Abstract
Purpose
The short-term displacement and reproducibility of the breast or chest wall, and the internal mammary (IM), infraclavicular (ICV), and supraclavicular (SCV) nodal regions have been assessed as a function of breath-hold state using an active breathing control (ABC) device for patients receiving loco-regional breast radiation therapy.
Methods
Ten patients were CT scanned using an ABC device at breath hold states of end exhale and 20%, 40%, 60%, and 80% of vital capacity (VC). Patients were scanned before treatment and at one-third and two-thirds of the way through treatment. A regional registration was performed for each target using a rigid-body transformation with mutual information as a metric.
Results
Between exhale and 40% of VC, the mean displacement was 0.27/0.34, 0.24/0.31, 0.22/0.19, and 0.13/0.19 cm anterior/superior for the breast or chest wall, and IM, ICV, and SCV nodes, respectively. At 80% of VC, the mean displacement from exhale was 0.84/.88, 0.76/.79, 0.70/0.79, and 0.54/0.56 cm anterior/superior for the breast or chest wall, and IM, ICV, and SCV nodes, respectively. The short-term reproducibility (standard deviation) was < 0.3 and ≤0.4 cm for 40% and 80% of VC, respectively. Displacements up to 1.9 cm were seen for individual patients.
Conclusions
The short-term reproducibility of target position is ≤0.4 cm using ABC for all structures for all breath hold states. This information can be used to guide treatment planning optimization studies that consider the effect of motion on target and normal tissue doses with and without active breathing control.
Keywords: radiation planning, breast cancer, heart, short-term reproducibility, active breathing control
INTRODUCTION
Randomized trials have demonstrated a significant improvement in overall survival in high-risk breast cancer patients treated with loco-regional radiotherapy.1–4 The regional nodes including the supraclavicular and internal mammary nodes were included in the target volume in many of these studies. While the degree of benefit from treatment of these nodes is unclear and is currently the subject of on-going international trials, the potential for cardiac and pulmonary toxicity, particularly with internal mammary node treatment, is clear in the absence of precise treatment planning techniques.2, 5–7 To evaluate the utility of techniques such as deep inspiration breath hold (DIBH) in reducing heart and lung doses, the overall displacement and short-term reproducibility of positioning of the breast, internal mammary (IM), infra-clavicular (ICV), and supra-clavicular (SCV) nodal regions with active breathing control (ABC) were measured as a function of breath hold state with respect to end exhale.
Previous studies have investigated the impact of ABC on the reproducibility of the lungs and the carina8 as well as the dosimetric impact of ABC, at normal exhale, normal inhale, and/or DIBH, to reduce dose to the heart and/or lungs for breast cancer patients.9–11 However, these studies did not investigate the reproducibility of positioning of all targets for breast cancer patients. Additional studies have focused on the reproducibility of using ABC for positioning of the lung12 and liver13,14 in other targeted radiation areas.
Therefore, the goals of this work are (1) to evaluate the displacement of the breast or chest wall and the IM, ICV, and SCV nodal regions as a function of breath hold state with respect to the end exhale state and (2) to verify the reproducibility of all targets using ABC for patients receiving loco-regional breast cancer irradiation. The first part of the study provides information about the magnitude of target displacement in relation to breathing state. This information represents a subset of the changes that occur during continuous normal breathing at defined points in the respiratory cycle, and is important since many patients are treated breathing freely, without any breathing instructions. For the second part of the study, we are especially interested in the reproducibility of position for targets at DIBH as studies suggest reduction in dose to normal tissues, particularly the heart, using DIBH techniques. A rigid body registration was done using mutual information as the metric to evaluate the short-term reproducibility of the breast or chest wall, the IM, ICV, and SCV nodal regions at multiple breath hold states.
