Semiconductor quantum dots (QDs) are light-emitting nanocrystals with unique optical and electronic properties that are not available from organic dyes or fluorescent proteins.1 In the near term, one of the most promising applications of these particles is for use as fluorescent probes for molecular, cellular, and in vivo imaging.2 However, a major problem is the large size of conventional QD probes, which adversely affects their molecular binding and in vivo biodistribution. This bulkiness is not an intrinsic problem of QD nanocrystals, but arises mainly from organic surface coatings used for encapsulation and stabilization. In fact, small 4−7 nm QDs have been shown to have hydrodynamic sizes (diameters) of 20−40 nm when coated with amphiphilic polymers.3 Small hydrophilic and cross-linked ligands such as thioglycerol and dihydrolipoic acid have been used to reduce the coating layer thickness,4-7 but the resulting dots often suffer from low colloidal stability, photobleaching, or low quantum yield problems. Consequently, these size-reduced QDs have found only limited utility in live cell and in vivo applications.2
Here we report a new class of multifunctional multidentate polymer ligands not only for minimizing the hydrodynamic size of QDs but also for overcoming the colloidal stability and photobleaching/signal brightness problems encountered in previous research. A major finding is that a mixed composition of thiol (-SH) and amine (-NH2) groups grafted to a linear polymer chain can lead to a highly compact QD with long-term colloidal stability, strong resistance to photobleaching, and high fluorescence quantum yield. In contrast to the standing brush-like conformations of PEGylated dihydrolipoic acid ligands and monovalent thiols, we believe that these multidentate polymer ligands can wrap around the QD in a closed “loops-and-trains” conformation.8 This structure is highly stable from a thermodynamic perspective, and is thus responsible for the excellent colloidal and optical properties observed. As a result, we have prepared a new generation of bright and stable CdTe QDs with small hydrodynamic diameters between 5.6 nm to 9.7 nm, with fluorescence emission tunable from the visible (515 nm) to the near infrared (720 nm). In addition to CdTe nanocrystals, we find that this new class of multidentate polymers is applicable to a broad range of core nanocrystals as well as core/shell nanostructures including CdS, ZnSe, CdSe/ZnS, and CdTe/CdS, more versatile and robust than amphiphilic phytochelatin peptides.9
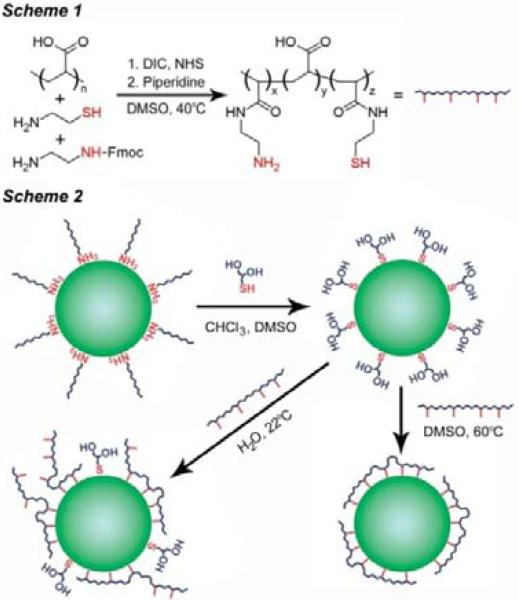
As depicted in Scheme 1, the multidentate polymer was synthesized by covalently modifying about 35% of the carboxylic acids of polyacrylic acid (PAA, MW ∼1800) with cysteamine and N-Fmocethylenediamine using diisopropylcarbodiimide (DIC) and N-hydroxysuccinimide (NHS). After deprotection of the amine with piperidine and purification, each polymer molecule contained approximately 3.5 active thiols and 3.0 active amines, as determined via Ellman's reagent and fluorescamine assays (see Supporting Information). For coating QDs, this balanced composition of amines and thiols was found to provide superior monodispersity, photostability, and fluorescence quantum yield compared to either amines or thiols alone. These multifunctional, multidentate polymers are soluble only in strongly polar solvents such as water, DMSO, and DMF. Because the CdTe QDs were prepared in a high temperature organic solvent using hydrophobic ligands (alkylamines, see Supporting Information), it is necessary to first exchange the native ligands with thioglycerol (Scheme 2). These polar monovalent ligands are then replaced with the multidentate ligand. A surprising finding is that stable, compactly coated QDs are produced only after heating (60−70°C) for 1−2 hours in DMSO under inert conditions. It is energetically favorable for the linear multidentate polymer to wrap around the QD in a closed configuration, but this highly ordered structure is kinetically slow to form at room temperature (see Scheme 2), so elevated temperatures are needed to speed up ligand exchange and loop closure.
Scheme 1.
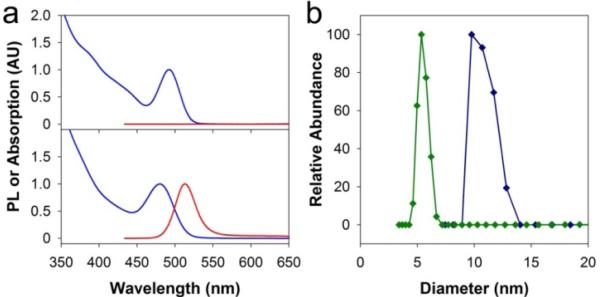
Figure 1 compares the optical properties and hydrodynamic sizes of CdTe QDs (2.5 nm) coated with a traditional amphiphilic polymer (octylamine-modified polyacrylic acid) or the mixed thiol/amine multidentate ligand. Although the amphiphilic polymer and the multidenate ligand are prepared from the same molecular-weight polyacrylic acid backbone, the QDs coated with the multidentate ligand are considerably smaller in size and also much brighter in fluorescence. Dynamic light scattering measurements show that the multidentate polymer coating is only 1.5−2 nm in thickness. This compact shell matches the geometric predictions of a polymer conformation with a high degree of adsorption on the QD surface, enabled by its high affinity and low molecular weight. In comparison, the coating thicknesses are on the order of 4−7 nm for amphiphilic polymers and even some monovalent molecular ligands.3b It is also worth noting that the CdTe QD is not protected with an electronically insulating inorganic shell (e.g., ZnS or CdS) and its fluorescence is retained with the multidentate polymer, but nearly completely quenched by the amphiphilic polymer.
Figure 1.
Comparison of optical and hydrodynamic properties of CdTe QDs (2.5 nm) solubilized in water with an amphiphilic polymer (octylamine-modified polyacrylic acid) or a multidentate polymer ligand. (a) Absorption (blue curves) and fluorescence emission (red curves) spectra of CdTe QDs with amphiphilic polymer (upper) or multidentate polymer (lower) coatings. (b) Dynamic light scattering size data of QDs with amphiphilic polymer (blue curve) and multidentate polymer (green curve) coatings. PL = photoluminescence, AU = arbitrary units. All samples were dissolved in phosphate buffered saline.
