Abstract
The developmental transition from vegetative growth to flowering in Arabidopsis is associated with a precipitous decline in the activity of leaf ascorbate peroxidase (APx), an enzymatic scavenger of hydrogen peroxide, and an increase in specific lipid peroxidation leading to the accumulation of 13-hydroperoxy-9,11,15 (Z,E,Z) octadecatrienoic acid (13 HOO-FA). The appearance of this specific isomer suggests that it is of enzymatic origin and may represent the activation of an oxylipin signaling pathway. We thus hypothesized that leaf 13-lipoxygenase (LOX) activity increases at the floral transition and leads to the observed elevation of 13-HOO-FA levels. Leaf protein extracts were prepared from seven distinct life stages of Arabidopsis plants and used to assay for LOX activity. We report that leaf 13-LOX enzymatic activity increases two- to three-fold from the vegetative stage to the immediate post-floral transition stage. We found two forms of LOX activity in cell extracts and show that the higher pH optimum form is the isoenzyme activated. This increase is correlated with a small increase in H2O2, perhaps resulting from the previously reported decline in leaf APx activity. Very low levels of exogenous H2O2 activate the induced form in vegetative leaf extracts in vitro, suggesting that the floral transition-dependent APx decline and subsequent H2O2 elevation are involved in activating plastid 13-LOX and thus a second messenger oxylipin pathway.
Keywords: lipoxygenase, ascorbate peroxidase, oxylipin pathway, floral transition
1. Introduction
A role for oxidative damage in the aging process, resulting from reduced species of oxygen (ROS) reacting with biomolecules, has been demonstrated in many organisms from yeast to man [1, 2]. Monocarpic plants undergo programmed aging, or senescence, where newly expressed genes direct the ordered dismantling of the leaf cellular apparatus for the nutritional storage benefit of the developing seeds [3]. Plant senescence also involves damage from ROS, which appears to be generated at a higher rate at older ages from increased H2O2-generating reactions. In addition, a programmed downregulation of the antioxidative capacity of leaf cells is operative. Thus, both H2O2 scavenging enzyme types, catalase and ascorbate peroxidase (APx), exhibit declines with leaf age in Arabidopsis. Catalase activity declines gradually in older leaves [4] while APx activity declines rapidly, and transiently, in leaves as the shoot apical meristem transitions to an inflorescence meristem [5, 4]. The link between flowering and senescence has been known for many years, perhaps millennia, and is certainly true in Arabidopsis. It is thus plausible that the signals that eventually lead the leaves down the senescence pathway are associated with the primary communicating signals between leaves and the meristem at the floral transition. The many common genes induced during developmental senescence and in the oxidative stress response suggest that the primary signals triggering the two pathways could be similar. Exogenous H2O2 addition activates the expression of many new genes also induced during natural senescence [6, 7]. H2O2 levels are elevated in leaves right at the floral transition [4], concomitant with the marked decline in APx activity. This floral transition-associated H2O2 burst is likely not associated with the increased ROS and ROS damage during the visible senescence stage, however. It appears only transiently, well before any chlorophyll breakdown, and predominates in the vascular transport tissues. Thus, it is more likely that this developmentally-controlled H2O2 burst generates a signal in leaves associated either with the induction of flowering or with altering leaf metabolism to prepare for the growing reproductive structures, which will ultimately result in leaf senescence.
Biochemical evidence suggests that a lipid-based signaling pathway is initiated as the transient H2O2 burst ensues, rather than a general ROS-mediated damage phenomenon. The lipid peroxidation rate is transiently elevated during the high H2O2 period, but the in vivo product isolated is 13-hydroperoxy-linolenic acid, not the isomeric mix of isomers that would result from H2O2 damage to the membrane [5]. This suggests that the enzyme 13-lipoxygenase (13-LOX) is activated in leaves at the floral transition. 13-LOX catalyzes the peroxidation of unsaturated carbon 13 of 18:2 or 18:3 fatty acids by the addition of molecular oxygen. This enzymatic reaction is the first (if acting on diacylglycerides [8]) or the second step (if acting on the free fatty acid) in the octadecanoate lipid signaling pathway. This pathway leads to the biosynthesis of numerous bioactive second messengers, including the jasmonates and other plant volatiles, that function as mobile signals to induce appropriate gene expression responses. The LOX genes themselves can be induced transcriptionally by the primary regulators, as evidenced in the wounding response, providing a feed forward mode to signal amplification (see [9] for review). The LOXs can also be activated post-transcriptionally. LOXs are metalloenzymes containing non-heme iron centers that can exist in the catalytically inactive ferrous state or the catalytically active ferric state. The lipid peroxide product can serve as the initiator to oxidize LOX-Fe2+ to LOX-Fe3+ and thus propagate the catalytic cycle [10]. However, H2O2 can also activate LOX-Fe2+ to LOX-Fe3+ [11], suggesting that one outcome of the transient leaf H2O2 burst is the activation of 13-LOX, and the consequent synthesis of a lipid-derived second messenger.
In this report, we test the hypothesis that 13-LOX is enzymatically activated and thus accounts for the observed transient lipid peroxidation in leaves at the floral transition. We show that leaf 13-LOX enzymatic activity increases two- to three-fold from the vegetative stage to the immediate post-floral transition stage. We found two forms of LOX activity in cell extracts, a low pH optimum and a higher pH optimum form. We show that the higher pH, chloroplastic form is the isoenzyme activated. Exogenous H2O2 can activate the higher pH form in vegetative leaf extracts, suggesting that the floral transition-dependent APx decline and subsequent H2O2 elevation are involved in activating 13-LOX and a second messenger oxylipin pathway.
2. Materials and methods
2.1. Plant Growth
Arabidopsis thaliana ecotype Columbia seeds (Lehle Seeds, Inc) were sown in 4:3:2 potting soil: vermiculite: perlite which were sterilized in an autoclave and treated with 0.05% fungicide (Daconil-2787) and 0.4% larvacide (Knock-Out Gnats, Abbott Lab) solution. Plants were induced to germinate simultaneously by incubating pots at 4°C for 2 days before transferring to growth chambers. Plants were irrigated with nutrient solution containing 0.0005 M KNO3, 0.0025 M KH2PO4, 0.004 M MgSO4·7 H2O, & 0.002 M Ca (NO3)2·4H2O twice a week and kept at 23°C under a 12 hour photoperiod with Sunlight fluorescent lights at 130 μmol/m2·sec−1.
