle cerebral artery occlusion in the rat pup. Pediatric Research. 1995;38:652–656. doi: 10.1203/00006450-199511000-00004. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Cole DJ, Osborne TN, Pearce WJ. Low dose L-NAME reduces infarct volume in the rat MCAO/reperfusion model. Journal of Neurosurgical Anesthesiology. 1993;5:241–249. doi: 10.1097/00008506-199310000-00004. [DOI] [PubMed] [Google Scholar]
- Buonocore G, Groenendaal F. Anti-oxidant strategies. Semin Fetal Neonatal Med. 2007 doi: 10.1016/j.siny.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Cao X, Phillis JW. a-Phenyl-tert-butyl-nitrone reduces cortical infarct and edema in rats subjected to focal ischemia. Brain Research. 1994;644:267–272. doi: 10.1016/0006-8993(94)91689-6. [DOI] [PubMed] [Google Scholar]
- Chan PH, Epstein CJ, Kinouchi H, Kamii H, Chen SF, Carlson E, Gafni J, Yang G, Reola L. Neuroprotective role of CuZn-superoxide dismutase in ischemic brain damage. Advances in Neurology. 1996;71:271–280. [PubMed] [Google Scholar]
- Chan PH, Kawase M, Murakami K, Chen SF, Li Y, Calagui B, Reola L, Carlson E, Epstein CJ. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. Journal of Neuroscience. 1998;18:8292–8299. doi: 10.1523/JNEUROSCI.18-20-08292.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Liao SL. Zinc toxicity on neonatal cortical neurons: involvement of glutathione chelation. Journal of Neurochemistry. 2003;85:443–453. doi: 10.1046/j.1471-4159.2003.01691.x. [DOI] [PubMed] [Google Scholar]
- Ditelberg JS, Sheldon RA, Epstein CJ, Ferriero DM. Brain injury after perinatal hypoxia-ischemia is exacerbated in copper/zinc superoxide dismutase transgenic mice. Pediatric Research. 1996;39:204–208. doi: 10.1203/00006450-199602000-00003. [DOI] [PubMed] [Google Scholar]
- Dringen R, Pawlowski PG, Hirrlinger J. Peroxide detoxification by brain cells. J Neurosci Res. 2005;79:157–165. doi: 10.1002/jnr.20280. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Oxidant mechanisms in neonatal hypoxia-ischemia. Developmental Neuroscience. 2001;23:198–202. doi: 10.1159/000046143. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. New England Journal of Medicine. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Ferriero DM, Holtzman DM, Black SM, Sheldon RA. Neonatal mice lacking neuronal nitric oxide synthase are less vulnerable to hypoxic-ischemic injury. Neurobiology of Disease. 1996;3:64–71. doi: 10.1006/nbdi.1996.0006. [DOI] [PubMed] [Google Scholar]
- Ferriero DM, Sheldon RA, Black SM, Chuai J. Selective destruction of nitric oxide synthase neurons with quisqualate reduces damage after hypoxia-ischemia in the neonatal rat. Pediatric Research. 1995;38:912–918. doi: 10.1203/00006450-199512000-00014. [DOI] [PubMed] [Google Scholar]
- Fullerton HJ, Ditelberg JS, Chen SF, Sarco DP, Chan PH, Epstein CJ, Ferriero DM. Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Annals of Neurology. 1998;44:357–364. doi: 10.1002/ana.410440311. [DOI] [PubMed] [Google Scholar]
- Garcia-Estrada J, Gonzalez-Perez O, Gonzalez-Castaneda RE, Martinez-Contreras A, Luquin S, de la Mora PG, Navarro-Ruiz A. An alpha-lipoic acid-vitamin E mixture reduces post-embolism lipid peroxidation, cerebral infarction, and neurological deficit in rats. Neurosci Res. 2003;47:219–224. doi: 10.1016/s0168-0102(03)00200-1. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Hayakawa T, Hattori H, Mikawa H. Inhibitor of nitric oxide synthesis reduces hypoxic-ischemic brain damage in the neonatal rat. Pediatric Research. 1994;35:10–14. doi: 10.1203/00006450-199401000-00003. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- Huie RE, Padmaja S. The reaction of no with superoxide. Free Radical Research Communications. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- Khan JY, Black SM. Developmental changes in murine brain antioxidant enzymes. Pediatric Research. 2003;54:77–82. doi: 10.1203/01.PDR.0000065736.69214.20. [DOI] [PubMed] [Google Scholar]
- Lafemina MJ, Sheldon RA, Ferriero DM. Acute hypoxia-ischemia results in hydrogen peroxide accumulation in neonatal but not adult mouse brain. Pediatr Res. 2006;59:680–683. doi: 10.1203/01.pdr.0000214891.35363.6a. [DOI] [PubMed] [Google Scholar]
- Mayer B, Klatt P, Werner ER, Schmidt K. Molecular mechanisms of inhibition of porcine brain nitric oxide synthase by the antinociceptive drug 7-nitro-indazole. Neuropharmacology. 1994;33:1253–1259. doi: 10.1016/0028-3908(94)90024-8. published erratum appears in. [DOI] [PubMed] [Google Scholar]; Neuropharmacology. 1995 Feb;34(2):243. [Google Scholar]