METHODS AND MATERIALS
Following approval of the Institutional Review Board, informed consent was obtained for patients who required loco-regional radiotherapy for treatment of breast cancer after breast conservative surgery (lumpectomy and axillary lymph node dissection) or after mastectomy. Patients were positioned supine on a carbon fiber breast board with head holder and above the head arm rests with indexed positions (Sinmed BV, The Netherlands). Patients were scanned using a high-speed 16-slice CT scanner (Lightspeed, General Electric, Milwaukee WI) in the treatment position on a flat table top. An ABC device (vMax, Sensormedics, Yorba Linda, CA) was used to control breath hold at breathing states of end exhale, and at 20%, 40%, 60%, and 80% of vital capacity (VC). Using a spirometer, the ABC device suspends breathing at a specific fraction of the patient’s lung volume determined from the measurement of the patient’s VC. Our observations of patient’s breathing showed that normal breathing demonstrated inhale peaks typically in the range of 20–40% of VC (above the expiratory reserve volume). Deep breathing was defined at 75–80% of VC, depending on the patients’ ability to maintain a breath hold multiple times at these levels. Patients were trained to use the device prior to receiving the first session CT scan. The series of scans were made prior to treatment (for planning), and after approximately 1/3 and 2/3 of the treatment period. Thus, a total of 15 datasets of approximately 140 images each were obtained per patient. Intra-venous contrast was administered for the first imaging session.
For this study, we utilized information obtained from the first 10 sequential patients enrolled in the study whose scans were adequate for analysis. Four of these patients had undergone mastectomy and 6 had undergone lumpectomy. The breast or chest wall and the IM, ICV, and SCV nodes were identified and contoured by a physician in UMPlan, our in-house treatment planning system.15
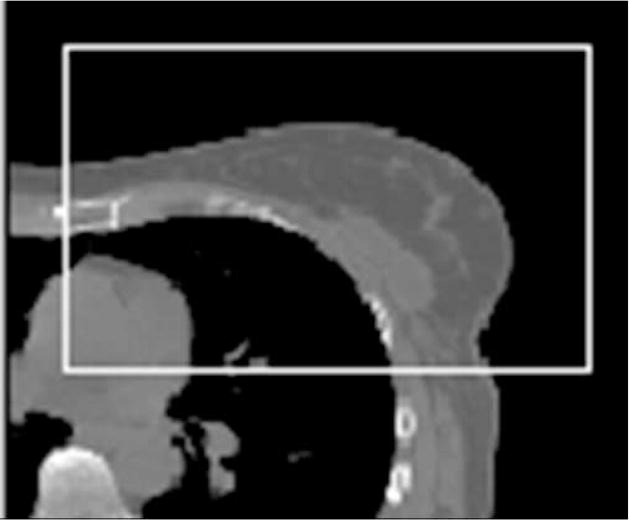
To permit regional alignment primarily based on the structures for which displacement was to be measured, a region-of-interest (ROI) was defined on the exhale dataset (used as the reference dataset) that was slightly larger than the contours (see Figure 1a for the breast region in one plane) in all dimensions. The voxels outside of this region on the reference data were excluded from calculation of the goodness-of-fit metric during alignment. The contrast range contributing to the MI metric was set to emphasize soft tissue differences for the regional alignment and to emphasize bone for the spine-to-spine alignment. For each region of interest, alignment to the end exhale state of each session was performed using a 3-D regional rigid alignment tool for translation and rotation developed at the Netherlands Cancer Institute.16 The alignment was reviewed visually on the axial, coronal, and sagittal planes (Figure 1b) to verify the goodness-of-fit based on anatomy within that ROI. The displacement as a function of breath-hold state was determined for a reference point placed approximately in the center of each region of interest on the exhale dataset.
Figure 1.
