Abstract
Traditional alcohol studies measure blood alcohol concentration to elucidate the biomedical factors that contribute to alcohol abuse and alcoholism. These measurements require large and expensive equipment, are labor intensive, and are disruptive to the subject. To alleviate these problems, we have developed an implantable, wireless biosensor that is capable of measuring alcohol levels for up to six weeks. Ethanol levels were measured in vivo in the interstitial fluid of a Wistar rat after administering 1 g/kg and 2 g/kg ethanol by intraperitoneal (IP) injection. The data were transmitted wirelessly using a biosensor selective for alcohol detection. A low-power piezoresistive microcantilever sensor array was used with a polymer coating suitable for measuring ethanol concentrations at 100% humidity over several hours. A hydrophobic, vapor permeable nanopore membrane was used to screen liquid and ions while allowing vapor to pass to the sensor from the subcutaneous interstitial fluid.
Keywords: microcantilevers, piezoresistive, ethanol, biosensor
I. Introduction
The development of sensors that will measure parts per billion of a particular chemical in an ever-changing environment is a very exciting research area. These sensors have applications to homeland security, industrial safety and quality control, and medical research and diagnosis. Some of these sensors use piezoresistive cantilevers as their active element[1–5]. Reporting data wirelessly is also very important for homeland security and industrial safety applications where monitoring chemical levels before entry into an area would be advantageous.
In the medical field, there has been a lot of interest in implantable biosensors for health monitoring. There are few reports of fully implantable biosensors that monitor chemicals or physical conditions wirelessly and in vivo. One such sensor was used to measure intracranial pressure.[6] This device is anticipated to last 45 days with a 3 V lithium battery. In vivo glucose monitoring has substantial ramifications for diabetic patients. Atanasov et al subcutaneously implanted a refillable glucose biosensor into dogs.[7] Their biosensor consists of an amperometric electrode system and an enzyme micro-bioreactor. Their device measures 5.0 × 7.0 × 1.5 cm and takes readings every 20 sec. Two of their sensors were able to survive for 25 days after implantation. More recently, wired coil-type glucose oxidase biosensors were implanted transcutaneously into rats to monitor glucose levels where the sensors performed well up to four weeks.[8] The researchers used a wired device to investigate sensor degradation issues. The wires had to be anchored to reduce the probability of the rats' removal of the sensor, but the sensors showed less variation in glucose sensitivity over time than prior biosensors.
Certain chemicals do not require the use of enzymes or antibodies to detect their presence. Other sensing materials such as polymers can be used. This is advantageous for long term implantation since enzymes and antibodies degrade over time. Ethanol is one chemical that can be monitored inside the body using a polymer as the sensing material. Since many materials that interact with ethanol also interact with water, a polymer that has a higher affinity for ethanol than water is crucial in detecting ethanol in a humid environment such as inside the body. By placing a sensor directly inside the body, the pervasiveness of ethanol after its consumption and its direct time correlation can be studied to aid in the understanding of alcoholism. We have developed a small implantable telesensor to study alcoholism. Future studies would incorporate the measurement of other significant compounds relevant to alcoholism such as acetaldehyde.
In this article we present in vivo measurements of alcohol levels in the subcutaneous interstitial fluid of a Wistar rat using a low-power, wireless, and implantable sensor capable of operating for up to six weeks. The active elements chosen for the sensor are commercially available piezoresistive microcantilevers in a bridge array. The microcantilevers have the requisite small size that is important with regard to implantation of any sensor. All of the associated electronics similarly must be miniaturized as much as possible. Therefore, surface mounted integrated circuits were used to obtain the most compact configuration. The resulting device was then placed in a capsule with a hydrophobic, vapor permeable membrane placed over an inlet. A mesh covered the filter to prevent biofouling. This sensor proved to be sufficiently small for implantation in laboratory rats.
II. Experiment
The active sensor utilizes piezoresistive microcantilever arrays (CantiTM Chip 4) manufactured by Cantion, Inc. (Lyngby, Denmark)[9]. The 4-cantilever array is fabricated with uniformly linearly doped (p type) silicon “wires” encapsulated in silicon nitride (Si3N4). The resulting embedded 4 kΩ resistive wire is laid down in a loop with connections to two bond pads for each cantilever. Each array consists of four silicon nitride cantilevers coated with a 2 nm chromium adhesion layer overlaid with 30 nm gold (Au) by vacuum physical vapor deposition. In this study, two cantilever arrays were used to configure two diagonal-type Wheatstone bridges as seen in Fig. 1.