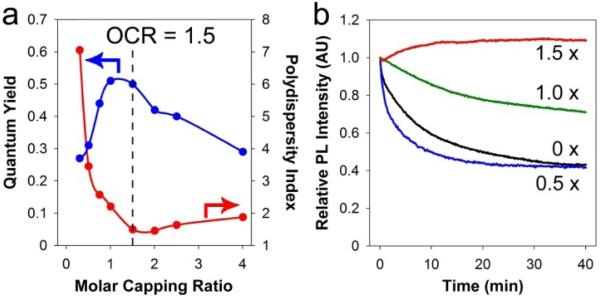
As shown in Figure 2, the fluorescence quantum yield, monodispersity and photostability of these polymer-coated QDs are strongly dependent on the molar capping ratio (MCR), which is calculated by dividing the sum of basic groups (amine and thiol) on the polymer by the sum of cadmium and tellurium atoms on the QD surface (see Supporting Information). When the MCR values are below 1.0, the amount of polymer is not sufficient to completely coat 2.5-nm CdTe QDs, resulting in polydisperse nanocrystals (as measured by the polydispersity index or PDI in gel filtration chromatograms). When the MCR values are above 2.0, the excess polymer leads to better size monodispersity and colloidal stability, but a reduced fluorescence quantum yield. Between these two limits is the optimal capping ratio (OCR) of approximately 1.5 (Figure 2A), yielding small, monodisperse nanocrystals (PDI < 1.5) with bright fluorescence (∼50% quantum yield) and exceptional photostability (Figure 2B). The OCR is QD size-dependent, and its value changes to 1.0 for 3.0 nm cores, and to 0.5 for 4.0 nm cores. The multidentate polymer-coated QDs are stable at room temperature for over 6 months, with no significant changes in gel filtration chromatograms. The quantum yield is also entirely retained under these conditions when stored in the dark. Furthermore, these dots can undergo dialysis for more than one week without deleterious effects, in contrast to QDs coated with monovalent ligands that generally aggregate within 2−3 hours.
Figure 2.
Effects of polymer capping ratios on QD properties. (a) QD (2.5 nm) fluorescence quantum yield (blue curve) and polydispersity index (red curve) as a function of molar capping ratio, and (b) photostability data at various capping ratios (MCR = 1.5, 1.0, or 0.5) and in the absence of polymer (MCR = 0). See text for details.
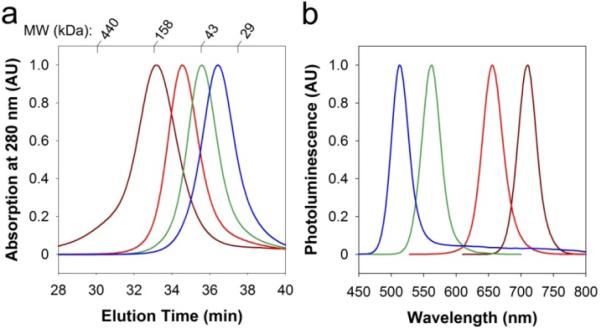
Figure 3 shows a size comparison of multidentate polymer-coated QDs (4 emission colors) with globular protein standards, as measured by gel filtration chromatography. The results demonstrate that the coated green-emitting QDs (515 nm) have a hydrodynamic size slightly larger than fluorescent proteins (MW = 27−30 kDa), while the yellow-emitting QDs (562 nm) dots are slightly smaller than serum albumin (MW = 66 kDa). Even the near-infrared emitting dots (720 nm) are similar to antibodies (MW = 150 kDa) in hydrodynamic size.
Figure 3.
(a) Gel filtration chromatograms of multidentate polymer coated CdTe QDs showing direct size comparison with protein standards (29, 43, 158, 440 kDa). (b) Fluorescence emission spectra from the corresponding QDs. The QD hydrodynamic sizes are 5.6 nm (2.5 nm core, blue), 6.6 nm (3.1 nm core, green), 7.8 nm (4.0 nm core, red), and 9.7 nm (6.0 nm core, brown).
In summary, we have reported a new strategy to minimize the hydrodynamic size of QDs by using multifunctional, multidentate polymer ligands. A novel finding is that a balanced composition of thiol and amine groups yields a highly compact coating for QDs, with a hydrodynamic thickness of only 1.5−2 nm. This has led to a new generation of highly bright and stable QDs with hydrodynamic diameters similar to proteins (5.6 nm to 9.7 nm) with tunable fluorescence emission from the visibleto the near infrared. These size-minimized QDs open new possibilities in multicolor molecular and cellular imaging at the level of single molecules and single nanoparticles.
Supplementary Material
SUPPORTING INFORMATION
Minimizing the Hydrodynamic Size of Quantum Dots with Multifunctional Multidentate Polymer Ligands
Andrew M. Smith and Shuming Nie*
Departments of Biomedical Engineering and Chemistry, Emory University and Georgia Institute of Technology, 101 Woodruff Circle Suite 2001, Atlanta, GA 30322
Materials. Polyacrylic acid (PAA, MW 1773), N-hydroxysuccinimide (NHS), N,N’-diisopropylcarbodiimide (DIC), cysteamine, β-mercaptoethanol (BME), 1-thioglycerol, dimethylsulfoxide (DMSO), dimethylformamide (DMF), cadmium oxide, tellurium, dioctylether (DOE), 5,5′-dithiobis(2-nitrobenzoic acid) (Ellman's reagent), glycine, L-cysteine, acetone, chloroform, methanol, hexane, and piperidine were purchased from Sigma. N-Fmocethylenediamine (Fmoc-EDA) was purchased from ABD Bioquest. Tetradecylphosphonic acid (TDPA) was obtained from Alfa Aesar. Oleylamine was from Acros Organics, trioctylphosphine (TOP) was from Strem, and fluorescamine was purchased from Invitrogen.
Polymer synthesis. PAA (1 g, 13.9 mmol carboxylic acids) was mixed with 25 mL DMSO in a 150 mL three-necked flask. After stirring for 24 hours at 35°C, freshly prepared anhydrous solutions of cysteamine (187 mg, 2.43 mmol) and Fmoc-EDA (686 mg, 2.43 mmol), each dissolved in 6 mL DMSO, were added. The solution was protected from light and bubbled with argon for 30 minutes at 35°C. After the addition of an anhydrous solution of NHS (1.12 mg, 9.71 mmol) in 6 mL DMSO, DIC (736 mg, 5.83 mmol) was slowly added over the course of 40 minutes during vigorous stirring. Bubbling was continued for 30 minutes, and then the reaction was allowed to proceed for 7 days at 40°C in the dark. Piperidine (18 mL) was then added, and the solution was stirred for four hours to deprotect the primary amines. BME (501 mg, 6.41 mmol) was added to quench the reaction, and the solution was stirred for 2 hours at 40°C, then cooled to room temperature and filtered. The mixture was condensed to ∼4 mL at 45°C under vacuum (∼40 Pa), and the polymer was precipitated with the addition of a 2:1 mixture of ice-cold acetone:chloroform, and isolated via centrifugation. The polymer was dissolved in ∼5 mL anhydrous DMF, filtered, and precipitated again with acetone-chloroform. This process was repeated three times, and the polymer was finally washed with acetone, dried under vacuum, and stored under argon. This modified polymer was a white powder, soluble in water, DMSO, DMF, or methanol, but insoluble in acetone, unlike PAA. If stored under air, this polymer darkened and became yellow-brown over the course of a few weeks, and also became increasingly difficult to dissolve in various solvents. This aging process coincided with a significant decrease in the number of active thiols per polymer, determined as described below. Therefore we concluded that this phenomenon is likely due to the formation of interpolymer disulfide crosslinks.