2.2. Preparation of Whole Cell Protein Extracts
Extracts were prepared by freezing 0.5 g of leaf tissue in liquid nitrogen and pulverizing to a fine powder before homogenizing with a Tissue Tearor in a homogenization buffer prepared with 0.2 M sodium phosphate and 0.99 mM disodium ethylenediamine tetraacetate (EDTA) in 1:2 ratio. The homogenate was centrifuged at 14,000 rpm at 4 °C for 15 minutes and the supernatant was collected and stored at −80°C. Plant whole cell extracts of leaf material were prepared from seven distinct life stages of Arabidopsis. The dye-binding assay was used to quantify total protein for all extracts [12].
2.3. Lipoxygenase Assay
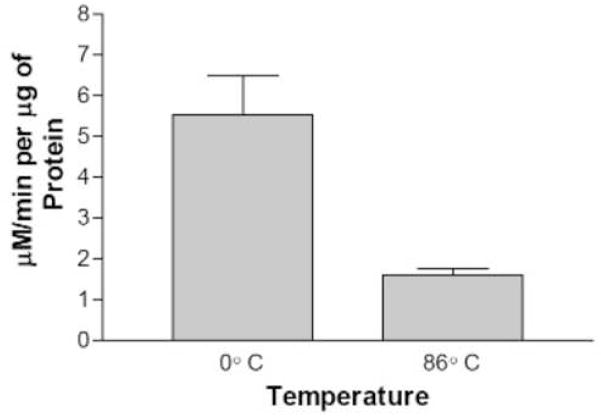
LOX activity was measured in the presence of linolenic acid and oxygen according to Axerold et al.[13]. The assay consisted of 50 mM Tris-HCl, pH 8.0, 10.2 mM linolenic acid, and various amounts of plant extract brought to a 1 ml final volume. The formation of a conjugated diene moiety was followed spectrophotometrically at 234 nm using an HP 8452A Diode Array Spectrophotometer. For heat denaturation, plant extracts of medium bolt and senescent plants were incubated at 86°C in a water bath for one hour with an equal aliquot of plant extract kept on ice for one hour. To ensure that the LOX activity resulted from a large, nondializable biomolecule, we subjected the extracts to dialysis prior to assaying. Plant extracts were dialyzed for one hour at 4°C with three changes of the homogenization buffer solution. A portion of the same plant extract was placed on ice for the same duration. After one hour, a LOX assay was performed as described above for both the dialyzed and non-dialyzed plant extract.
2.4. Activation of LOX with Hydrogen Peroxide
Crude protein extracts of pre-bolt and medium bolt stage were incubated with hydrogen peroxide concentrations including 10 μM, 50 μM, & 100 μM for 2 minutes and subsequently assayed for LOX activity. In addition, a LOX assay was used to detect non-enzymatic oxidation of the substrate due to hydrogen peroxide compared to substrate alone. Percent activation or inhibition of LOX activity was determined at pH 5.0 using a sodium citrate buffer and pH 7.4 using a sodium phosphate buffer for both extracts mentioned above.
2.5. Isolation of Arabidopsis chloroplasts
Chloroplasts were prepared as described [14] with slight modifications. Briefly, extracts were prepared using ~8g of leaf tissue and homogenized using a Tissue Tearor for 3–4 sec in 20 ml of Isolation buffer (0.3M sorbitol, 5 mM MgCl2, 5 mM EGTA, 5 mM EDTA, 20 mM HEPES/KOH, pH 8.0, and 10 mM NaHCO3). The homogenate was filtered through a double layer of Miracloth. The debris retained in the filter was returned to a beaker, another 20 ml of isolation buffer added, and homogenization was repeated. This homogenization procedure was carried out a total of five times. The supernatant solutions were considered the cytosol-enriched fraction. The homogenates were then combined together, centrifuged at 2962 rpm for 5 min. at 4°C and the pellet was resuspended in ~500μL of isolation buffer. The resuspended chloroplasts were loaded onto a two-step Percoll gradient or a linear Percoll gradient. The two-step Percoll gradient was carried out in 20-ml Corex tubes. The bottom layer of the two-step gradient consisted of 2.55 ml Percoll solution (95% (w/v) Percoll, 3% (w/v) PEG 6000, 1% (w/v) Ficoll, 1% (w/v) BSA) plus 0.45 ml of gradient mixture (25 mM HEPES-NaOH, pH 8.0, 10 mM EDTA, and 5 % (w/v) sorbitol). The top layer consisted of 2.94 ml Percoll solution plus 4.06 ml gradient mixture. The two-step gradient was centrifuged using a swing-out rotor at 3373 rpm for 10 min. at 4°C. The upper band contained broken chloroplasts and the lower band contained intact chloroplasts. To each band, 30 ml of HMS buffer (50mM HEPES, 3 mM MgSO4, 0.3 M sorbitol) was added and the tubes were inverted to wash off the Percoll. Then, the tubes were centrifuged using a swing-out rotor at 2962 rpm for 5 min, at 4°C. The pellet was resuspended in ~500 μL of HMS buffer with 1% NP40 for extraction.
Amperometric detection of hydrogen peroxide
A CHI 832 electroanalyzer (CH Instruments, Austin, TX) was used for the amperometric measurements. Sample injection was carried out with a six port rotary valve (Valco Instruments Co., Houston, TX) and a syringe pump (Harvard Apparatus, Holliston, MA). The dual gold electrode and the rotary valve were housed in a faraday cage (Bioanalytical Systems, West Lafavette, IN). A 3-mm dual gold electrode (Bioanalytical Systems, Inc, West Lafayette, IN) was used as the working electrode. It was polished down to 0.3 μM with Al2O3 slurry and then sonicated in an ultrasonic water bath with ethanol, acetone and deioinzed water, respectively. Peroxdidase redox polymer (PRP), which has been shown to be highly sensitive for H2O2 detection [15] was then immobilized on the dual gold electrode and the resultant electrode was assembled onto the cell that also contained a platinum wire auxiliary electrode and an Ag/AgCl reference electrode. The PRP-modified electrode was fabricated by coating 2 μL of PRP on the gold dual electrodes and stored in a vacuum dessicator overnight to dry. Four concentrations of H2O2 standard solutions were injected into the flow cell with the PRP-modified electrode potential held at 0.2 V vs. Ag/AgCl and a calibration curve was obtained by plotting the current versus the H2O2 concentration. Equal volumes of equal protein concentration whole-cell leaf extracts were then injected for H2O2 measurements.