Fig. 1.
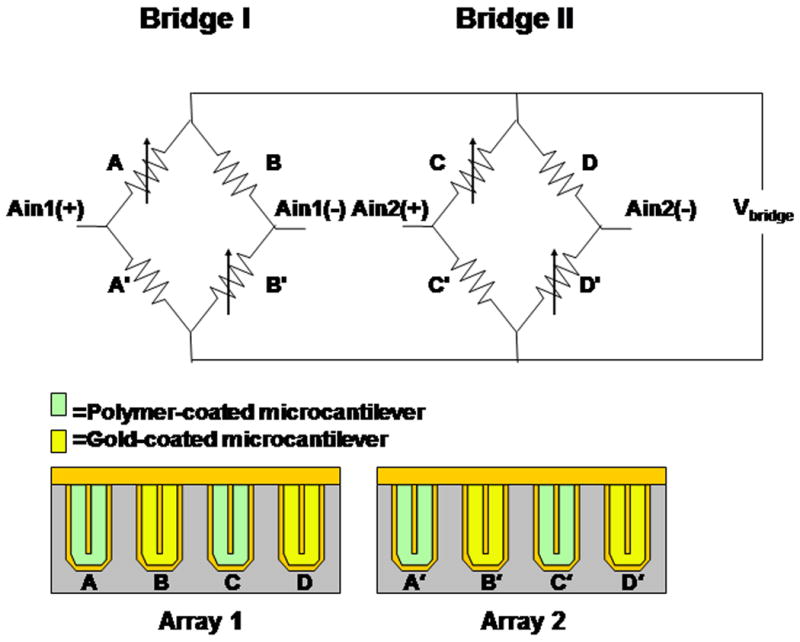
Two cantilever arrays form two diagonal-type Wheatstone bridges. Each cantilever has two electrical contacts. One of these contacts from all four cantilevers in one array are connected to a common bond pad. A voltage is applied across the common bond pads of the two arrays. Voltage changes due to surface stress of the cantilevers are monitored using the outputs Ain1 and Ain2 which are sent to the AD7792 analog-to-digital converter.
The power is provided by two series 1.5 V silver oxide batteries. A precision voltage regulator allows 1.22 V across the bridge so that adequate signal levels can be delivered to an AD7792 analog-to-digital converter (ADC) by Analog Devices (Norwood, MA)[10]. The very low-power ADC consists of a differential amplifier and a 16 bit sigma-delta ADC. The differential bridge voltage as a function of cantilever resistance is converted to a serial data stream where it is sent to an ultra low-power 16-bit RISC microprocessor. The microprocessor Manchester encodes the data. The microprocessor also contains the initial conditions for how often data is read, the gain setting, and capsule ID number. In this article, the gain was 128 and the data was read every 8 seconds. The microprocessor can be programmed to alter the gain and/or data collection interval. By increasing the time between reads, the battery life can be extended for up to six weeks. The battery lasts about one week at the current data read interval. In addition, the microprocessor is capable of providing an internal temperature measurement.
The encoded data is sent to the RF transmitter (Melexis FSK transmitter IC) where it is converted to a frequency shift key (FSK) data stream. The data is transmitted at 403 MHz, which is in accordance with the Medical Implant Communications Service per FCC 47CFR Part 95. The antenna is two loops of wire that circumscribes the PC board. It is connected to the main PC board containing the electronic components via gold pin standoffs. The ground plane of the PC board provides the ground plane for the antenna. Individually selected reactive components are used in order to provide impedance matching. The length of the antenna wire was not optimized for transmission due to the limited capsule size. A receiver, called a base station, decodes the Manchester encoded FSK and converts it to RS232. The capsule can be up to 15 ft from the base station for adequate transmission. The architecture of the system is diagrammed in Fig. 2.
Fig. 2.