Determination of reactive amines and thiols. The modified polymer was assayed for reactive amines and thiols using fluorescamine and Ellman's reagent, respectively. For amine determination, a 10 mg/mL solution of fluorescamine in DMSO was freshly prepared, and glycine standards (100 nM – 1 mM) were prepared in deionized water. The assay was initiated by mixing 411.3 μL water, 50 μL sample or standard, 25 μL of 1 M sodium borate buffer (pH 8.5), and 13.7 μL fluorescamine solution. After 20 minutes of reaction in the dark, the fluorescence intensity at 470 nm, with 380 nm excitation, was measured. The polymer was assayed immediately after dissolution at 10 μg/mL in 20 mM sodium hydroxide. For thiol determination, a 2 mM stock solution of Ellman's reagent in 50 mM sodium acetate buffer (pH 4.7), and L-cysteine standards (10 μM – 100 mM) in deionized water were freshly prepared at 4°C. The assay was initiated by mixing 850 μL water, 10 μL sample or standard, 100 μL of 1 M Tris buffer (pH 8.5), and 50 μL Ellman's reagent solution. After 10 minutes of reaction, the optical density at 412 nm was measured. The polymer was assayed immediately after dissolution in 20 mM sodium hydroxide at 500 μg/mL. Standard curves allowed the determination of the molar amount of thiol or amine per gram of polymer. These values were converted to moles of functional group per polymer chain using the molecular weight of the modified polymer (∼2200 Da), determined via gel filtration chromatography, which correlated strongly with theoretical calculations.
Synthesis of CdTe nanocrystals. CdO (25.7 mg, 0.2 mmol), TDPA (122 mg, 0.44 mmol), and DOE (2 mL) were added to a three-necked flask and heated to 250°C under argon until complete dissolution of CdO. After cooling to room temperature, oleylamine (1 g, 3.74 mmol) and 6.5 mL DOE were added. The solution was heated to reflux under vacuum (∼20 Pa, ∼65°C) for 1 hour and then heated to 300°C under argon flow. A second solution, containing tellurium (12.76 mg, 0.1 mmol), TOP (2 mL), and DOE (3 mL), was injected into the cadmium precursor solution, and the growth temperature was set to 265°C. Using this method, highly monodipserse nanocrystals could be grown between 2.5 and 3.5 nm diameter after reaction times between 20 seconds and 10 minutes. To grow larger nanocrystals, additional cadmium and tellurium precursors were sequentially injected dropwise into the reaction solution, starting at 4 minutes after the first injection. After reaching the desired size, the reaction mixture was cooled to room temperature, diluted with 85 mL hexane, and centrifuged to remove most of the excess cadmium precursor. The QDs were isolated using at least six hexane-methanol extractions. On the final extraction, the QDs were condensed to ∼ 1 mL through the addition of methanol. These QDs were then diluted to ∼20 mL with chloroform, bubbled with argon for 30 minutes and stored at 4°C in the dark. QD size was determined from its known correlation with the first exciton peak wavelength,1 and verified via TEM.
Ligand exchange with 1-thioglycerol. Purified CdTe QDs (2.5 nm) in chloroform (7 mL, ∼150 uM) were added to a three-necked Ligand exchange with 1-thioglycerol flask connected to a Schlenk line. Under intense stirring, neat 1-thioglycerol was added dropwise until the first visible sign of flocculation. Then 4 mL of DMSO was added dropwise. An excess of 1-thioglycerol (3 mL) was then added, and chloroform was removed under vacuum at 25°C. After stirring for an additional 2 hours at 25°C under argon, the QDs were precipitated with the addition of an ice-cold mixture of acetone:chloroform (1:1, 193 mL total). Following centrifugation, the pellet was washed with acetone and dried under vacuum.
Coating quantum dots with the multidentate polymer ligand. Two techniques were used to coat 1-thioglycerol QDs with the modified polymer, as depicted in Scheme 2. In the first method, CdTe QDs coated with 1-thioglycerol were suspended in basic water (50 mM sodium hydroxide), centrifuged at 7000g for 10 minutes, and then filtered to remove aggregated nanocrystals. Various amounts of polymer dissolved in basic water were added to the QDs, which were then gently mixed (Figure S1). Using this method, addition of an excess of ligand resulted in complete precipitation of the QD over the course of several hours, likely due to crosslinking of the QDs mediated by the multidentate ligand. In the second method, QDs coated with 1-thioglycerol were suspended in DMSO and centrifuged at 7000g for 10 minutes to remove possible nanocrystal aggregates. The nanocrystals were diluted to ∼5−20 μM for smaller sizes (2.5−3.5 nm), or ∼2−5 μM for larger nanocrystals. The QDs were then degassed extensively at room temperature and charged with argon. An anhydrous DMSO solution of the polymer (∼5 mg/mL) was added under vigorous stirring. The solution was then heated to 60°C for 90 minutes for smaller QDs (2.5−3.5 nm), or 70−75°C for 120 minutes for larger nanocrystals. In the absence of the polymer, the nanocrystals aggregated and precipitated from solution during heating. Indeed, the multidentate polymer greatly enhances the thermal stability of these nanocrystals, as there was no evidence of Ostwald ripening of 2.5 nm cores up to ∼130°C. After cooling the QDs to room temperature, ice-cold aqueous sodium hydroxide (50 mM, twice the volume of DMSO) was slowly added, and the solution was stirred for 2 hours. The QDs were then extensively dialyzed against basic water for 2−3 days using 25 kDa molecular weight cutoff dialysis tubing (Spectra/Por). Figure S2 depicts the optical properties of these QDs and their hydrodynamic sizes at the different stages of this coating procedure. Figure S3 shows a representative TEM of 2.5 nm QDs coated with the multidentate polymer ligand. The gel filtration chromatograms in Figure S4 demonstrate the dramatic difference in QD monodispersity resulting from the two different coating techniques described above.
Supporting Figure S1. Photographs of 4 nm thioglycerol-stabilized CdTe nanocrystals in water, 6 months after addition of the multidentate polymer ligand at various molar capping ratios as indicated on the vials. From left to right, the molar capping ratio is 0, 0.1, 0.5, 0.75, 1, 1.5, and 2.
Supporting Figure S2. Absorption (a), photoluminescence (b), and dynamic light scattering spectra (c) of 2.5 nm CdTe QDs in chloroform, DMSO after ligand exchange with 1-thioglycerol, and in water after coating with the multidentate polymer. Note that a small amount of deep trap emission arises upon coating with 1-thioglycerol, and remains after exchange with the polymer. Photoluminescence spectra were obtained with 420 nm excitation.
Supporting Figure S3. TEM of 2.5 nm CdTe QDs coated with the multidentate polymer in water.
Supporting Figure S4. Comparison between gel filtration chromatograms of 6.0 nm CdTe QDs coated with the multidentate polymer with MCR = 0.75, using the two procedures indicated in Scheme 2. The QDs coated in DMSO were purified extensively prior to testing. The QDs coated in water were tested 3 weeks after mixing, and were not purified in order to prevent destabilization of labile aggregates.
Calculation of molar capping ratio. The amount of polymer added per QD was standardized with reference to the number of QD surface atoms. This relationship was used in order to shed light on the mechanism of interaction between the QDs and the polymer, and to simplify the extrapolation of the polymer coating procedure to other nanocrystalline materials, without the need for extensive optimization. The molar capping ratio (MCR) was reported as the number of thiol and amine groups per surface atom. Therefore
where nSH and nNH2 are the numbers of thiols and amines on the polymer ligand, respectively, and nCd and nTe are the number of cadmium and tellurium surface atoms on the QDs. For example, a 2.5 nm CdTe QD has ∼95 total surface atoms (the calculation of the number of surface atoms per QD is described below), and one polymer chain contains roughly 6.5 basic groups (3.5 thiols and 3.0 amines). Therefore, the optimal capping ratio (OCR) value of 1.5x denotes the addition of ∼22 polymer chains per QD, or roughly 48 mg of polymer per μmol of QDs. Indeed, this is a very small amount of polymer for such a large number of QDs. With elevated temperature this reaction is highly efficient, as nearly all of the polymer binds to the QDs (no detectable free amines were found in the dialysate during purification). Figure S5 depicts several gel filtration chromatograms of 2.5 nm QDs coated with the multidentate ligand at different MCR values. These chromoatograms were used to extract the polydispersity index values plotted in Figure 2. Similar trends in QD size distributions were also observed for larger QDs coated with the multidentate ligands. However, as stated in the main body of this manuscript, the OCR was found to decrease with increasing size of the nanocrystal core. This trend is indicative of the size-dependent differences in nanocrystal surface curvature, the intrinsic degree of flexibility of the polymer, and the increasing availability of more than one free orbital per surface atom with decreasing nanocrystal size.