EPR analysis of whole leaf tissue
Leaf tissue was frozen in liquid nitrogen and then ground to a fine powder. Each EPR tube was packed with approximately 0.25 g of leaf tissue and the tubes stored at −80°C until experiments were run. EPR measurements were performed on a Bruker EMX spectrometer. The temperature was regulated at 15 K with an Oxford ESR 900 continuous flow helium cryostat. Signals were observed using a microwave frequency of 9.4 GHz, microwave power of 1.9 mW, modulation frequency of 100 kHz, and modulation amplitude of 1.00G. The Spectra were analyzed using Bruker WinEPR System software.
3. Results
3.1. Leaf Lipoxygenase Activity during the Arabidopsis Life Span
During the vegetative to reproductive stage transition in Arabidopsis, leaf lipid peroxidation increases coincident with a decline in leaf ascorbate peroxidase activity and an increase in H2O2. This lipid peroxidation is not random as might be expected from chemical attack of the membrane by the elevated levels of H2O2. Instead one major product, a 13-hydroxy-9,11,15 (Z,E,Z) octadecatrienoic acid (13 HOO-FA) was observed [5]. We thus hypothesize that most of the lipid peroxidation at the floral transition is enzymatic in nature and mediated by a 13-specific lipoxygenase. It is known that LOX-generated hydroperoxy fatty acids are further metabolized through a variety of pathways and that these pathways ultimately produce bioactive molecules, that can initiate the transcription of genes, such as PR1 and perhaps senescence activating genes [16]. To test this, we measured LOX activity in the presence of linolenic acid and oxygen in leaf extracts prepared from various stages of the life span of Arabidopsis plants. These stages included pre-bolt (prior to the formation of any visible inflorescences), short bolt (< 1 cm length inflorescence), medium bolt (~1 cm), long bolt (3 cm), flowering, and seeding. Figure 1 shows a representative sample of LOX specific activity at the indicated stages for one set of plants. The experiment was repeated four times with similar results. A minimum of a two-fold increase in LOX specific activity was observed between the pre-bolt and short bolt stage, corresponding to the time of repressed APx activity and enhanced lipid peroxidation rates [5]. The elevated LOX activity continued as the bolts extended to a length of 3 cm. By the time that flowers appeared through the seeding phase, apparent LOX activity was elevated even more. Much of this activity though, as shown below for senescing samples, was non-enzymatic in nature.
Fig. 1.
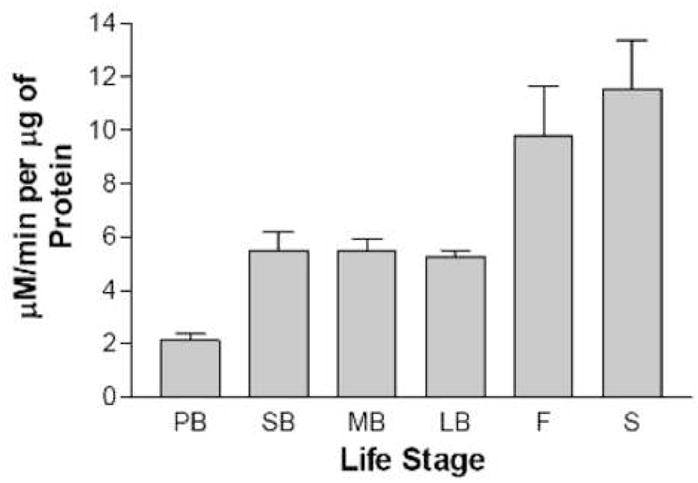
LOX specific activity as a function of life stage. Whole-cell leaf extracts prepared from PB, pre-bolt; SB, short bolt; MB, medium bolt; LB, long bolt; F, flowering; and S, seeding stage plants were assayed for LOX activity. The bars represent the average of three independent LOX determinations and the error bars represent the standard deviation in the data.
3.2 Identification of LOX isomers in Arabidopsis leaves
A variety of LOX enzymes have been observed in many plant species that catalyze the oxygenation of linolenic acid at carbon 9 or 13. The genetic sequences of at least six lipoxygenases are known in Arabidopsis [17, 18], three of which have chloroplast targeting signals. It is expected that perhaps three lipoxygenases reside in the chloroplast due to the presence of a transit peptide sequence similar to those found in known chloroplast proteins [17]. Other LOXs are speculated to reside in the cytosol [18]. The LOX assay results in Fig. 1 report overall activity in whole cell extracts. To identify possible isoforms of LOX, assays were performed as a function of pH and the results are shown in Figure 2. LOX activity for the medium bolt leaf extract displayed two distinct LOX activity peaks centered around pH 5.0 and pH 7.5. LOX activity for the pre-bolt leaf extract had only one LOX activity peak, at pH 5.0, and corresponded to the same LOX activity peak as in the medium bolt sample. To insure that the LOX activity maximum at pH 7.5 existed only in the medium bolt but not the pre-bolt leaf extract, a more narrow pH range between 7 to 8 was chosen to conduct the LOX assays and the results are shown in Figure 3. These data suggested that there was a second LOX activity maximum for medium bolt, which was not present in pre-bolt. A five-fold lipoxygenase increase was present at pH 7.4 in the medium bolt sample compared to the pre-bolt sample at a similar pH value. Control experiments where non-enzymatic lipid peroxidation was measured showed that, over the pH range used in Figs 2 and 3, very little differences were detected (data not shown). The data thus suggest that much of the increase in LOX activity in the post-bolt sample was due to the increase in the higher pH isoform of the enzyme.
Fig. 2.
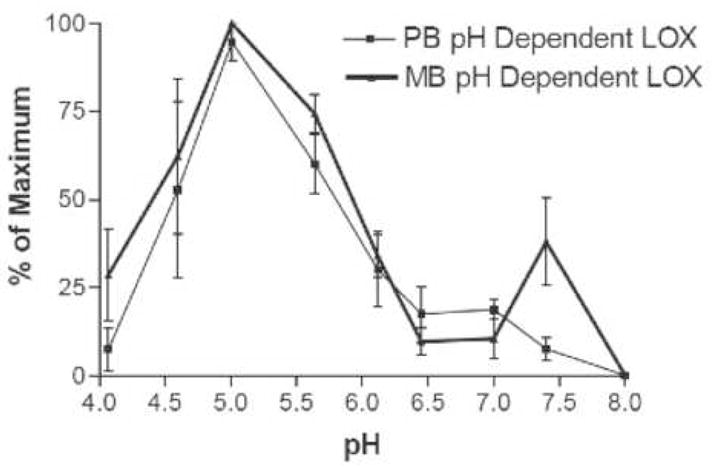
LOX specific activity as a function of pH and life stage. Whole-cell extracts prepared from pre-bolt and medium bolt leaves were assayed for LOX activity at the pHs indicated. The results are expressed as a percentage of maximum activity. Thin line, pre-bolt leaves; heavy line, medium bolt leaves. The error bars represent the standard deviation of the data over three independent determinations (i.e, using extracts from three different sets of plants).