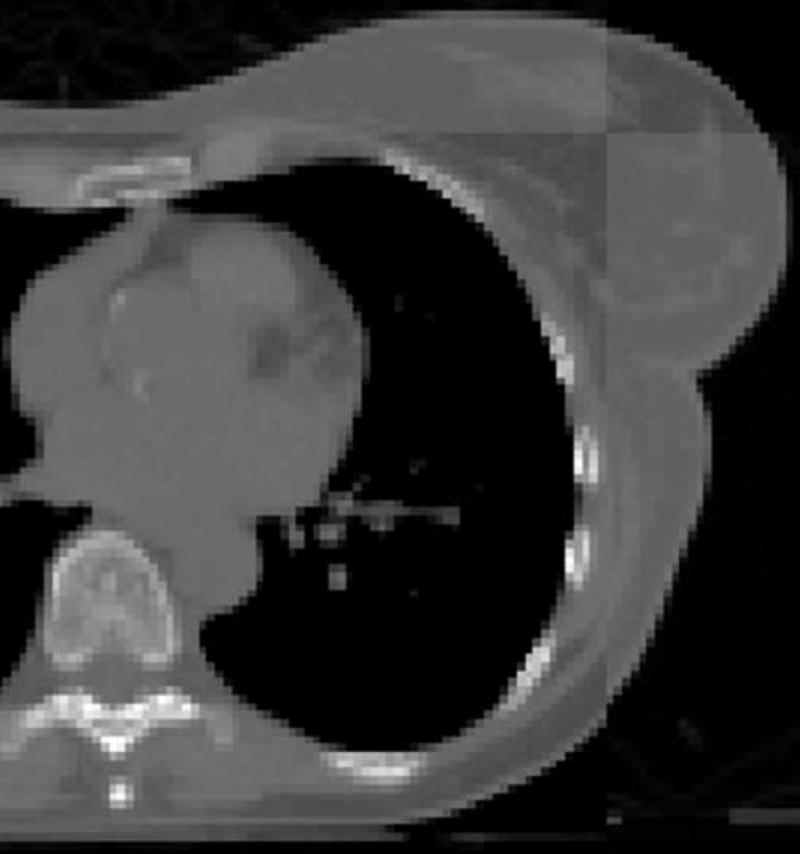
(a) Cropped region for alignment of the breast on an axial slice of the exhale dataset for one patient. (b) Checkerboard view showing alignment between exhale and 20% states for one patient.
The short-term reproducibility was assessed by measuring the displacement of each region of interest after rigid body registration. The 20, 40, 60, and 80% VC scans of session 1 were registered to the session 1 exhale scan. Similarly, the 20, 40, 60, and 80% VC scans of session 2 were registered to the session 2 exhale scan. This process was repeated for the scans from session 3. The mean intra-session displacement was determined with respect to the end exhale scan set of each session. The short-term reproducibility for each breath hold state was determined from the standard deviation of measurements from all patients over the 3 scan sessions.
RESULTS
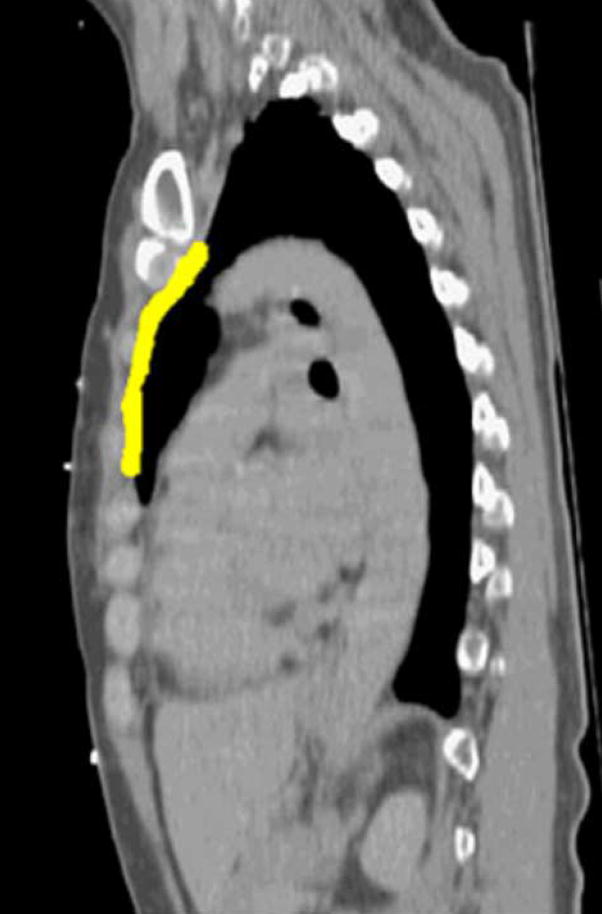
For the ten patients studied, the mean displacement of the breast and nodal targets with respect to position at end exhale was anisotropic, with minimal (but measurable) left-right displacement, and larger average displacements in the anterior-posterior (A-P) and inferior-superior (I-S) directions (up to 0.9 cm) over all breathing states as shown in Table I. At each breath hold state, the mean and SD are determined for 30 data points. Figure 2 shows an example of the range of displacement in the sagittal plane for the IM node region (highlighted).
Table I.