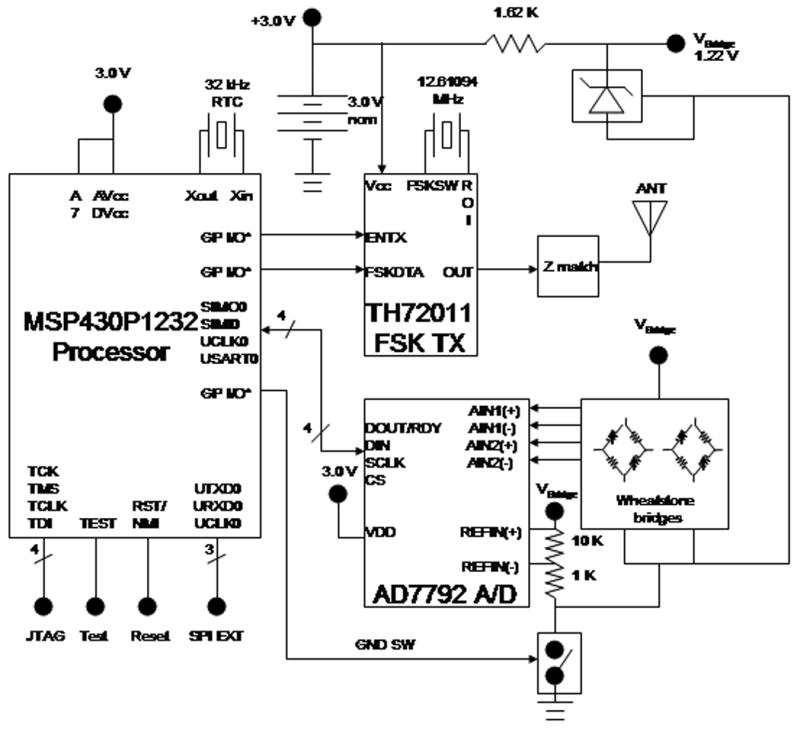
A diagram of the biosensor components. Voltage signals from the Wheatstone bridges are sent to an AD7792 analog-to-digital converter. The microprocessor Manchester encodes the data which is sent to an RF transmitter. The RF transmitter converts the encoded data to a frequency shift key data stream. The data is transmitted at 403 MHz.
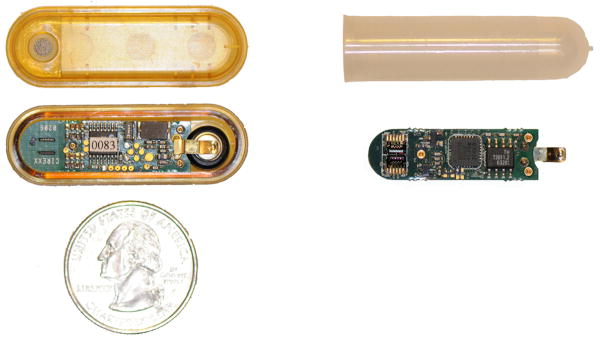
In initial experiments the electronic boards were incorporated into an FDA-approved capsule housing, as seen in Fig. 3, which measures 4.3 cm in length and 1.4 cm in width. This capsule housing and the electronic boards were manufactured by Flextronics, Inc[11]. The capsule housing has rounded corners to avoid damaging tissue when implanted. The sensor arrays are placed at one end of the capsule where they are exposed to the environment via an opening on the topside of the capsule housing. A titanium frit is sealed to a hydrophobic, vapor permeable nanopore membrane, and the membrane complex is then sealed to the opening. The exposed membrane is about 3 mm by 3 mm. The titanium frit prevents biofouling, and the hydrophobic membrane allows vapor to pass while blocking liquid, thus exposing the sensors only to ethanol and other volatiles. For this experiment, we used a General Electric Osmonics, Inc. (Minnetonka, MN) nylon membrane, which has a 0.1 μm pore size[12].
Fig. 3.
A photo of the original capsule design next to the modified capsule.
The cantilever array electrical connections to the PC board were found to experience failure after a lengthy exposure to 100% relative humidity. This failure was attributed to the sensitivity of the conductive epoxy to water vapor. To circumvent this problem, the original PC board was modified. The cantilever arrays were removed by dipping the arrays in methylene chloride and detaching them with a razor blade. The conductive epoxy and stabilizing underfill that was used to attach the arrays to the PC board were carefully removed under a microscope, using a razor blade to expose the underlying gold bond pads. 100 nm gold was sputtered onto the gold bond pads to create a cleaner surface to wire bond new cantilever arrays to the PC board. The new cantilever arrays were first attached to the PC board using a cyanoacrylate adhesive. Then the cantilever arrays were wire bonded to the PC board using aluminum wire. Due to the modification, the arrays were placed upside down on the PC board. The original design allowed the cantilevers to be exposed by a slot in the PC board. The original enclosure for the PC board could no longer be used since the arrays were no longer where the vapor could reach the sensor area. A more crude enclosure had to be used, which was used in previous wired experiments[13]. This enclosure was a polypropylene centrifuge tube which was shortened to about 4.5 cm. The inner diameter measured 1 cm. The nanopore membrane (13 mm in diameter) was sealed to the opening of the centrifuge tube with a polypropylene mesh.