Fig. 3.
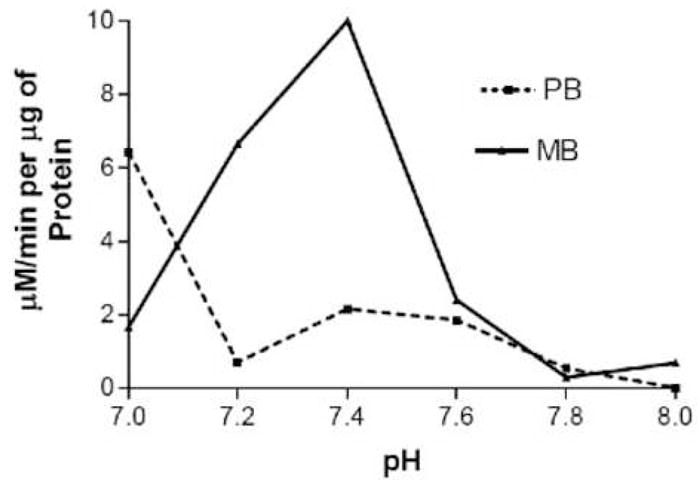
LOX specific activity at higher pHs. Whole-cell extracts prepared from pre-bolt and medium bolt leaves were assayed for LOX activity at the pHs indicated. The results are expressed as specific activity. Solid line, pre-bolt leaves; dashed line, medium bolt leaves.
In order to determine the cellular localization of the two LOX isoforms, we prepared cytoplasmic and chloroplast extracts from pre-bolt and post-bolt plants and measured LOX activity at the two pH optima. At either pH, all of the activity was found in the intact chloroplast fraction, with none being detected in either broken chloroplasts or in the cytoplasmic fraction. In addition, the pH 7.4 activity was only detected in post-bolt chloroplasts and noen was detected in pre-bolt chloroplasts. Thus, the developmentally induced, higher pH LOX isoform is localized to the chloroplast and could be the product of the LOX2 gene, although it is possible that other LOX isoforms identified by sequence, e.g. LOX3, and LOX3-related genes, could also account for the observed activity. This result suggests that the floral transition is associated with the activation of an oxylipin pathway. In defense responses, the oxylipin signaling pathway is known to be initiated in the chloroplast by the LOX2 protein [19].
3.3 Activation of chloroplast LOX activity by hydrogen peroxide
Previously, a decline in APx, an enzymatic scavenger of H2O2, was measured at the transition state from the vegetative to the reproductive stage [5]. This decline in APX is expected to raise the levels of H2O2, which can react directly with membrane lipids. However, since the lipid peroxides present in immediate post-bolt leaves are predominantly 13-hydroperoxy fatty acids [5], we hypothesize that the elevated H2O2 itself activates LOX activity. We thus tested whether H2O2 incubation can activate LOX in our low-APX extracts. Leaf extracts from pre-bolt and medium bolt plants were thus incubated with low concentrations of H2O2, from 10 μM to 100 μM, for two minutes and then assayed for LOX activity. The results are plotted in Fig. 4. The medium bolt sample at both pH 5.0 and pH 7.4 was relatively insensitive to these concentrations of H2O2. In contrast, the pre-bolt sample at pH 7.4 showed at least a two-fold increase in LOX specific activity at 10 μM hydrogen peroxide. A LOX activity increase was also observed for the pre-bolt sample at pH 5.0 but it was not as dramatic as the increase seen at pH 7.4. Under these conditions, non-enzymatic lipid peroxidation, i.e. the formation of the lipid peroxide product in the presence of H2O2 but in the absence of extract protein, was negligible. Thus, the products measured in Fig. 4 result from LOX catalysis.
Fig. 4.
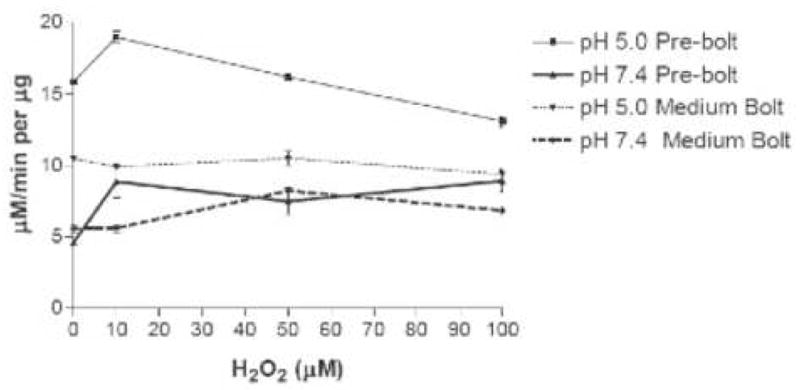
LOX specific activity following incubation of leaf extracts with hydrogen peroxide. Curves represent LOX activity after a 2-minute incubation with hydrogen peroxide at the indicated concentrations. LOX activity was measured at pH 5.0 (thin lines) or pH 7.4 (heavy lines). Error bars represent standard deviation in the data.
We attempted to directly measure the H2O2 concentration in these extracts using cyclic voltammetry and differential pulse voltammetry, which is at least three times more sensitive than the former. Both techniques failed in detecting H2O2 in the extracts, while standard H2O2 was detected down to the 0.5 mM range (data not shown). Thus, either the endogenous H2O2 in the extracts already reacted prior to our experiments or, more likely, the levels were below 0.5 mM. We thus used amperommetry using an electrode coated with horseradish peroxidase to amplify the H2O2 signal [15]. The data are presented in Fig. 5. As can be seen, only the small bolt sample gave a peak current, suggesting that H2O2 is elevated to detectable levels at this stage. Thus, our data lend credence to the idea that APx decline allows a fairly small, but measurable, H2O2 increase to initiate an oxylipin pathway through LOX activation. It thus appears that the response of LOX to 10 μM H2O2 shown in Fig. 4 is significant and relevant to the expected low endogenous H2O2 concentration.