Supporting Figure S5. Gel filtration chromatograms of 2.5 nm CdTe QDs coated with different amounts of the multidentate polymer, in phosphate buffered saline. The coating procedure was performed at 60°C in DMSO. The amount of polymer added is indicated by the MCR values on the right. Note the presence of higher molecular weight aggregates (shorter retention times) for MCR values below the optimal capping ratio (1.5x). The 0x nanocrystals were not heated, but instead were dialyzed against borate buffer for 30 minutes prior to injection into the column. All other nanocrystal samples were dialyzed for 2 hours.
Coating quantum dots with the amphiphilic polymer. Purified CdTe QDs (2.5 nm, 10 nmol) in chloroform were mixed with a chloroform solution of 40% octylamine-modified polyacrylic acid (10 μmol polymer chains). The QDs were slowly dried under vacuum to a film that was readily soluble in phosphate buffered saline. The resulting QDs were purified via ultracentrifugation. During this process, the QD absorption spectra were unchanged, but the fluorescence was entirely quenched (Figure 1a). Small QDs (<4 nm) coated with these amphiphilic polymers were substantially more polydisperse than nanoparticles containing larger QD cores. Figure S6 depicts gel filtration chromatograms of 2.5 nm CdTe QDs coated with this amphiphilic polymer, as well as QDs coated with 1-thioglycerol.
Supporting Figure S6. Gel filtration chromatogram of 2.5 nm CdTe QDs coated in 40%-octylamine-modified PAA (blue) or 1-thioglycerol (red). A large excess of thioglycerol, which elutes at ∼45 minutes, was found to be necessary to stabilize these QDs during chromatography.
Calculation of number of surface atoms per nanocrystal. Determination of surface atom density on nanocrystals can be difficult, and imprecise, especially for very small particles that cannot be easily characterized microscopically. Nevertheless, reasonable accuracy can be obtained by using theoretical calculations informed by empirical data. In this work, the CdTe nanocrystals that we prepared (2.5−6 nm diameter) were found to be in the zinc blende crystal structure (x-ray diffraction and high-resolution TEM data will be reported elsewhere), which is consistent with similar CdTe synthesis reports using phosphonic acid ligands.2 This data allows the use of the bulk density and interplanar distances of zinc blende CdTe in our calculations. From TEM, the nanocrystals were found to be relatively spherical, with slight elongation along the [111] direction in larger nanocrystals (aspect ratio 1.4 or less). Because of the near-spherical nature of growth, it is likely that a variety of crystalline facets are exposed on individual nanocrystals, each with a range of planar densities of atoms. It is also likely that there is a distribution of different facets exposed across an assembly of nanocrystals. Therefore one may obtain an effective average number of surface atoms per nanocrystal by averaging the surface densities of commonly exposed facets in zinc blende nanocrystals over the calculated surface area of the nanocrystal. In this work we chose to use the commonly observed (111), (100), and (110) zinc blende planes, which are representative of the lattice structure, with both polar and nonpolar surfaces. For this calculation, we defined a surface atom as an atom (either Cd2+ or Te2−) located on a nanocrystal facet with one or more unpassivated orbitals. Some facets, such as Cd2+-terminated {111} faces, have closely underlying Te2− atoms that are less than 1 Å beneath the surface plane. These atoms reside in the voids between Cd2+ atoms, and thus are likely to be sterically accessible from the surface, but because they are completely passivated, they were not included in our definition.
First we calculated the average distance between parallel planes of atoms for zinc blende CdTe (Table S1). This average interplanar distance, d, is therefore the distance between the plane of surface atoms and the next underlying plane of atoms. In the [100] and [110] directions, all adjacent planes are equidistant, whereas this distance varies between neighboring planes in the [111] direction, and thus we calculated an “average” interplanar distance. We also calculated the planar density of atoms on each facet, although this data was not directly used in our calculation (Table S1).
Supporting Table S1. Characteristics of three lattice planes of the zinc blende CdTe crystal structure. The interplanar distance, d, used in the surface atom calculation, is tabulated, along with several other parameters of interest.
Next we calculated the effective volume of surface atoms within each QD. We assumed a spherical geometry, and used the interplanar distance d as the thickness of one monolayer of surface atoms in each nanocrystal. In this calculation, the surface volume was used, rather than the surface area, in order to yield a more realistic determination of surface atoms in very small nanocrystals (< 2 nm). For these high surface area nanocrystals, use of the surface area generally resulted in a surface atom number that was larger than the total number of atoms in each nanocrystal. Therefore
where VSA is the volume of surface atoms per QD, r is the QD radius, and d is the average interplanar distance from Table S1.
We assumed that this spherical shell of surface atoms was the same density as bulk zinc blende CdTe, and therefore the number of surface atoms per nanocrystal could be calculate as
where nSA is the number of surface atoms per QD, D is the bulk density of zinc blende CdTe (5.85 g cm-3), NA is Avogadro's number, MW is the molecular weight of CdTe, and 2 is a factor accounting for 2 atoms per molecule of CdTe.
Table S2 displays the calculated number of surface atoms on a single, spherical CdTe QD that is hypothetically terminated solely by {111}, {100}, or {110} planes for various nanocrystal sizes. These values may be averaged, depending on available empirical information. For instance, HRTEM analysis of some of our larger CdTe nanocrystal samples revealed that the {110} planes were frequently parallel to the TEM grid. Therefore it is likely that these QDs were faceted along this plane, allowing them to adopt this orientation on their substrate during solvent evaporation.3 In this case, more weight can be given to these facets in the averaging calculation. For our calculations, we could obtain very little structural information about these very small nanocrystals, and therefore we simply averaged these three representative values together.
Supporting Table S2. Total number of atoms and total number surface atoms in various sizes of CdTe nanocrystals. The surface atom count is listed for each surface facet of interest, as well as its numerical average. This later value was used to calculate the molar capping ratio, described above.
Several methods have been reported in the literature to determine the number of surface atoms on nanocrystals without the use of complex energy-minimization computations.4 We compared several different calculation methods based on a quasi-spherical particles, as well as methods we developed to predict the number of surface atoms on different polyhedral shapes with various lattice facets. For all of these methods, we obtained strongly correlated results. A substantial difference (greater than 15%) was only observed in comparison with empirically unrealistic shapes with 8 sides or less (cubes or tetrahedrons). The specific method used for this work was chosen for its simplicity and its ease of quickly incorporating empirical knowledge of known facets.
We note two factors that could yield errors in this calculation and complicate the interpretation of the MCR value, with respect to the interaction between the nanocrystal surface atoms and the multidentate ligand. (1) Nanocrystals of various sizes have been theoretically and experimentally shown to have reconstructed surface atoms that may minimize the total energy of the crystal.5 Because of the very high fraction of atoms that reside on the surfaces of the small nanocrystals used in this study (∼39% for 2.5 nm QDs), surface reconstruction is likely. (2) Many lattice directions, such as the (100) direction, are terminated by atoms with two unpassivated orbitals, which could theoretically bind to two ligands. Atoms with more than one exposed binding site are even more likely to be present on the smallest QDs, which have such highly curved surfaces that a surface ‘facet’ may not even be an appropriate term for their description.