Selectivity is achieved in part by using a polymer that has an exceptionally high partition coefficient for ethanol versus water. Two cantilevers on each array were spot coated with methyl phenyl mercapto propyl silicone (MCP20 OV17) in toluene provided by Seacoast Science, Inc. (Carlsbad, CA)[14]. The coating process was achieved using a hollow borosilicate microcapillary tube that was pulled to a fine tip comparable in size to the cantilever using a gas flame[15]. The microcapillary tube was connected to long tubing that ended at a bulb syringe to aid in the deposition process. The microcapillary tube was mounted on a xyz translation stage. A microscope was used to view the cantilever and microcapillary tube. The cantilevers were coated by depositing one drop of polymer solution on each cantilever. The thiol-modified polymer should aid in the attachment of the polymer to the gold surface of the cantilever via the sulfur group.
III. Results and Discussion
The telesensor was taken to Indiana University Purdue University at Indianapolis (IUPUI) where it was surgically implanted under the skin at the nape of a male outbred Wistar rat. Animals used in this study were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All research protocols were approved by the Institutional Animal Care and Use Committee and are in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, NIH, and the Guide for the Care and Use of Laboratory Animals of the National Research Council, 1996. The Wistar rat was implanted with the sensor at 8:12 AM. After a surgical recovery period, the sensor was monitored in the rat. Once stabilization was achieved, the rat was given an IP injection at 11:56 AM of 20% ethanol at a dose of 1 g/kg ethanol where the rat had a mass of 405 g. Immediately following the IP injection, the sensor signal had a positive slope as seen in Fig. 4. There was a response from the sensor although it was not considerably larger than the background noise. This response could be enhanced in the future by waiting a longer time for the sensor to stabilize in the rat. There may also be drift due to the exposure of the electronics to the water vapor. The original capsule design can be modified to allow wire bonded arrays to be attached to the PC board while maintaining isolation of the sensor chamber from the electronics.
Fig. 4.
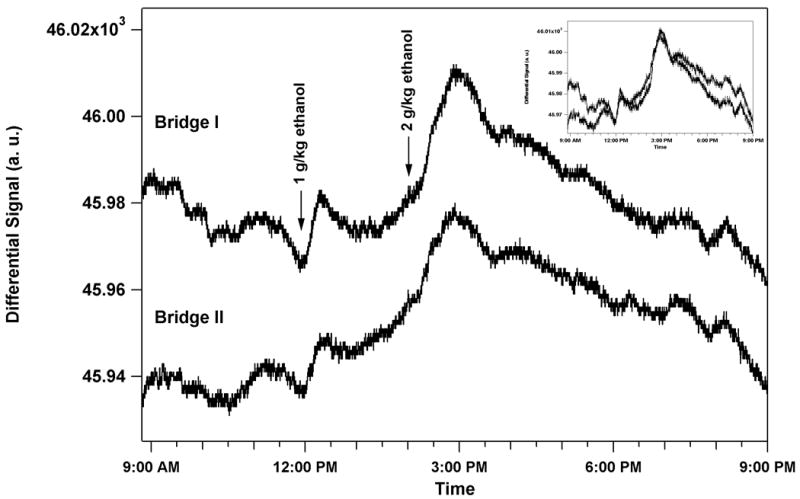
The left-hand axis shows sensor output versus time. An ethanol dose of 1 g/kg was given to the rat by IP injection at 11:56 AM, 3 h 44 min after surgical implantation. Immediately following the IP injection, the sensor signal had a positive slope and peaked 20 min. after injection. The signal gradually decreased as the alcohol was metabolized. A second ethanol dose of 2 g/kg ethanol was given by IP injection at 2:02 PM, 2 h 4 min after the first dose. The sensor signal peaked 55 min after the injection. The upper graph shows the sensor output from Bridge I while the lower graph shows the sensor output from Bridge II. Bridge II is offset so that both bridges could be plotted with the same scale. An inset shows the overlap of the two bridges.