Fig. 5.
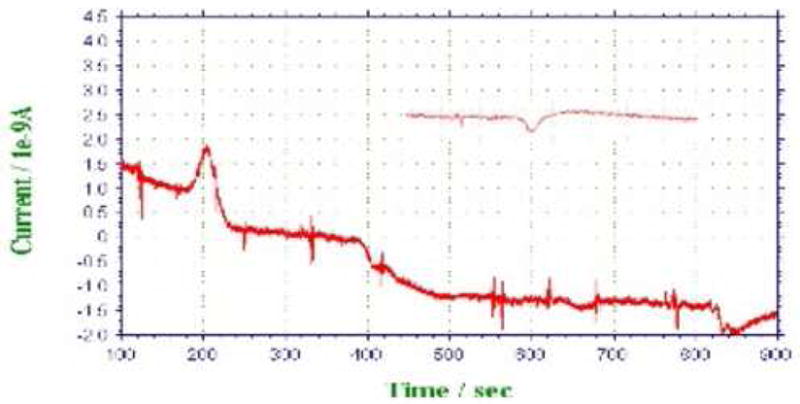
Amperommetric detection of H2O2 in leaf extracts. Fifty μL of equal protein concentration whole-cell extract from short bolt, medium bolt, long bolt, and flowering plants were pumped into the detection cell at 120, 330, 553, and 772 s, respectively. The extracts are expected to enter the cell after 80 s. The inset shows a pre-bolt sample.
3.5 Lipid peroxidation during senescence is mostly non-enzymatic
We found that LOX activity measured in extracts prepared from senescing plants (leaves at least 50% yellow, but not dried) was more variable than at other stages, but much higher than any other stage. Apparent LOX activity during senescence was three-to four-fold higher than at any other stage and as much as twenty-fold higher than in pre-bolt plants. To determine if this increase in substrate oxidation was due to an enzyme or by some non-enzymatic means, a heat denaturing experiment was conducted. It is well known that most proteins are denatured and their enzymatic activity diminished when exposed to heat. This has been shown directly for LOX [20]. We thus subjected both a medium-bolt and a senescent extract to an 86°C incubation for one hour and measured the remaining LOX activity. The data in Fig. 6 show that the incubation with heat using the medium bolt extract significantly reduced LOX specific activity at least four-fold compared to incubating on ice for the same time period. On the other hand, the senescent leaf extract only decreased about 30% in activity by heat. This result combined with the large increase of lipoxygenase enzyme activity at senescence, led us to speculate that the very high lipid peroxidation observed in senescent leaf extracts was perhaps due to non-enzymatic oxidation of the substrate catalyzed by some species present only in senescing leaves.
Fig. 6.
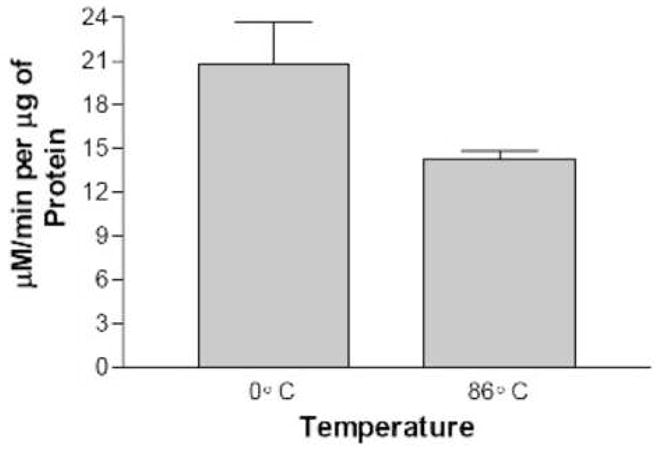
LOX specific activity following high temperature incubation. Leaf extracts prepared from medium bolt plants, panel A, or senescing plants, panel B, were incubated for 1 hr either on ice or at 86°C before LOX activity measurement. The assays were performed in triplicate with the standard deviation of the data shown.
3.6 EPR-active species are highly elevated in senescent leaf material
To gain evidence for a senescent-specific molecular species that might be responsible for the observed elevated lipid peroxidation, we used EPR to detect organic radicals or transition metal complexes. Plants were grown to the indicated stages and equal masses of leaf material rapidly frozen, crushed, transferred to EPR tubes and signals measured. The EPR spectra of pre-bolt, small bolt, flowering and senescent stage plants are shown in Fig. 7. The peak expected for lipoxygenase is at g = 6, or 1200 G, which was undetectable in our whole-leaf samples. Throughout the pre-bolt to the flowering stages, there was relative uniformity in the spectra showing a small peak at g = 1.94. This peak has been previously reported and belongs to Cu2+ and Mn 2+ complexes and organic radicals. Senescent stage leaves, on the other hand, gave spectra with major increases at g = 4.28 and at g = 1.94. The peak at g = 4.28 belongs to high-spin Fe(III) in a rhombic configuration. Intensity for all EPR spectra was set to 1000, therefore the senescent stage showed a very large increase in peak size relative to the other stages.
Fig. 7.