Displacement and short-term reproducibility for motion of the breast/chestwall and relevant nodal regions based on measurements for 10 patients using active breathing control.
| Breast/chest wall Motion | ||||||
|---|---|---|---|---|---|---|
| Breathing State (%) | Left-Right (cm) | Anterior-Posterior (cm) | Inferior-Superior (cm) | |||
| Displacement | SD | Displacement | SD | Displacement | SD | |
| 20 | 0.02 | 0.06 | 0.01 | 0.18 | 0.03 | 0.23 |
| 40 | 0.02 | 0.09 | −0.27 | 0.31 | −0.34 | 0.29 |
| 60 | 0.02 | 0.12 | −0.55 | 0.35 | −0.67 | 0.33 |
| 80 | 0.05 | 0.14 | −0.84 | 0.40 | −0.88 | 0.29 |
| Internal Mammary Nodes | ||||||
| Breathing State (%) | Left-Right (cm) | Anterior-Posterior (cm) | Inferior-Superior (cm) | |||
| Displacement | SD | Displacement | SD | Displacement | SD | |
| 20 | 0.01 | 0.09 | 0.01 | 0.18 | 0.03 | 0.22 |
| 40 | 0.04 | 0.16 | −0.24 | 0.24 | −0.31 | 0.28 |
| 60 | 0.03 | 0.12 | −0.57 | 0.30 | −0.62 | 0.30 |
| 80 | 0.04 | 0.19 | −0.76 | 0.24 | −0.79 | 0.25 |
| Infraclavicular Nodes | ||||||
| Breathing State (%) | Left-Right (cm) | Anterior-Posterior (cm) | Inferior-Superior (cm) | |||
| Displacement | SD | Displacement | SD | Displacement | SD | |
| 20 | 0.00 | 0.05 | 0.04 | 0.16 | 0.05 | 0.15 |
| 40 | 0.02 | 0.10 | −0.22 | 0.25 | −0.19 | 0.22 |
| 60 | 0.04 | 0.16 | −0.50 | 0.29 | −0.42 | 0.20 |
| 80 | 0.03 | 0.18 | −0.70 | 0.25 | −0.56 | 0.20 |
| Supraclavicular Nodes | ||||||
| Breathing State (%) | Left-Right (cm) | Anterior-Posterior (cm) | Inferior-Superior (cm) | |||
| Displacement | SD | Displacement | SD | Displacement | SD | |
| 20 | 0.04 | 0.07 | 0.04 | 0.11 | 0.06 | 0.10 |
| 40 | 0.05 | 0.13 | −0.13 | 0.19 | −0.03 | 0.10 |
| 60 | 0.05 | 0.14 | −0.32 | 0.23 | −0.16 | 0.11 |
| 80 | 0.08 | 0.17 | −0.54 | 0.34 | −0.25 | 0.12 |
Figure 2.
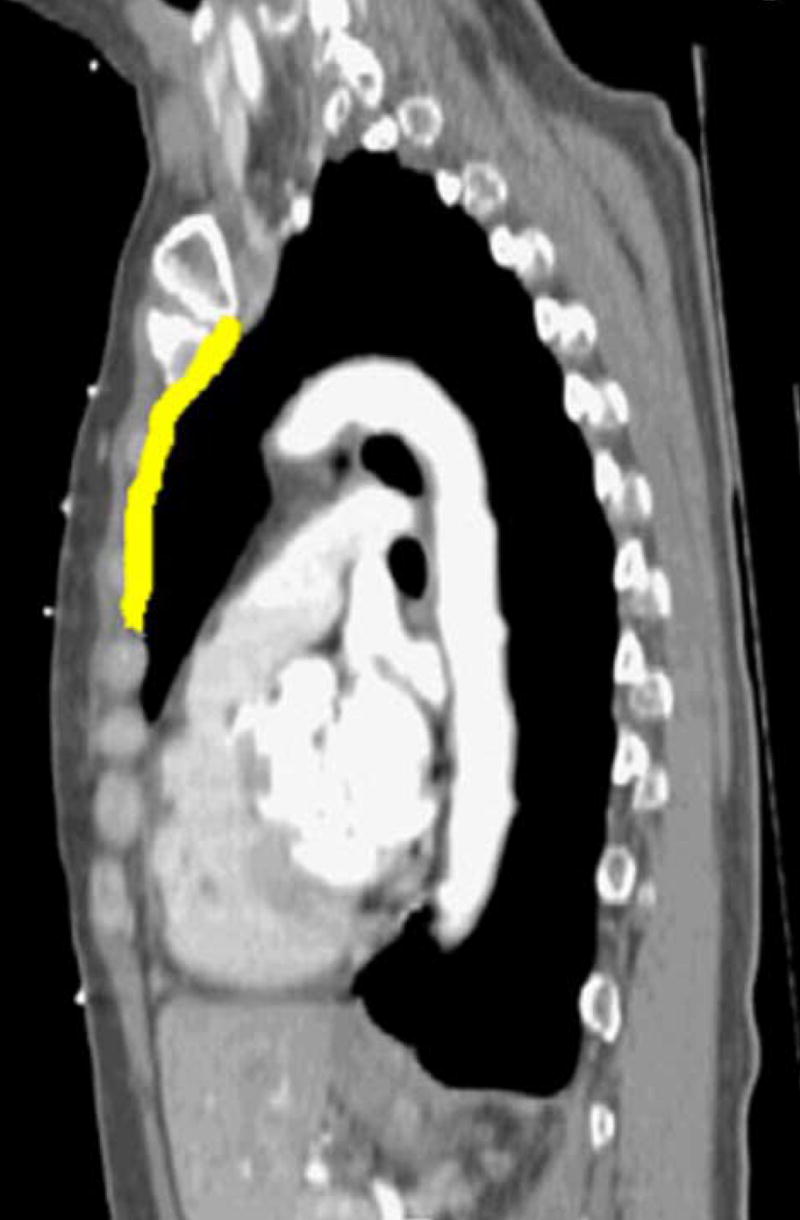
Motion of IM nodes from (a) exhale to (b) 80% of vital capacity in a sagittal plane for one patient and scan session.