The rat was given a second dose of 2 g/kg alcohol IP injection at 2:02 PM. The sensor response peaked 55 min after the injection. This response was much larger and lasted a much longer time. Both time courses of alcohol administration followed the time course of other alcohol concentration measurements in the subcutaneous fluid of the Wistar rat[16]. Also note that both bridges give the same response.
The sensor was left implanted overnight. The rat was given a 2 g/kg dose of ethanol by IP injection at 9:24 AM the following morning. Bridge II of the sensor showed a deflection of about the same magnitude as the previous day as seen in Fig. 5. The peak response occurred 56 min after injection. The rat was again given a 1 g/kg alcohol IP injection at 4:34 PM on Day 2. The sensor still responded to alcohol where the peak response occurred about 45 min after injection. This response time is longer than the first 1 g/kg injection on Day 1.
Fig. 5.
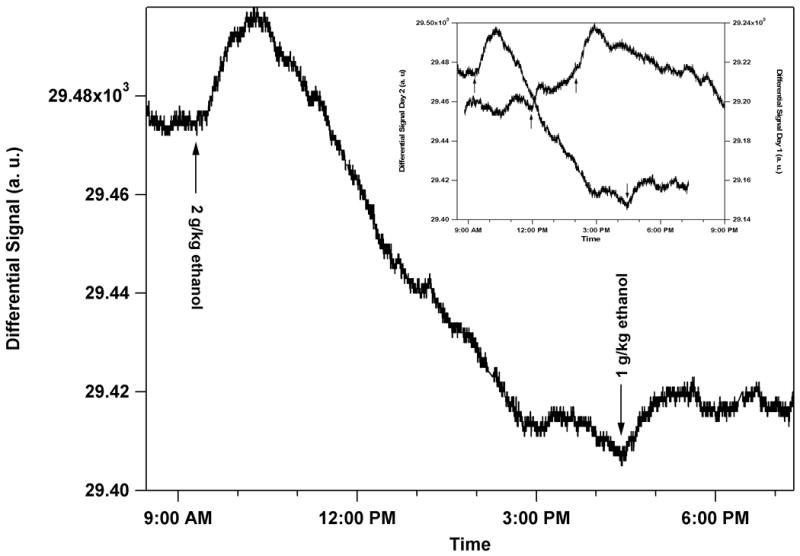
The left-hand axis shows sensor output versus time. An ethanol dose of 2 g/kg was given to the rat by IP injection at 9:24 AM the following morning after surgical implantation. Immediately following the IP injection, the sensor signal had a positive slope and peaked 56 min after injection. A second ethanol dose of 1 g/kg ethanol was given by IP injection at 4:34 PM. The sensor signal peaked 48 min after the injection. The graph shows the sensor output from Bridge II. An inset shows the overlap of Bridge II from Days 1 and 2 where the magnitude of the scales on both axis are the same. In this figure, no offsets are used.
As can be seen in Fig. 5, the drift was larger on Day 2. This drift will have to be further investigated but is most likely due to the wire-bonded connections. Similar drift has been seen in previous capsules as the epoxy swelled with humidity. Eventually the connections break all together. Since this capsule has fragile wire bonds, the bonds were most likely slowly lifting off from the pad. In addition, the electronics themselves may be reacting to the humid environment since the electronics are not isolated from the sensor chamber in this setup. Bridge I also showed deflection, but the drift was much larger so no quantitative data could be extracted.
The internal temperature of the rat was also measured. The rat's temperature does change with alcohol administration. Most of the temperature changes should cancel out in the Wheatstone bridge since all of the cantilevers consist of the same material. However, there will still be a small amount of temperature drift since two of the cantilevers are coated with the polymer. This drift is much smaller than the alcohol signal. The temperature would have to change from room temperature to body temperature to achieve such a large signal deflection as observed in the 2 g/kg ethanol IP injection. Thus, the observed signal change is not due to temperature effects alone.
Other alcohol sensors were also tested. Due to the lack of a pristine surface to wire bond the arrays to the PC board, some sensors did not perform as well. Some mounted cantilever arrays manufactured by Cantion, Inc. were also used on the capsules. These arrays were soldered to the capsule PC board by taking advantage of the electrical connections for balancing resistors. Although there was some response from the alcohol on at least one of these sensors, the background noise and drift is much larger for these capsules. This result is not surprising since the length of the wire connections is longer. Overall, the wire bonded sensors were very stable and hold the most promise for successful implantable sensors.