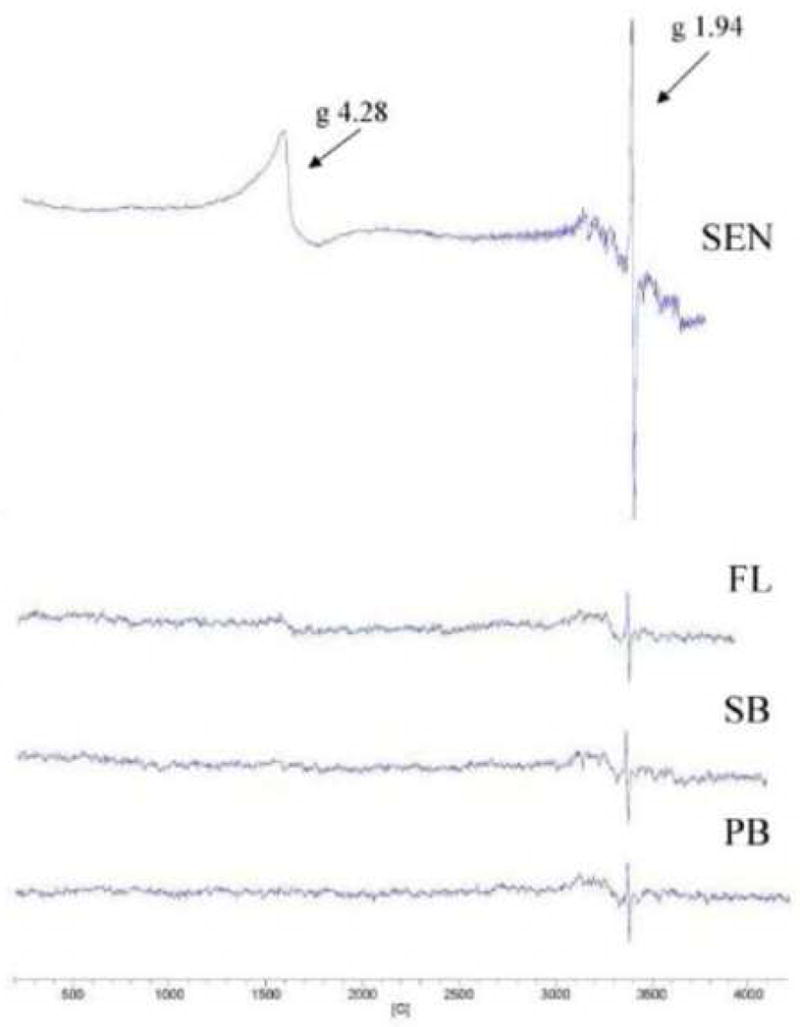
EPR spectra of whole-leaf samples as a function of life stage. Spectra were recorded for equal quantities of PB, pre-bolt; SB, short bolt; FL, flowering; and SEN, senescent leaves. G values for the major signals are indicated.
4. Discussion
This study presents evidence for a developmentally timed induction of LOX activity in leaves at the onset of the floral transition of the shoot apex. Measured LOX activity increases at least two-fold comparing pre-flowering to immediate post-floral transition plants. Further increases are evident at later developmental stages with an apparent massive increase occurring during senescence. These results are in concordance with another study in Arabidopsis that showed a general increase in LOX protein levels as leaf development proceeds [21]. However, that study did not report the developmental state of the principal shoot meristem when leaf LOX measurements were taken, as we report here. In addition, the immunoblotting technique used should depict overall LOX protein levels without distinguishing catalytically active from inactive LOX enzyme. On the other hand, our results suggest that, even with LOX protein present, LOX activity appears to depend on the developmental stage of the plant, suggesting that its activity is regulated post-translationally. Indeed, we found that an enhancement of LOX activity occurs by short exposure of a low activity, pre-bolt extract to low concentrations of H2O2. Thus, we show here that the transition of the shoot apex from a vegetative to an inflorescence mersitem, an event promoted by FT protein and perhaps other signals emanating from the leaves [22, 23], is associated with an increased capacity of the leaves to synthesize oxylipins. The senescence-associated LOX activity increase appears to be of non-enzymatic origin since heat inactivation only decreases the observed activity by about a third. This result was expected as Berger et al. [24] also observed predominant non-enzymatic lipid oxidation during senescence of Arabidopsis leaves by measuring ratios of regioisomers. The large increase in apparent non-enzymatic lipid peroxidation is associated with large increases in EPR-active Cu2+ and/or Mn 2+ complexes and rhombic Fe(III). The exact nature of these molecular species is unclear but has been observed in necrotic plant tissues [25]. Atherton et al. [26] observed similar spectra in dessicated plant tissues and suggested that a single organic, quinone-derived free radical is responsible and ubiquitously present in all senescing tissues, an assertion that was later called into question [27]. As senescence involves the active degradation of leaf proteins, many of which, especially in chloroplast membranes, are metalloenzymes, it is not surprising that oxidized metal ion species would accumulate in senescing leaves. It is interesting to note that one of the most highly up-regulated genes during Arabidopsis leaf senescence is SAG12, which encodes a copper transporter, suggesting a role for copper metabolism in senescence physiology [28, 29].
In crude leaf extracts we detect at least two LOX isoforms, one with a pH optimum at 5.0 and another with an optimum at 7.4. The lower pH form is present in leaf extracts at all developmental stages while the higher pH form is undetectable before the floral transition but increases after the transition. Thus, it is the pH 7.4 LOX that is induced in leaves. Interestingly, the pH 7.4 LOX isoform is actually present in pre-bolt leaf extracts, since incubating with H2O2 results in detectable activity. It appears then that LOX induction at the floral transition is due to oxidative activation of the pre-existing higher pH enzyme, perhaps by H2O2 itself, which we confirmed is possible by demonstrating the activation of the higher pH enzyme in pre-bolt extracts using very low concentrations of H2O2. It is well documented that H2O2 is able to activate LOX by oxidizing the Fe2+ to Fe3+, which is the active form of LOX [30]. The rpeaction involves an initial rate-limiting step of hydrogen abstraction from the 11th carbon of its substrate, linolenic acid, producing a radical that rapidly reacts with molecular oxygen and eventually forms a 13-(S)-HPOTE [31]. The resting state of LOX involves a Fe 3+-OH moiety and is converted to the active Fe 2−OH2 by a hydroperoxide [30]. It is speculated that H2O2 can oxidize the Fe2+ to an Fe3+ iron peroxo species that then gets protonated to regenerate the Fe 3+-OH [31].