Characterization and instrumentation. Dynamic light scattering measurements were performed on a Brookhaven Instruments 90Plus Particle Size Analyzer. Before analysis, nanoparticle samples (1−100 μM, depending on the core size) were first centrifuged at 7000g for 10 minutes and then filtered through a 0.2 μm filter. Ultracentrifugal isolation of aqueous solutions of nanocrystals was performed on a Beckman Coulter Optima TLX Ultracentrifuge, typically at 100,000 rpm for 1 hour. Absorption spectra were obtained with a Shimadzu spectrophotometer with 1 nm slit widths. Photoluminescence spectra were obtained using a spectrofluorometer from Photon Technology International. The excitation source was a xenon lamp, the detector was a photomultiplier tube, and the spectrometer slit widths were typically 2 nm. Photostability measurements were performed by continuously recording emission spectra (400−620 nm) from 200 nM solutions of QDs with continuous high intensity 390 nm excitation (16 nm excitation spectrometer slit widths). Quantum yield was measured by comparison to the organic dye Atto 520 (dissolved in ethanol) using previously published methods.6 Transmission electron microscopy was performed by Dr. Hong Yi at the Electron Microscopy Core Facility at Emory University. Inductively-coupled plasma-mass spectrometry (ICP-MS) was performed with a PlasmaQuad 3 at the Center for Applied Isotope Studies at the University of Georgia. Gel filtration chromatography was performed on a Superose 6 10/300 GL column, with 280 nm absorption monitored on an AKTAprime plus system (GE Healthcare). The flow rate was 0.5 mL/min and the following protein standards were used for molecular weight determination: Ferritin (440 kDa), Aldolase (158 kDa), Ovalbumin (43 kDa), and carbonic anhydrase (29 kDa). Polydispersity index (PDI) was calculated from chromatograms using conventional techniques for polymer characterization, with the formula PDI = Mw / Mn. The PDI for pure protein solutions was typically 1.25−1.35.
References
(1) Yu, W. W.; Qu, L. H.; Guo, W. H.; Peng, X. G. Chem. Mater. 2003, 15, 2854−2860.
(2) Yu, W. W.; Wang, Y. A.; Peng, X. G. Chem. Mater. 2003, 15, 4300−4308.
(3) Li, R. F.; Lee, J.; Yang, B. C.; Horspool, D. N.; Aindow, M.; Papadimitrakopoulos, F. J. Am. Chem. Soc. 2005, 127, 2524−2532.
(4) Kirchner, C.; Liedl, T.; Kudera, S.; Pellegrino, T.; Javier, A. M.; Gaub, H. E.; Stolzle, S.; Fertig, N.; Parak, W. J. Nano Lett. 2005, 5, 331−338; Katari, J. E. B.; Colvin, V. L.; Alivisatos, A. P. J. Phys. Chem. 1994, 98, 4109−4117.
(5) Aruguete, D. M.; Marcus, M. A.; Li, L. S.; Williamson, A.; Fakra, S.; Gygi, F.; Galli, G. A.; Alivisatos, A. P. J. Phys. Chem. C 2007, 111, 75−79; Puzder, A.; Williamson, A. J.; Zaitseva, N.; Galli, G.; Manna, L.; Alivisatos, A. P. Nano Lett. 2004, 4, 2361−2365; Rempel, J. Y.; Trout, B. L.; Bawendi, M. G.; Jensen, K. F. J. Phys. Chem. B 2005, 109, 19320−19328; Ding, Y.; Wang, Z. L. Surf. Sci. 2007, 601, 425−433.
(6) Himel, C. M.; Mayer, R. T. Anal. Chem. 1970, 42, 130−132.
Acknowledgement
This work was supported by grants from the National Institutes of Health (P20 GM072069, R01 CA108468, and U01HL080711, U54CA119338). A.M.S. acknowledges the Whitaker Foundation for generous fellowship support.
Footnotes
Supporting Information Available: Detailed methods and calculations, as well as spectroscopic and chromatographic data with various coatings. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a Gao XH, Yang L, Petros JA, Marshall FF, Simons JW, Nie SM. Curr. Opin. Biotechnol. 2005;16:1–10. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]; b Alivisatos AP, Gu WW, Larabell C. Annu. Rev. Biomed. Eng. 2005;7:55–76. doi: 10.1146/annurev.bioeng.7.060804.100432. [DOI] [PubMed] [Google Scholar]
- 2.a Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Nature Mater. 2005;4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]; b Smith AM, Duan HW, Mohs AM, Nie SM. Adv. Drug Delivery Rev. 2008;60:1226–1240. doi: 10.1016/j.addr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a Smith AM, Duan HW, Rhyner MN, Ruan G, Nie SM. Phys. Chem. Chem. Phys. 2006;8:3895–3903. doi: 10.1039/b606572b. [DOI] [PubMed] [Google Scholar]; b Pons T, Uyeda HT, Medintz IL, Mattoussi H. J. Phys. Chem. B. 2006;110:20308–20316. doi: 10.1021/jp065041h. [DOI] [PubMed] [Google Scholar]; c Pellegrino T, Manna L, Kudera S, Liedl T, Koktysh D, Rogach AL, Keller S, Radler J, Natile G, Parak WJ. Nano Lett. 2004;4:703–303. [Google Scholar]
- 4.a Rogach AL, Franzl T, Klar TA, Feldmann J, Gaponik N, Lesnyak V, Shavel A, Eychmuller A, Rakovich YP, Donegan JF. J. Phys. Chem. C. 2007;111:14628–14637. [Google Scholar]; b Lovric J, Bazzi HS, Cuie Y, Fortin GRA, Winnik FM, Maysinger D. J. Mol. Med. 2005;83:377–385. doi: 10.1007/s00109-004-0629-x. [DOI] [PubMed] [Google Scholar]; c Nabiev I, et al. Nano Lett. 2007;7:3452–3461. doi: 10.1021/nl0719832. [DOI] [PubMed] [Google Scholar]
- 5.a Susumu K, Uyeda HT, Medintz IL, Pons T, Delehanty JB, Mattoussi H. J. Am. Chem. Soc. 2007;129:13987–13996. doi: 10.1021/ja0749744. [DOI] [PubMed] [Google Scholar]; b Liu W, Howarth M, Greytak AB, Zheng Y, Nocera DG, Ting AY, Bawendi MG. J. Am. Chem. Soc. 2008;130:1274–1284. doi: 10.1021/ja076069p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubois F, Mahler B, Dubertret B, Doris E, Mioskowski C. J. Am. Chem. Soc. 2007;129:482–483. doi: 10.1021/ja067742y. [DOI] [PubMed] [Google Scholar]
- 7.a Guo W, Li JJ, Wang YA, Peng XG. J. Am. Chem. Soc. 2003;125:3901–3909. doi: 10.1021/ja028469c. [DOI] [PubMed] [Google Scholar]; b Kim S, Bawendi MG. J. Am. Chem. Soc. 2003;125:14652–14653. doi: 10.1021/ja0368094. [DOI] [PubMed] [Google Scholar]; c Kim SW, Kim S, Tracy JB, Jasanoff A, Bawendi MG. J. Am. Chem. Soc. 2005;127:4556–4557. doi: 10.1021/ja043577f. [DOI] [PubMed] [Google Scholar]
- 8.a Chakraborty AK, Golumbfskie A. J. Annu. Rev. Phys. Chem. 2001;52:537–573. doi: 10.1146/annurev.physchem.52.1.537. [DOI] [PubMed] [Google Scholar]; b Wang MF, Felorzabihi N, Guerin G, Haley JC, Scholes GD, Winnik MA. Macromolecules. 2007;40:6377–6384. [Google Scholar]; c Wang XS, Dykstra TE, Salvador MR, Manners I, Scholes GD, Winnik MA. J. Am. Chem. Soc. 2004;126:7784–7785. doi: 10.1021/ja0489339. [DOI] [PubMed] [Google Scholar]
- 9.Pinaud F, King D, Moore H-P, Weiss S. J. Am. Chem. Soc. 2004;126:6115–6123. doi: 10.1021/ja031691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
Minimizing the Hydrodynamic Size of Quantum Dots with Multifunctional Multidentate Polymer Ligands
Andrew M. Smith and Shuming Nie*
Departments of Biomedical Engineering and Chemistry, Emory University and Georgia Institute of Technology, 101 Woodruff Circle Suite 2001, Atlanta, GA 30322
Materials. Polyacrylic acid (PAA, MW 1773), N-hydroxysuccinimide (NHS), N,N’-diisopropylcarbodiimide (DIC), cysteamine, β-mercaptoethanol (BME), 1-thioglycerol, dimethylsulfoxide (DMSO), dimethylformamide (DMF), cadmium oxide, tellurium, dioctylether (DOE), 5,5′-dithiobis(2-nitrobenzoic acid) (Ellman's reagent), glycine, L-cysteine, acetone, chloroform, methanol, hexane, and piperidine were purchased from Sigma. N-Fmocethylenediamine (Fmoc-EDA) was purchased from ABD Bioquest. Tetradecylphosphonic acid (TDPA) was obtained from Alfa Aesar. Oleylamine was from Acros Organics, trioctylphosphine (TOP) was from Strem, and fluorescamine was purchased from Invitrogen.