IV. Conclusion
In vivo measurements of ethanol content in the interstitial fluid of a Wistar rat injected with alcohol was attained with a wireless, implantable, low-power, microcantilever sensor array. The biosensor continued acquiring data over an extended time period after implantation. The implantable sensor was shown to accurately portray the time evolution of ethanol absorption and elimination. This sensor has useful applications for alcoholism studies where previous experiments showed that less than 100 ppm ethanol can be detected[17].
This telesensor can be made more compact by utilizing recently improved technology. It can also be used in many different environments to study many different analytes. Volatile analytes can make use of the hydrophobic membrane while nonvolatile analytes can be monitored by removing the membrane so that the cantilevers are in an ambient or fluid environment. In the case of a fluid environment, the electronics must be protected adequately.
The cantilevers can have different coatings to detect specific compounds. In an allied effort to measure cocaine concentrations in the interstitial fluid of laboratory rats with the same telesensor, thiolated aptamers were used[18]. These were bound to a thin gold coating on the microcantilevers and were specific for binding cocaine. A detection limit of 1 ng/ml was determined. The report of this data is hoped to be presented in a future paper and the key result is mentioned here to note the versatility of the telesensor. The results suggest a broad range of potentially significant measurements.
Depending upon the requisite duty cycle and the available battery technology, the data could be reported by an implanted telesensor over an extended period of time. Known difficulties would include problems with highly reactive analytes such as acetaldehyde as reversibility is then jeopardized. Poisoning of the surface also must be prevented by the use of adequate filters such as have been demonstrated in the present work. Additional problems are encountered when multiple compounds are targeted as this requires different coatings on different microcantilevers in an array, and while this problem can be solved with specialized, high-resolution, coating systems, the necessity of coating large numbers of sensors would require a higher degree of automation than at present.
Acknowledgments
This research was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) under Contract No. N01AA23012.
Biographies
Christine P. Cheney is presently a post-doctoral research associate at the Department of Physics and Astronomy, University of Tennessee, Knoxville, where she studies novel materials and complex electron systems using ultrafast laser spectroscopy and angle-resolved photoemission spectroscopy. She obtained her Ph.D. in condensed matter physics from Vanderbilt University, Nashville, TN. Research interests: microsensors, vibrational and electronic dynamical processes localized at surfaces, interfaces, and in the bulk.
Bernadeta R. Srijanto has recently received her Ph.D. degree in Electrical Engineering at the University of Tennessee, Knoxville. She worked in the Applied Physics Laboratories at the Department of Physics, University of Tennessee while pursuing the degree. Research interests: microsensors, scanning probe microscopy, instrumentations, digital systems, and logic design (VHDL).
David L. Hedden is a senior research associate at the Department of Physics and Astronomy, University of Tennessee, Knoxville. After seven years in the US Navy as an electronic technician he earned a BA in Philosophy and an MS in Applied Mathematics from Appalachian State University. Research interests: microsensors for biological, chemical, and explosive materials, scanning probe microscopy, alpha particle detection and analysis, and instrumentation.
Anthony C. Gehl has an MSE and BSEET degrees from Purdue University and has recently joined the staff at Oak Ridge National Laboratory (ORNL) where he conducts research for the Solar Technologies Program. From 2001 through July 2008 Tony was a member of the research staff of the Physics and Astronomy Dept. at University of Tennessee where he was involved with micro-cantilever based detection of explosives (at ORNL), and of cocaine and alcohol in the body (at UT).
Thomas L. Ferrell is a research professor at the Applied Physics Laboratories, Department of Physics and Astronomy at the University of Tennessee, Knoxville. He earned his Ph.D. in Physics in 1969 from Clemson University. Research interests: microsensors, surface science, scanning probe microscopy and spectroscopy, surface plasmons, surface-enhanced raman scattering, medical imaging, theoretical condensed-matter physics.
Jonathan Schultz is part of the research staff at the Institute of Psychiatric Research, Indiana University School of Medicine. He has his MA degree from Indiana University in Psychology. Research interest: addiction models
Eric A. Engleman has a Ph.D. in Medical Neurobiology from Indiana University in 1992. He is an Assistant Research Professor at the Indiana University School of Medicine, Department of Psychiatry. Research interests: The neurobiology of addictions, In vivo microdialysis, psychopharmacology and neurochemistry
William J. McBride is a Professor at the Institute of Psychiatric Research, Indiana University School of Medicine. Research interests: neurobiological mechanisms involved in high alcohol-drinking behavior, neurobiological factors mediating the development of adolescent alcohol-drinking behavior, neural basis of alcohol relapse, serotonin mechanisms in the development of alcohol tolerance, and neuronal mechanisms in alcohol reinforcement.