By fractionation of subcellular compartments, we demonstrate that the inducible, higher pH form of LOX resides in the chloroplast. The activity could represent the product of the LOX2 gene, which contains a predicted chloroplast targeting signal and is known to reside in the chloroplast [32]. LOX2 is responsible for the generation of jasmonic acid (JA) and methyl jasmonate, well-known lipid derived signaling molecules operative in defense responses whose synthesis is initiated in the cholorplast. Given the apparent specificity of LOX2 for JA synthesis in response to wounding, however, it is unclear why it should be induced in leaves during the floral transition, although JA is necessary for flower maturation [33]. Of the seven lipoxygenase genes in Arabidopsis, LOX2, LOX3 and two LOX3-like proteins are predicted to have chloroplast targeting signals. Thus, the inducible, higher pH chloroplast LOX could be LOX3 or a LOX3-like paralogue, suggesting that perhaps an oxylipin other than JA is being synthesized in leaves right at the floral transition. In fact, even during the wound response, it appears that an oxylipin other than JA may spread throughout the plant [34], likely by binding to the lipid transfer protein DIR1 [35], in systemic acquired resistance (SAR). Our results suggest that the developmental transition to flowering is also associated with the induced synthesis of lipid signals through a plastid LOX-initiated biosynthetic pathway. The pathway potentially leads to the synthesis of jasmonic acid, which could play a role in the floral transition, or alternatively a different oxylipin biosynthetic pathway may be initiated leading to the production of a heretofore unrecognized lipid derived signal. It is known that the LOX pathway branches immediately after the LOX reaction into several different directions to make a whole variety of compounds likely to have bioactivity [9]. It is also clear from the Arabidopsis genome that the lipid transfer protein gene family [36] in Arabidopsis has at least 25 members, all of which are apoplastic and one of which is the potential SAR mobile carrier DIR1 [35]. Thus, it is likely that plant signaling at a distance may include the regulated synthesis of specific lipids at source sites and the subsequent apoplastic delivery of those bioactive compounds to target sites.
What accounts for the transient increase in leaf LOX activity at the floral transition? We measured a small but distinct increase in H2O2 at the same stage and thus hypothesize that the H2O2 itself activates plastid LOX. We further speculate that the H2O2 increase is due to the developmental decline in ascorbate peroxidase (APx) activity, shown by us [5] and more recently confirmed [4]. APx activity falls to nearly undetectable levels in leaves at the floral transition. Since APx catalyzes the removal of H2O2 by converting it to H2O and O2, we expect that the H2O2 concentration would increase during the low APx period, which we show in this study and was also reported by others [4]. APx activity is also known to decline during the hypersensitive response [37], presumably to allow the H2O2 concentration to increase as part of the defense signaling pathway, and declines prior to the observation of senescence symptoms in daylily and Gladiolus [38, 39]. Thus, APx inhibition may be a general mechanism that allows H2O2 to serve an immediate cellular signaling function. In the developmental case of the transition to flowering, the APx decline and subsequent H2O2 increase may be responsible for the observed increase in plastid LOX activity. Since plastid LOX catalyzes an early step in oxylipin synthesis, it seems likely that the floral transition involves the production of oxylipin signals that could be operative either locally within the leaf or at a distance following transport. Such signals could contribute to the floral transition at the shoot apex and/or alter gene expression in leaves to support the newly developing reproductive structures.
Acknowledgments
This work was supported by grants from the National Institutes of Health Minority Biomedical Research Support Program (R25 GM06133), the NIH MBRS-Support of Continuous Research Excellence Program (S06 GM08101), NIH Bridges to the Doctorate Program (R25 GM054939), the NIH Research Infrastructure in Minority Institutions Program (P20 MD001824), and the National Science Foundation Collaborative Research at Undergraduate Institutions Program (DBI9710796).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finch CE. Longevity, senescence, and the genome. The University of Chicago Press; Chicago.: 1990. [Google Scholar]
- 2.Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 3.Leopold AC. Senescence in plant development. Science. 1961;134:1727–1732. doi: 10.1126/science.134.3492.1727. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann P, Heinlein C, Orendi G, Zentgraf U. Senescence-specific regulation of catalases in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ. 2006;29:1049–1060. doi: 10.1111/j.1365-3040.2005.01459.x. [DOI] [PubMed] [Google Scholar]
- 5.Ye Z, Rodriguez R, Tran A, Hoang H, de los Santos D, Brown S, Vellanoweth R. The developmental transition to flowering represses ascorbate peroxidase activity and induces enzymatic lipid peroxidation in leaf tissue in Arabidopsis thaliana. Plant Science. 2000;158:115–127. doi: 10.1016/s0168-9452(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 6.Desikan R, Neill SJ, Hancock JT. Hydrogen peroxide-induced gene expression in Arabidopsis thaliana. Free Radic Biol Med. 2000;28:773–778. doi: 10.1016/s0891-5849(00)00157-x. [DOI] [PubMed] [Google Scholar]
- 7.Desikan R, AH-MS, Hancock JT, Neill SJ. Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 2001;127:159–172. doi: 10.1104/pp.127.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stelmach BA, Muller A, Hennig P, Gebhardt S, Schubert-Zsilavecz M, Weiler EW. A novel class of oxylipins, sn1-O-(12-oxophytodienoyl)-sn2-O-(hexadecatrienoyl)-monogalactosyl Diglyceride, from Arabidopsis thaliana. J Biol Chem. 2001;276:12832–12838. doi: 10.1074/jbc.M010743200. [DOI] [PubMed] [Google Scholar]
- 9.Feussner I, Wasternack C. The lipoxygenase pathway. Annu Rev Plant Biol. 2002;53:275–297. doi: 10.1146/annurev.arplant.53.100301.135248. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn H, Borchert A. Regulation of enzymatic lipid peroxidation: the interplay of peroxidizing and peroxide reducing enzymes. Free Radic Biol Med. 2002;33:154–172. doi: 10.1016/s0891-5849(02)00855-9. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni AP, Mitra A, Chaudhuri J, Byczkowski JZ, Richards I. Hydrogen peroxide: a potent activator of dioxygenase activity of soybean lipoxygenase. Biochem Biophys Res Commun. 1990;166:417–423. doi: 10.1016/0006-291x(90)91961-q. [DOI] [PubMed] [Google Scholar]