The mean displacement and SD of the breast or chest wall was 0.27 (0.31) cm anterior, and 0.34 (0.29) cm superior at 40% of VC compared to 0.84 (0.40) cm anterior and 0.88 (0.29) cm superior at 80% of VC. For the IM node region, the mean displacement and SD was 0.24 (0.24) cm anterior and 0.31 (0.28) cm superior at 40% of VC compared to 0.76 (0.24) cm anterior and 0.79 (0.25) cm superior at 80% of VC. For the ICV, the mean displacement and SD was 0.22 (0.25) cm anterior and 0.19 (0.22) cm superior at 40% VC compared to 0.70 (0.25) cm anterior and 0.56 (0.20) cm superior at 80% VC. Less displacement and slightly better reproducibility were seen for the SCV, where the mean displacement and SD were 0.13 (0.19) cm anterior and 0.03 (0.10) cm superior at 40% of VC compared to 0.54 (0.34) cm anterior and 0.25 (0.12) cm superior at 80% of VC.
The maximum displacements seen for individual patients at 80% of VC are presented in Table II. Such patients may receive a greater benefit to the use of ABC to improve the reproducibility of positioning of the targets at DIBH.
Table II.
Maximum displacements at a breath hold state of 80% of VC with respect to end exhale.
| Target | Displacement (cm) | ||
|---|---|---|---|
| Left-Right | Anterior-Posterior | Inferior-Superior | |
| Breast or chestwall | 0.33 | −1.6 | −1.3 |
| IM nodes | −0.6 | −1.5 | −1.9 |
| ICV nodes | −0.23 | −1.1 | −0.9 |
| SCV nodes | −0.32 | −1.0 | −0.7 |
To demonstrate variations of displacements for individual patients, the mean and standard deviations for each target for patients who had minimal and maximal average displacements at 80% of VC are presented in Tables III and IV, respectively. The short-term reproducibility of target positioning was up to 0.2 cm for the patient with minimum overall displacement of the treatment targets (< 0.4 cm) and ranged from 0.22–0.51 cm for the patient with maximum overall displacement of the treatment targets (up to 1.3 cm anteriorly and 1.57 cm superiorly). Slightly less maximum displacement (up to 0.72 cm anteriorly and 0.24 cm superiorly) was seen for the SCV nodes.
Table III.