Polymer synthesis. PAA (1 g, 13.9 mmol carboxylic acids) was mixed with 25 mL DMSO in a 150 mL three-necked flask. After stirring for 24 hours at 35°C, freshly prepared anhydrous solutions of cysteamine (187 mg, 2.43 mmol) and Fmoc-EDA (686 mg, 2.43 mmol), each dissolved in 6 mL DMSO, were added. The solution was protected from light and bubbled with argon for 30 minutes at 35°C. After the addition of an anhydrous solution of NHS (1.12 mg, 9.71 mmol) in 6 mL DMSO, DIC (736 mg, 5.83 mmol) was slowly added over the course of 40 minutes during vigorous stirring. Bubbling was continued for 30 minutes, and then the reaction was allowed to proceed for 7 days at 40°C in the dark. Piperidine (18 mL) was then added, and the solution was stirred for four hours to deprotect the primary amines. BME (501 mg, 6.41 mmol) was added to quench the reaction, and the solution was stirred for 2 hours at 40°C, then cooled to room temperature and filtered. The mixture was condensed to ∼4 mL at 45°C under vacuum (∼40 Pa), and the polymer was precipitated with the addition of a 2:1 mixture of ice-cold acetone:chloroform, and isolated via centrifugation. The polymer was dissolved in ∼5 mL anhydrous DMF, filtered, and precipitated again with acetone-chloroform. This process was repeated three times, and the polymer was finally washed with acetone, dried under vacuum, and stored under argon. This modified polymer was a white powder, soluble in water, DMSO, DMF, or methanol, but insoluble in acetone, unlike PAA. If stored under air, this polymer darkened and became yellow-brown over the course of a few weeks, and also became increasingly difficult to dissolve in various solvents. This aging process coincided with a significant decrease in the number of active thiols per polymer, determined as described below. Therefore we concluded that this phenomenon is likely due to the formation of interpolymer disulfide crosslinks.
Determination of reactive amines and thiols. The modified polymer was assayed for reactive amines and thiols using fluorescamine and Ellman's reagent, respectively. For amine determination, a 10 mg/mL solution of fluorescamine in DMSO was freshly prepared, and glycine standards (100 nM – 1 mM) were prepared in deionized water. The assay was initiated by mixing 411.3 μL water, 50 μL sample or standard, 25 μL of 1 M sodium borate buffer (pH 8.5), and 13.7 μL fluorescamine solution. After 20 minutes of reaction in the dark, the fluorescence intensity at 470 nm, with 380 nm excitation, was measured. The polymer was assayed immediately after dissolution at 10 μg/mL in 20 mM sodium hydroxide. For thiol determination, a 2 mM stock solution of Ellman's reagent in 50 mM sodium acetate buffer (pH 4.7), and L-cysteine standards (10 μM – 100 mM) in deionized water were freshly prepared at 4°C. The assay was initiated by mixing 850 μL water, 10 μL sample or standard, 100 μL of 1 M Tris buffer (pH 8.5), and 50 μL Ellman's reagent solution. After 10 minutes of reaction, the optical density at 412 nm was measured. The polymer was assayed immediately after dissolution in 20 mM sodium hydroxide at 500 μg/mL. Standard curves allowed the determination of the molar amount of thiol or amine per gram of polymer. These values were converted to moles of functional group per polymer chain using the molecular weight of the modified polymer (∼2200 Da), determined via gel filtration chromatography, which correlated strongly with theoretical calculations.
Synthesis of CdTe nanocrystals. CdO (25.7 mg, 0.2 mmol), TDPA (122 mg, 0.44 mmol), and DOE (2 mL) were added to a three-necked flask and heated to 250°C under argon until complete dissolution of CdO. After cooling to room temperature, oleylamine (1 g, 3.74 mmol) and 6.5 mL DOE were added. The solution was heated to reflux under vacuum (∼20 Pa, ∼65°C) for 1 hour and then heated to 300°C under argon flow. A second solution, containing tellurium (12.76 mg, 0.1 mmol), TOP (2 mL), and DOE (3 mL), was injected into the cadmium precursor solution, and the growth temperature was set to 265°C. Using this method, highly monodipserse nanocrystals could be grown between 2.5 and 3.5 nm diameter after reaction times between 20 seconds and 10 minutes. To grow larger nanocrystals, additional cadmium and tellurium precursors were sequentially injected dropwise into the reaction solution, starting at 4 minutes after the first injection. After reaching the desired size, the reaction mixture was cooled to room temperature, diluted with 85 mL hexane, and centrifuged to remove most of the excess cadmium precursor. The QDs were isolated using at least six hexane-methanol extractions. On the final extraction, the QDs were condensed to ∼ 1 mL through the addition of methanol. These QDs were then diluted to ∼20 mL with chloroform, bubbled with argon for 30 minutes and stored at 4°C in the dark. QD size was determined from its known correlation with the first exciton peak wavelength,1 and verified via TEM.
Ligand exchange with 1-thioglycerol. Purified CdTe QDs (2.5 nm) in chloroform (7 mL, ∼150 uM) were added to a three-necked Ligand exchange with 1-thioglycerol flask connected to a Schlenk line. Under intense stirring, neat 1-thioglycerol was added dropwise until the first visible sign of flocculation. Then 4 mL of DMSO was added dropwise. An excess of 1-thioglycerol (3 mL) was then added, and chloroform was removed under vacuum at 25°C. After stirring for an additional 2 hours at 25°C under argon, the QDs were precipitated with the addition of an ice-cold mixture of acetone:chloroform (1:1, 193 mL total). Following centrifugation, the pellet was washed with acetone and dried under vacuum.