- 12.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 13.Axerold B, Cheesbrough TM, Laakso S. Lipoxygenase from Soybeans. Methods in Enzymology. 1981:441–451. [Google Scholar]
- 14.Aronsson H, Jarvis P. A simple method for isolating import-competent Arabidopsis chloroplasts. FEBS Lett. 2002;529:215–220. doi: 10.1016/s0014-5793(02)03342-2. [DOI] [PubMed] [Google Scholar]
- 15.Vreeke M, Maidan R, Heller A. Hydrogen Peroxide and b-Nicotinamide Adenine Dinucleotide Sensing Amperometric Electrodes Based on Electrical Connection of Horseradish Peroxidase Redox Centers to Electrodes through a Three-Dimensional Electron Relaying Polymer Network. Anal Chem. 1992;64:3084–3090. [Google Scholar]
- 16.Weber H. Fatty acid-derived signals in plants. Trends Plant Sci. 2002;7:217–224. doi: 10.1016/s1360-1385(02)02250-1. [DOI] [PubMed] [Google Scholar]
- 17.Bell E, Mullet JE. Characterization of an Arabidopsis lipoxygenase gene responsive to methyl jasmonate and wounding. Plant Physiol. 1993;103:1133–1137. doi: 10.1104/pp.103.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melan MA, Dong X, Endara ME, Davis KR, Ausubel FM, Peterman TK. An Arabidopsis thaliana lipoxygenase gene can be induced by pathogens, abscisic acid, and methyl jasmonate. Plant Physiol. 1993;101:441–450. doi: 10.1104/pp.101.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farmer EE, Weber H, Vollenweider S. Fatty acid signaling in Arabidopsis. Planta. 1998;206:167–174. doi: 10.1007/s004250050388. [DOI] [PubMed] [Google Scholar]
- 20.Anthon GE, Barrett DM. Thermal Inactivation of Lipoxygenase and hydroperoxytrienoic acid lyase in tomatoes. Food Chemistry. 2003;81:275–279. [Google Scholar]
- 21.Hause B, Stenzel I, Miersch O, Wasternack C. Occurrence of the allene oxide cyclase in different organs and tissues of Arabidopsis thaliana. Phytochemistry. 2003;64:971–980. doi: 10.1016/s0031-9422(03)00447-3. [DOI] [PubMed] [Google Scholar]
- 22.Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G. FT Protein Movement Contributes to Long-Distance Signaling in Floral Induction of Arabidopsis. Science. 2007 doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 23.Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. Hd3a Protein Is a Mobile Flowering Signal in Rice. Science. 2007 doi: 10.1126/science.1141753. [DOI] [PubMed] [Google Scholar]
- 24.Berger S, Weichert H, Porzel A, Wasternack C, Kuhn H, Feussner I. Enzymatic and non-enzymatic lipid peroxidation in leaf development. Biochim Biophys Acta. 2001;1533:266–276. doi: 10.1016/s1388-1981(01)00161-5. [DOI] [PubMed] [Google Scholar]
- 25.Monk LS, McPhail DB, Goodman BA, Davies HV. An electron spin resonance investigation of internal rust spot, a physiological disorder of the potato tuber. Free Radic Res Commun. 1989;5:345–350. doi: 10.3109/10715768909073417. [DOI] [PubMed] [Google Scholar]
- 26.Atherton NM, Hendry GA, Mobius K, Rohrer M, Torring JT. A free radical ubiquitously associated with snenscence in plants: evidence for a quinone. Free Radic Res Comm. 1993;19:297–301. doi: 10.3109/10715769309056518. [DOI] [PubMed] [Google Scholar]
- 27.Goodman BA, Deighton N, Glidewell SM, Wood CB, Pritchard HW, Benson EE. Do EPR spectra show the presence of a unique and ubiquitous quinone-derived free radical that is associated with senescence in plants? Free Radic Res. 1995;23:187–192. doi: 10.3109/10715769509064031. [DOI] [PubMed] [Google Scholar]
- 28.Quirino BF, Normanly J, Amasino RM. Diverse range of gene activity during Arabidopsis thaliana leaf senescence includes pathogen-independent induction of defense-related genes. Plant Mol Biol. 1999;40:267–278. doi: 10.1023/a:1006199932265. [DOI] [PubMed] [Google Scholar]
- 29.Ouelhadj A, Kaminski M, Mittag M, Humbeck K. Receptor-like protein kinase HvLysMR1 of barley (Hordeum vulgare L.) is induced during leaf senescence and heavy metal stress. J Exp Bot. 2007;58:1381–1396. doi: 10.1093/jxb/erl304. [DOI] [PubMed] [Google Scholar]
- 30.Skrzypczak-Jankun E, Bross RA, Carroll RT, Dunham WR, Funk MO., Jr Three-dimensional structure of a purple lipoxygenase. J Am Chem Soc. 2001;123:10814–10820. doi: 10.1021/ja011759t. [DOI] [PubMed] [Google Scholar]
- 31.Knapp MJ, Seebeck FP, Klinman JP. Steric control of oxygenation regiochemistry in soybean lipoxygenase-1. J Am Chem Soc. 2001;123:2931–2932. doi: 10.1021/ja003855k. [DOI] [PubMed] [Google Scholar]
- 32.Bell E, Creelman RA, Mullet JE. A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc Natl Acad Sci U S A. 1995;92:8675–8679. doi: 10.1073/pnas.92.19.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagpal P, Ellis CM, Weber H, Ploense SE, Barkawi LS, Guilfoyle TJ, Hagen G, Alonso JM, Cohen JD, Farmer EE, Ecker JR, Reed JW. Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development. 2005;132:4107–4118. doi: 10.1242/dev.01955. [DOI] [PubMed] [Google Scholar]
- 34.Chaturvedi R, Krothapalli K, Makandar R, Nandi A, Sparks AA, Roth MR, Welti R, Shah J. Plastid omega3-fatty acid desaturase-dependent accumulation of a systemic acquired resistance inducing activity in petiole exudates of Arabidopsis thaliana is independent of jasmonic acid. Plant J. 2007 doi: 10.1111/j.1365-313X.2007.03400.x. [DOI] [PubMed] [Google Scholar]
- 35.Maldonado AM, Doerner P, Dixon RA, Lamb CJ, Cameron RK. A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature. 2002;419:399–403. doi: 10.1038/nature00962. [DOI] [PubMed] [Google Scholar]
- 36.Arondel V, Vergnolle C, Cantrel C, Kader JC. Lipid transfer proteins are encoded by a small multigene family in Arabidopsis thaliana. Plant Science. 2000;157:1–12. doi: 10.1016/s0168-9452(00)00232-6. [DOI] [PubMed] [Google Scholar]
- 37.Mittler R, Feng X, Cohen M. Post-transcriptional suppression of cytosolic ascorbate peroxidase expression during pathogen-induced programmed cell death in tobacco. Plant Cell. 1998;10:461–473. doi: 10.1105/tpc.10.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panavas T, Rubinstein B. Oxidative events during programmed cell death of daylily (Hemerocallis hybrid) petals. Plant Science. 1998;133:125–138. [Google Scholar]
- 39.Hossain Z, Mandal AK, Kumar Datta S, Krishna Biswas A. Decline in ascorbate peroxidase activity--a prerequisite factor for tepal senescence in gladiolus. J Plant Physiol. 2006;163:186–194. doi: 10.1016/j.jplph.2005.03.004. [DOI] [PubMed] [Google Scholar]