Average displacement and standard deviation for a single patient at 80% of VC with minimal movement.
| Target | Left-Right (cm) | Anterior-Posterior (cm) | Inferior-Superior (cm) | |||
|---|---|---|---|---|---|---|
| Displacement | SD | Displacement | SD | Displacement | SD | |
| Breast or chestwall | 0.09 | 0.09 | −0.14 | 0.09 | −0.29 | 0.13 |
| IM nodes | −0.02 | 0.15 | −0.36 | 0.15 | −0.36 | 0.21 |
| ICV nodes | −0.06 | 0.19 | −0.31 | 0.21 | −0.30 | 0.10 |
| SCV nodes | −0.11 | 0.08 | 0.04 | 0.12 | −0.01 | 0.09 |
Table IV.
Average displacement and standard deviation for a single patient at 80% of VC with maximal movement.
| Target | Left-Right (cm) | Anterior-Posterior (cm) | Inferior-Superior (cm) | |||
|---|---|---|---|---|---|---|
| Displacement | SD | Displacement | SD | Displacement | SD | |
| Breast or chestwall | 0.04 | 0.27 | −1.3 | 0.51 | −1.57 | 0.49 |
| IM nodes | −0.07 | 0.45 | −1.29 | 0.45 | −1.27 | 0.22 |
| ICV nodes | −0.18 | 0.4 | −1.03 | 0.38 | −0.87 | 0.36 |
| SCV nodes | 0.03 | 0.25 | −0.72 | 0.37 | −0.24 | 0.14 |
DISCUSSION
When using ABC, we found that the mean displacement and reproducibility of IM position in all directions to be approximately 0.2 –0.4 cm (Table I) at breath hold states ranging from exhale to 40% of VC. To account for the systematic baseline shift that occurs over the course of treatment, due to normal variation of the inhale volume over time, other approaches, such as imaging, are required for target localization. Due to the small variability in displacement, ABC does not seem to offer any advantages at shallow breath hold states. When considering the displacement from end exhalation to DIBH (80% of VC), the targets moved up to 1.9 cm. Again, due to changes in the baseline position of exhale with time, localization approaches are required prior to treatment. Because the reproducibility (σ) of positioning with ABC at DIBH for all targets ranged between 0.14–0.34 cm (Table I), these values allow for smaller margins and allow for treatment planning advantages in minimizing the amount of heart and lung in the treatment fields.
Remouchamps et al. focused on quantifying the overall movement of the lung and chest wall for radiotherapy of breast cancer patients with ABC immobilization at moderate deep inspiration breath hold.8 They evaluated motion and reproducibility for ABC patients immobilized with and without an alpha cradle and found the lung reproducibility to be approximately 0.22 cm.8 When they performed a regional analysis, they found better immobilization for the upper two-thirds of the lungs. The results presented here for a regional alignment are comparable to the findings from that study (0.3 cm).
The detailed displacement and reproducibility information obtained from this study can be used to create appropriate margins for PTVs for patients receiving loco-regional breast cancer therapy using an ABC device to control patient breath hold. For example, this information could be incorporated into approaches with a margin recipe to determine the appropriate PTV for a given confidence interval.17, 18 Schwarz et al. have evaluated the impact of geometrical uncertainties on lung cancer tumors using periodic models for breathing.19 The information from this study can also be used to evaluate optimization approaches, such as the Multiple Instance Geometry Approximation,20 that derive more robust treatment plans by taking known or expected motions into account.
CONCLUSIONS
These data demonstrate that the short-term reproducibility of breast or chest wall, IM, ICV, and SCV node position via active breathing control at various breathing states is less than 0.4 cm. This information can be used to guide treatment planning optimization studies that are designed to consider the effect of motion on target and normal tissue doses with and without active breathing control.
Acknowledgments
This work was sponsored by NIH R21 CA099254 and P01-CA59827. The authors would like to thank Barb Harber and Monika Benedict-Blue for their assistance in this study.
Footnotes
This work was presented in part at the Annual Meeting of the American Society for Therapeutic Radiology and Oncology, October 2004 (Atlanta, Georgia).