Coating quantum dots with the multidentate polymer ligand. Two techniques were used to coat 1-thioglycerol QDs with the modified polymer, as depicted in Scheme 2. In the first method, CdTe QDs coated with 1-thioglycerol were suspended in basic water (50 mM sodium hydroxide), centrifuged at 7000g for 10 minutes, and then filtered to remove aggregated nanocrystals. Various amounts of polymer dissolved in basic water were added to the QDs, which were then gently mixed (Figure S1). Using this method, addition of an excess of ligand resulted in complete precipitation of the QD over the course of several hours, likely due to crosslinking of the QDs mediated by the multidentate ligand. In the second method, QDs coated with 1-thioglycerol were suspended in DMSO and centrifuged at 7000g for 10 minutes to remove possible nanocrystal aggregates. The nanocrystals were diluted to ∼5−20 μM for smaller sizes (2.5−3.5 nm), or ∼2−5 μM for larger nanocrystals. The QDs were then degassed extensively at room temperature and charged with argon. An anhydrous DMSO solution of the polymer (∼5 mg/mL) was added under vigorous stirring. The solution was then heated to 60°C for 90 minutes for smaller QDs (2.5−3.5 nm), or 70−75°C for 120 minutes for larger nanocrystals. In the absence of the polymer, the nanocrystals aggregated and precipitated from solution during heating. Indeed, the multidentate polymer greatly enhances the thermal stability of these nanocrystals, as there was no evidence of Ostwald ripening of 2.5 nm cores up to ∼130°C. After cooling the QDs to room temperature, ice-cold aqueous sodium hydroxide (50 mM, twice the volume of DMSO) was slowly added, and the solution was stirred for 2 hours. The QDs were then extensively dialyzed against basic water for 2−3 days using 25 kDa molecular weight cutoff dialysis tubing (Spectra/Por). Figure S2 depicts the optical properties of these QDs and their hydrodynamic sizes at the different stages of this coating procedure. Figure S3 shows a representative TEM of 2.5 nm QDs coated with the multidentate polymer ligand. The gel filtration chromatograms in Figure S4 demonstrate the dramatic difference in QD monodispersity resulting from the two different coating techniques described above.
Supporting Figure S1. Photographs of 4 nm thioglycerol-stabilized CdTe nanocrystals in water, 6 months after addition of the multidentate polymer ligand at various molar capping ratios as indicated on the vials. From left to right, the molar capping ratio is 0, 0.1, 0.5, 0.75, 1, 1.5, and 2.
Supporting Figure S2. Absorption (a), photoluminescence (b), and dynamic light scattering spectra (c) of 2.5 nm CdTe QDs in chloroform, DMSO after ligand exchange with 1-thioglycerol, and in water after coating with the multidentate polymer. Note that a small amount of deep trap emission arises upon coating with 1-thioglycerol, and remains after exchange with the polymer. Photoluminescence spectra were obtained with 420 nm excitation.
Supporting Figure S3. TEM of 2.5 nm CdTe QDs coated with the multidentate polymer in water.
Supporting Figure S4. Comparison between gel filtration chromatograms of 6.0 nm CdTe QDs coated with the multidentate polymer with MCR = 0.75, using the two procedures indicated in Scheme 2. The QDs coated in DMSO were purified extensively prior to testing. The QDs coated in water were tested 3 weeks after mixing, and were not purified in order to prevent destabilization of labile aggregates.
Calculation of molar capping ratio. The amount of polymer added per QD was standardized with reference to the number of QD surface atoms. This relationship was used in order to shed light on the mechanism of interaction between the QDs and the polymer, and to simplify the extrapolation of the polymer coating procedure to other nanocrystalline materials, without the need for extensive optimization. The molar capping ratio (MCR) was reported as the number of thiol and amine groups per surface atom. Therefore
where nSH and nNH2 are the numbers of thiols and amines on the polymer ligand, respectively, and nCd and nTe are the number of cadmium and tellurium surface atoms on the QDs. For example, a 2.5 nm CdTe QD has ∼95 total surface atoms (the calculation of the number of surface atoms per QD is described below), and one polymer chain contains roughly 6.5 basic groups (3.5 thiols and 3.0 amines). Therefore, the optimal capping ratio (OCR) value of 1.5x denotes the addition of ∼22 polymer chains per QD, or roughly 48 mg of polymer per μmol of QDs. Indeed, this is a very small amount of polymer for such a large number of QDs. With elevated temperature this reaction is highly efficient, as nearly all of the polymer binds to the QDs (no detectable free amines were found in the dialysate during purification). Figure S5 depicts several gel filtration chromatograms of 2.5 nm QDs coated with the multidentate ligand at different MCR values. These chromoatograms were used to extract the polydispersity index values plotted in Figure 2. Similar trends in QD size distributions were also observed for larger QDs coated with the multidentate ligands. However, as stated in the main body of this manuscript, the OCR was found to decrease with increasing size of the nanocrystal core. This trend is indicative of the size-dependent differences in nanocrystal surface curvature, the intrinsic degree of flexibility of the polymer, and the increasing availability of more than one free orbital per surface atom with decreasing nanocrystal size.
Supporting Figure S5. Gel filtration chromatograms of 2.5 nm CdTe QDs coated with different amounts of the multidentate polymer, in phosphate buffered saline. The coating procedure was performed at 60°C in DMSO. The amount of polymer added is indicated by the MCR values on the right. Note the presence of higher molecular weight aggregates (shorter retention times) for MCR values below the optimal capping ratio (1.5x). The 0x nanocrystals were not heated, but instead were dialyzed against borate buffer for 30 minutes prior to injection into the column. All other nanocrystal samples were dialyzed for 2 hours.
Coating quantum dots with the amphiphilic polymer. Purified CdTe QDs (2.5 nm, 10 nmol) in chloroform were mixed with a chloroform solution of 40% octylamine-modified polyacrylic acid (10 μmol polymer chains). The QDs were slowly dried under vacuum to a film that was readily soluble in phosphate buffered saline. The resulting QDs were purified via ultracentrifugation. During this process, the QD absorption spectra were unchanged, but the fluorescence was entirely quenched (Figure 1a). Small QDs (<4 nm) coated with these amphiphilic polymers were substantially more polydisperse than nanoparticles containing larger QD cores. Figure S6 depicts gel filtration chromatograms of 2.5 nm CdTe QDs coated with this amphiphilic polymer, as well as QDs coated with 1-thioglycerol.
Supporting Figure S6. Gel filtration chromatogram of 2.5 nm CdTe QDs coated in 40%-octylamine-modified PAA (blue) or 1-thioglycerol (red). A large excess of thioglycerol, which elutes at ∼45 minutes, was found to be necessary to stabilize these QDs during chromatography.
Calculation of number of surface atoms per nanocrystal. Determination of surface atom density on nanocrystals can be difficult, and imprecise, especially for very small particles that cannot be easily characterized microscopically. Nevertheless, reasonable accuracy can be obtained by using theoretical calculations informed by empirical data. In this work, the CdTe nanocrystals that we prepared (2.5−6 nm diameter) were found to be in the zinc blende crystal structure (x-ray diffraction and high-resolution TEM data will be reported elsewhere), which is consistent with similar CdTe synthesis reports using phosphonic acid ligands.2 This data allows the use of the bulk density and interplanar distances of zinc blende CdTe in our calculations. From TEM, the nanocrystals were found to be relatively spherical, with slight elongation along the [111] direction in larger nanocrystals (aspect ratio 1.4 or less). Because of the near-spherical nature of growth, it is likely that a variety of crystalline facets are exposed on individual nanocrystals, each with a range of planar densities of atoms. It is also likely that there is a distribution of different facets exposed across an assembly of nanocrystals. Therefore one may obtain an effective average number of surface atoms per nanocrystal by averaging the surface densities of commonly exposed facets in zinc blende nanocrystals over the calculated surface area of the nanocrystal. In this work we chose to use the commonly observed (111), (100), and (110) zinc blende planes, which are representative of the lattice structure, with both polar and nonpolar surfaces. For this calculation, we defined a surface atom as an atom (either Cd2+ or Te2−) located on a nanocrystal facet with one or more unpassivated orbitals. Some facets, such as Cd2+-terminated {111} faces, have closely underlying Te2− atoms that are less than 1 Å beneath the surface plane. These atoms reside in the voids between Cd2+ atoms, and thus are likely to be sterically accessible from the surface, but because they are completely passivated, they were not included in our definition.