- McLean C, Ferriero D. Mechanisms of hypoxic-ischemic injury in the term infant. Seminars in Perinatology. 2004;28:425–432. doi: 10.1053/j.semperi.2004.10.005. [DOI] [PubMed] [Google Scholar]
- McLean CW, Mirochnitchenko O, Claus CP, Noble-Haeusslein LJ, Ferriero DM. Overexpression of glutathione peroxidase protects immature murine neurons from oxidative stress. Developmental Neuroscience. 2005;27:169–175. doi: 10.1159/000085989. [DOI] [PubMed] [Google Scholar]
- McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Ped Neurol. 2004;30:227–235. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Mischel RE, Kim YS, Sheldon RA, Ferriero DM. Hydrogen peroxide is selectively toxic to immature murine neurons in vitro. Neuroscience Letters. 1997;231:17–20. doi: 10.1016/s0304-3940(97)00531-4. [DOI] [PubMed] [Google Scholar]
- Muramatsu K, Sheldon RA, Black SM, Taeuber M, Ferriero DM. Nitric oxide synthase activity and inhibition after neonatal hypoxia-ischemia in the mouse. Brain Research Developmental Brain Research. 2000;123:119–127. doi: 10.1016/s0165-3806(00)00088-2. [DOI] [PubMed] [Google Scholar]
- Palmer C, Roberts RL, Bero C. Deferoxamine posttreatment reduces ischemic brain injury in neonatal rats. Stroke. 1994;25:1039–1045. doi: 10.1161/01.str.25.5.1039. [DOI] [PubMed] [Google Scholar]
- Sarco D, Becker J, Palmer C, Sheldon RA, Ferriero DM. The neuroprotective effect of deferoxamine in the hypoxic-ischemic immature mouse brain. Neuroscience Letters. 2000;282:113–116. doi: 10.1016/s0304-3940(00)00878-8. [DOI] [PubMed] [Google Scholar]
- Sheldon RA, Almli L, Ferriero DM. Copper/zinc superoxide dismutase transgenic brain in neonatal hypoxia-ischemia. Methods Enzymol. 2002;353:389–397. doi: 10.1016/s0076-6879(02)53063-9. [DOI] [PubMed] [Google Scholar]
- Sheldon RA, Aminoff A, Lee CL, Christen S, Ferriero DM. Hypoxic Preconditioning Reverses Protection After Neonatal Hypoxia-Ischemia in Glutathione Peroxidase Transgenic Murine Brain. Pediatr Res. 2007 doi: 10.1203/pdr.0b013e318053664c. [DOI] [PubMed] [Google Scholar]
- Sheldon RA, Jiang X, Francisco C, Christen S, Vexler ZS, Tauber MG, Ferriero DM. Manipulation of antioxidant pathways in neonatal murine brain. Pediatric Research. 2004;56:656–662. doi: 10.1203/01.PDR.0000139413.27864.50. [DOI] [PubMed] [Google Scholar]
- Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Research. 1998;810:114–122. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- Spanggord H, Sheldon RA, Ferriero DM. Cysteamine eliminates nitric oxide synthase activity but is not protective to the hypoxic-ischemic neonatal rat brain. Neuroscience Letters. 1996;213:41–44. doi: 10.1016/0304-3940(96)12848-2. [DOI] [PubMed] [Google Scholar]
- Wang H, Cheng E, Brooke S, Chang P, Sapolsky R. Over-expression of antioxidant enzymes protects cultured hippocampal and cortical neurons from necrotic insults. J Neurochem. 2003;87:1527–1534. doi: 10.1046/j.1471-4159.2003.02123.x. [DOI] [PubMed] [Google Scholar]
- White AR, Cappai R. Neurotoxicity from glutathione depletion is dependent on extracellular trace copper. Journal of Neuroscience Research. 2003;71:889–897. doi: 10.1002/jnr.10537. [DOI] [PubMed] [Google Scholar]
- Wolz P, Krieglstein J. Neuroprotective effects of alpha-lipoic acid and its enantiomers demonstrated in rodent models of focal cerebral ischemia. Neuropharmacology. 1996;35:369–375. doi: 10.1016/0028-3908(95)00172-7. [DOI] [PubMed] [Google Scholar]
- Yue TL, Gu JL, Lysko PG, Cheng HY, Barone FC, Feuerstein G. Neuroprotective effects of phenyl-t-butyl-nitrone in gerbil global brain ischemia and in cultured rat cerebellar neurons. Brain Research. 1992;574:193–197. doi: 10.1016/0006-8993(92)90816-r. [DOI] [PubMed] [Google Scholar]
- Zemlyak I, Nimon V, Brooke S, Moore T, McLaughlin J, Sapolsky R. Gene therapy in the nervous system with superoxide dismutase. Brain Res. 2006;1088:12–18. doi: 10.1016/j.brainres.2006.02.109. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Pahlmark K, Smith ML, Siesjö BK. Delayed treatment with the spin trap alpha-phenyl-N-tert-butyl nitrone (PBN) reduces infarct size following transient middle cerebral artery occlusion in rats. Acta Physiologica Scandinavica. 1994;152:349–350. doi: 10.1111/j.1748-1716.1994.tb09816.x. [DOI] [PubMed] [Google Scholar]