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 2.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen HM, Overgaard M, Grau C, et al. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol. 2006;24:2268–2275. doi: 10.1200/JCO.2005.02.8738. [DOI] [PubMed] [Google Scholar]
- 5.Corn BW, Trock BJ, Goodman RL. Irradiation-related ischemic heart disease. J Clin Oncol. 1990;8:741–750. doi: 10.1200/JCO.1990.8.4.741. [DOI] [PubMed] [Google Scholar]
- 6.Fuller SA, Haybittle JL, Smith RE, et al. Cardiac doses in post-operative breast irradiation. Radiother Oncol. 1992;25:19–24. doi: 10.1016/0167-8140(92)90190-6. [DOI] [PubMed] [Google Scholar]
- 7.Paszat LF, Mackillop WJ, Groome PA, et al. Mortality from myocardial infarction following postlumpectomy radiotherapy for breast cancer: a population-based study in Ontario, Canada. Int J Radiat Oncol Biol Phys. 1999;43:755–762. doi: 10.1016/s0360-3016(98)00412-x. [DOI] [PubMed] [Google Scholar]
- 8.Remouchamps VM, Letts N, Yan D, et al. Three-dimensional evaluation of intra- and interfraction immobilization of lung and chest wall using active breathing control: a reproducibility study with breast cancer patients. Int J Radiat Oncol Biol Phys. 2003;57:968–978. doi: 10.1016/s0360-3016(03)00710-7. [DOI] [PubMed] [Google Scholar]
- 9.Sixel KE, Aznar MC, Ung YC. Deep inspiration breath hold to reduce irradiated heart volume in breast cancer patients. Int J Radiat Oncol Biol Phys. 2001;49:199–204. doi: 10.1016/s0360-3016(00)01455-3. [DOI] [PubMed] [Google Scholar]
- 10.Remouchamps VM, Vicini FA, Sharpe MB, et al. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55:392–406. doi: 10.1016/s0360-3016(02)04143-3. [DOI] [PubMed] [Google Scholar]
- 11.Frazier RC, Vicini FA, Sharpe MB, et al. Impact of breathing motion on whole breast radiotherapy: a dosimetric analysis using active breathing control. Int J Radiat Oncol Biol Phys. 2004;58:1041–1047. doi: 10.1016/j.ijrobp.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Wilson EM, Williams FJ, Lyn BE, et al. Validation of active breathing control in patients with non-small-cell lung cancer to be treated with CHARTWEL. Int J Radiat Oncol Biol Phys. 2003;57:864–874. doi: 10.1016/s0360-3016(03)00712-0. [DOI] [PubMed] [Google Scholar]
- 13.Dawson LA, Brock KK, Kazanjian S, et al. The reproducibility of organ position using active breathing control (ABC) during liver radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51:1410–1421. doi: 10.1016/s0360-3016(01)02653-0. [DOI] [PubMed] [Google Scholar]
- 14.Eccles C, Brock KK, Bissonnette JP, et al. Reproducibility of liver position using active breathing coordinator for liver cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64:751–759. doi: 10.1016/j.ijrobp.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 15.Fraass BA, McShan DL. The use of computers in radiation therapy. Elsevier Science Publishers BV; 1987. 3-D treatment planning: I. Overview of a clinical planning system; pp. 273–276. [Google Scholar]
- 16.Wolthaus JW, van Herk M, Muller SH, et al. Fusion of respiration-correlated PET and CT scans: correlated lung tumour motion in anatomical and functional scans. Phys Med Biol. 2005;50:1569–1583. doi: 10.1088/0031-9155/50/7/017. [DOI] [PubMed] [Google Scholar]
- 17.van Herk M, Remeijer P, Lebesque JV. Inclusion of geometric uncertainties in treatment plan evaluation. Int J Radiat Oncol Biol Phys. 2002;52:1407–1422. doi: 10.1016/s0360-3016(01)02805-x. [DOI] [PubMed] [Google Scholar]
- 18.van Herk M, Remeijer P, Rasch C, et al. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–1135. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz M, Van der Geer J, Van Herk M, et al. Impact of geometrical uncertainties on 3D CRT and IMRT dose distributions for lung cancer treatment. Int J Radiat Oncol Biol Phys. 2006;65:1260–1269. doi: 10.1016/j.ijrobp.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 20.McShan DL, Kessler ML, Vineberg K, et al. Inverse plan optimization accounting for random geometric uncertainties with a multiple instance geometry approximation (MIGA) Med Phys. 2006;33:1510–1521. doi: 10.1118/1.2191016. [DOI] [PubMed] [Google Scholar]