First we calculated the average distance between parallel planes of atoms for zinc blende CdTe (Table S1). This average interplanar distance, d, is therefore the distance between the plane of surface atoms and the next underlying plane of atoms. In the [100] and [110] directions, all adjacent planes are equidistant, whereas this distance varies between neighboring planes in the [111] direction, and thus we calculated an “average” interplanar distance. We also calculated the planar density of atoms on each facet, although this data was not directly used in our calculation (Table S1).
Supporting Table S1. Characteristics of three lattice planes of the zinc blende CdTe crystal structure. The interplanar distance, d, used in the surface atom calculation, is tabulated, along with several other parameters of interest.
Next we calculated the effective volume of surface atoms within each QD. We assumed a spherical geometry, and used the interplanar distance d as the thickness of one monolayer of surface atoms in each nanocrystal. In this calculation, the surface volume was used, rather than the surface area, in order to yield a more realistic determination of surface atoms in very small nanocrystals (< 2 nm). For these high surface area nanocrystals, use of the surface area generally resulted in a surface atom number that was larger than the total number of atoms in each nanocrystal. Therefore
where VSA is the volume of surface atoms per QD, r is the QD radius, and d is the average interplanar distance from Table S1.
We assumed that this spherical shell of surface atoms was the same density as bulk zinc blende CdTe, and therefore the number of surface atoms per nanocrystal could be calculate as
where nSA is the number of surface atoms per QD, D is the bulk density of zinc blende CdTe (5.85 g cm-3), NA is Avogadro's number, MW is the molecular weight of CdTe, and 2 is a factor accounting for 2 atoms per molecule of CdTe.
Table S2 displays the calculated number of surface atoms on a single, spherical CdTe QD that is hypothetically terminated solely by {111}, {100}, or {110} planes for various nanocrystal sizes. These values may be averaged, depending on available empirical information. For instance, HRTEM analysis of some of our larger CdTe nanocrystal samples revealed that the {110} planes were frequently parallel to the TEM grid. Therefore it is likely that these QDs were faceted along this plane, allowing them to adopt this orientation on their substrate during solvent evaporation.3 In this case, more weight can be given to these facets in the averaging calculation. For our calculations, we could obtain very little structural information about these very small nanocrystals, and therefore we simply averaged these three representative values together.
Supporting Table S2. Total number of atoms and total number surface atoms in various sizes of CdTe nanocrystals. The surface atom count is listed for each surface facet of interest, as well as its numerical average. This later value was used to calculate the molar capping ratio, described above.
Several methods have been reported in the literature to determine the number of surface atoms on nanocrystals without the use of complex energy-minimization computations.4 We compared several different calculation methods based on a quasi-spherical particles, as well as methods we developed to predict the number of surface atoms on different polyhedral shapes with various lattice facets. For all of these methods, we obtained strongly correlated results. A substantial difference (greater than 15%) was only observed in comparison with empirically unrealistic shapes with 8 sides or less (cubes or tetrahedrons). The specific method used for this work was chosen for its simplicity and its ease of quickly incorporating empirical knowledge of known facets.
We note two factors that could yield errors in this calculation and complicate the interpretation of the MCR value, with respect to the interaction between the nanocrystal surface atoms and the multidentate ligand. (1) Nanocrystals of various sizes have been theoretically and experimentally shown to have reconstructed surface atoms that may minimize the total energy of the crystal.5 Because of the very high fraction of atoms that reside on the surfaces of the small nanocrystals used in this study (∼39% for 2.5 nm QDs), surface reconstruction is likely. (2) Many lattice directions, such as the (100) direction, are terminated by atoms with two unpassivated orbitals, which could theoretically bind to two ligands. Atoms with more than one exposed binding site are even more likely to be present on the smallest QDs, which have such highly curved surfaces that a surface ‘facet’ may not even be an appropriate term for their description.
Characterization and instrumentation. Dynamic light scattering measurements were performed on a Brookhaven Instruments 90Plus Particle Size Analyzer. Before analysis, nanoparticle samples (1−100 μM, depending on the core size) were first centrifuged at 7000g for 10 minutes and then filtered through a 0.2 μm filter. Ultracentrifugal isolation of aqueous solutions of nanocrystals was performed on a Beckman Coulter Optima TLX Ultracentrifuge, typically at 100,000 rpm for 1 hour. Absorption spectra were obtained with a Shimadzu spectrophotometer with 1 nm slit widths. Photoluminescence spectra were obtained using a spectrofluorometer from Photon Technology International. The excitation source was a xenon lamp, the detector was a photomultiplier tube, and the spectrometer slit widths were typically 2 nm. Photostability measurements were performed by continuously recording emission spectra (400−620 nm) from 200 nM solutions of QDs with continuous high intensity 390 nm excitation (16 nm excitation spectrometer slit widths). Quantum yield was measured by comparison to the organic dye Atto 520 (dissolved in ethanol) using previously published methods.6 Transmission electron microscopy was performed by Dr. Hong Yi at the Electron Microscopy Core Facility at Emory University. Inductively-coupled plasma-mass spectrometry (ICP-MS) was performed with a PlasmaQuad 3 at the Center for Applied Isotope Studies at the University of Georgia. Gel filtration chromatography was performed on a Superose 6 10/300 GL column, with 280 nm absorption monitored on an AKTAprime plus system (GE Healthcare). The flow rate was 0.5 mL/min and the following protein standards were used for molecular weight determination: Ferritin (440 kDa), Aldolase (158 kDa), Ovalbumin (43 kDa), and carbonic anhydrase (29 kDa). Polydispersity index (PDI) was calculated from chromatograms using conventional techniques for polymer characterization, with the formula PDI = Mw / Mn. The PDI for pure protein solutions was typically 1.25−1.35.
References
(1) Yu, W. W.; Qu, L. H.; Guo, W. H.; Peng, X. G. Chem. Mater. 2003, 15, 2854−2860.
(2) Yu, W. W.; Wang, Y. A.; Peng, X. G. Chem. Mater. 2003, 15, 4300−4308.
(3) Li, R. F.; Lee, J.; Yang, B. C.; Horspool, D. N.; Aindow, M.; Papadimitrakopoulos, F. J. Am. Chem. Soc. 2005, 127, 2524−2532.
(4) Kirchner, C.; Liedl, T.; Kudera, S.; Pellegrino, T.; Javier, A. M.; Gaub, H. E.; Stolzle, S.; Fertig, N.; Parak, W. J. Nano Lett. 2005, 5, 331−338; Katari, J. E. B.; Colvin, V. L.; Alivisatos, A. P. J. Phys. Chem. 1994, 98, 4109−4117.
(5) Aruguete, D. M.; Marcus, M. A.; Li, L. S.; Williamson, A.; Fakra, S.; Gygi, F.; Galli, G. A.; Alivisatos, A. P. J. Phys. Chem. C 2007, 111, 75−79; Puzder, A.; Williamson, A. J.; Zaitseva, N.; Galli, G.; Manna, L.; Alivisatos, A. P. Nano Lett. 2004, 4, 2361−2365; Rempel, J. Y.; Trout, B. L.; Bawendi, M. G.; Jensen, K. F. J. Phys. Chem. B 2005, 109, 19320−19328; Ding, Y.; Wang, Z. L. Surf. Sci. 2007, 601, 425−433.
(6) Himel, C. M.; Mayer, R. T. Anal. Chem. 1970, 42, 130−132.