Sean O'Connor obtained his M.D. in 1978 from the University of Connecticut School of Medicine, Farmington, CT. He is a Professor of Psychiatry and Biomedical Engineering at the Institute of Psychiatric Research, Indiana University School of Medicine. Research interests: Characterizing the human brains response to alcohol, brain imaging and electrical recording, and neurobiology of alcoholism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boisen A, Thaysen J, Jensenius H, Hansen O. Environmental sensors based on micromachined cantilevers with integrated read-out. Ultramicroscopy. 2000;82:11–16. doi: 10.1016/s0304-3991(99)00148-5. [DOI] [PubMed] [Google Scholar]
- 2.Seo H, Jung S, Jeon S. Detection of formaldehyde vapor using mercaptophenol-coated piezoresistive cantilevers. Sens Actuators B. 2007;126:522–526. [Google Scholar]
- 3.Dauksaite V, Lorentzen M, Besenbacher F, Kjems J. Antibody-based protein detection using piezoresistive cantilever arrays. Nanotechnology. 2007;18:125503. [Google Scholar]
- 4.Wee KW, Kang GY, Park J, Kang JY, Yoon DS, Park JH, Kim TS. Novel electrical detection of label-free disease marker proteins using piezoresistive self-sensing micro-cantilevers. Biosens Bioelectron. 2005;20:1932–1938. doi: 10.1016/j.bios.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Porter TL, Vail TL, Eastman MP, Stewart R, Reed J, Venedam R, Delinger W. A solid-state sensor platform for the detection of hydrogen cyanide gas. Sens Actuators B. 2007;123:313–317. [Google Scholar]
- 6.Kawoos U, Tofighi M, Warty R, Kralick F, Rosen A. In-vitro and in-vivo trans-scalp evaluation of an intracranial pressure implant at 2.4 GHz. IEEE Trans Microw Theory Tech. 2008;56:2356–2365. [Google Scholar]
- 7.Atanasov P, Yang S, Salehi C, Ghindilis AL, Wilkins E, Schade D. Implantation of a refillable glucose monitoring-telemetry device. Biosensors and Bioelectronics. 1997;12:669–680. doi: 10.1016/s0956-5663(97)00025-0. [DOI] [PubMed] [Google Scholar]
- 8.Yu B, West L, Moussy Y, Moussy F. Transcutaneous implantation methods for improving the long-term performance of glucose sensors in rats. IEEE Sens Journ. 2008;8:97–103. [Google Scholar]
- 9.www.cantion.com
- 10.www.analogdevices.com
- 11.www.flextronics.com
- 12.www.gewater.com
- 13.Cheney CP, Wig A, Farahi RH, Gehl A, Hedden DL, Ferrell TL, Ji D, Bell R, McBride WR, O'Connor S. In vivo real-time ethanol vapor detection in the interstitial fluid of a Wistar rat using piezoresistive microcantilevers. Appl Phys Lett. 2007;90:013901. [Google Scholar]
- 14.www.seacoastscience.com
- 15.Many thanks to Ramya Desikan and Byron Kilbour for their guidance and assistance with the spot coater.
- 16.Engleman EA, Ingraham CM, Franklin KM, Keith CM, McClaren JA, Schultz J, Morzorati SL, O'Connor S, Thielen RJ, Murphy JM, McBride WJ. In Vivo Time-Course Changes in Ethanol Levels Sampled With Subcutaneous Microdialysis. Alcohol Clin Exp Res. 2008;32:435–442. doi: 10.1111/j.1530-0277.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- 17.Cheney CP, Wig A, Gehl A, Hedden DL, Farahi RH, Hunter SR, Ferrell TL. Ethanol vapor detection in saline solution using piezoresistive microcantilevers. Rev Sci Instrum. 2006;77:095101. [Google Scholar]
- 18.B. Srijanto, C. P. Cheney, D. L. Hedden, A. Gehl, T. L. Ferrell, J. Schultz, E. A. Engleman, W. J. McBride, and S. O'Connor. In Preparation.