Abstract
Monoclonal antibody CS-17 is a TSH receptor (TSHR) inverse agonist (suppresses constitutive activity) and a TSH antagonist. Elucidation of the CS-17 epitope will provide insight into TSHR structure and function. Present information on its epitope conflicts with recent data regarding another TSHR inverse agonist antibody. To characterize further the CS-17 epitope, we exploited the observation that CS-17 does not recognize a chimeric receptor with TSHR hinge region residues 261–289 replaced with homologous rat LH receptor residues (13 mismatches). We generated individual and double TSHR mutations corresponding to these mismatches. On flow cytometry, only T273L/R274V reduced CS-17 recognition. No mutation affected TSH-stimulated cAMP generation. Because the immunogen for CS-17 generation was highly glycosylated, we also investigated whether the glycan moiety at N198, topologically adjacent to Y195 (a previously identified epitopic component), could contribute to the CS-17 epitope. Elimination of this N-linked glycan (mutations of N198 and T200) abrogated CS-17 binding without altering TSH responsiveness. However, studies with tunicamycin suggested that these mutations affected CS-17 binding by altering the polypeptide backbone rather than eliminating the glycan moiety. TSHR residues N198 and T200, like Y195, are on the convex facet of the leucine-rich domain. In summary, the present data indicate that the discontinuous epitope of CS-17, a TSHR inverse agonist and TSH antagonist, includes a component in the hinge region as well as the convex surface of the TSHR leucine-rich domain. These findings expand our present concept of glycoprotein hormone binding and function.
The discontinuous epitope of CS-17, a TSH receptor mAb with inverse agonist and TSH antagonist activities, includes a component in the TSH receptor hinge, expanding our present concept of glycoprotein hormone binding and function.
The TSH receptor (TSHR) plays a critical role in thyroid function in health and disease (reviewed in Refs. 1 and 2). The physiological negative feedback mechanism between thyroid hormones and TSH secretion by the pituitary contributes to the maintenance of euthyroidism. This equilibrium is subverted by thyroid-stimulating autoantibodies that activate the TSHR in Graves’ disease leading to hyperthyroidism (3,4). More rarely, competitive antagonists of TSH binding to its receptor cause hypothyroidism (5). The TSHR also maintains relatively high activity even in the absence of ligand (6). Such constitutive activity may reduce the efficacy of TSH suppressive therapy for metastatic well-differentiated thyroid carcinomas. TSHR somatic (7) or germ line (8) mutations can up-regulate constitutive activity leading to “toxic” thyroid adenomas or familial, nonautoimmune hyperthyroidism.
Besides agonists (thyroid-stimulating autoantibodies) and antagonists (TSH blocking antibodies), some TSHR antibodies function as inverse agonists, defined as the ability to reduce ligand-independent TSHR constitutive activity. However, in general pharmacological terms, these functions are not clearly separable. A weak agonist can be an antagonist, and many antagonists are not “neutral” but also have inverse agonist activity (reviewed in Ref. 9). Two monoclonal TSHR antibodies with inverse agonist activity have recently been reported: murine CS-17 (10) and human 5C9 (11). Both are also TSH antagonists. TSHR inverse agonists may be of clinical value, e.g. in well-differentiated thyroid carcinoma (10). Because of ligand-independent constitutive activity, even total suppression of TSH with high doses of T4 can only partially reduce TSHR activity in residual thyroid carcinoma cells. Understanding the mechanism by which TSHR antibodies with inverse agonist activity suppress constitutive activity can also provide valuable insight into the TSHR structure-function relationship. To attain this goal, it is necessary to identify the epitopes for the TSHR inverse agonist antibodies; not an easy task because, like most antibodies to large proteins (12), both CS-17 and 5C9 have conformational, discontinuous epitopes that cannot be localized by binding to synthetic peptides (10,11). Although TSHR mutagenesis studies have identified a few amino acid residues likely to be involved in the binding sites for both monoclonal antibodies (mAb) (11,13), much additional work is required to more fully delineate these epitopes.
The approximate location of the CS-17 epitope on the TSHR ectodomain has been deduced from several lines of evidence. First, CS-17 was generated by immunization with TSHR residues 22–289 (residues 1–21 being the signal peptide) (10). This immunogen includes the entire TSHR leucine-rich domain (LRD) and the proximal portion of the hinge region, also known as the signaling specificity domain (SSD) (14). Together, the LRD and proximal SSD comprise the TSHR A subunit, formed by intramolecular cleavage of TSHR on the cell surface into disulfide linked A and B subunits, with deletion of an imprecisely defined loop (C-peptide region) (reviewed in Ref. 2). Second, studies on CS-17 recognition of chimeric receptors, in which segments of the human TSHR are substituted with the homologous regions of the rat LH receptor (LHR), further narrowed the CS-17 epitope to residues 170–289 (10). Finally, based on the fortuitous observation that CS-17 recognizes the human but not the porcine TSHR, replacing human with porcine amino acids within the residue 170–289 “window” revealed TSHR Y195 to be important for CS-17 binding (13). Mutation of residues Q235 and S243 together (but not individually) reduced CS-17 binding to a much lesser extent.
Identification of TSHR residue Y195 in the epitope of mAb CS-17 had interesting implications for understanding TSHR structure and function. This residue is on the convex surface of the LRD. In contrast, binding of mAb 5C9 (also a TSH binding antagonist and a functional inverse agonist) was diminished by mutagenesis of residues on the opposite, concave, facet of the TSHR LRD (11). The present study reinforces the deduction that CS-17, a TSH antagonist, binds to the convex surface of the TSHR LRD, an observation that poses questions regarding the prevalent concept for the location of the glycoprotein hormone binding sites (15,16). Moreover, we provide the first evidence that a TSHR inverse agonist interacts with the enigmatic SSD (or hinge region) linking the TSHR LRD with the hepta-helical membrane-spanning segment.
Materials and Methods
TSHR mutation and expression
Introduction of the wild-type TSHR cDNA (17) (with the H601 polymorphism converted to Y601) into the vector pcDNA5/FRT (Invitrogen Corp., Carlsbad, CA) was described previously, as was the TSHR with the combined Q235Y/S243P mutation (13). The mutations described below in the text (Results) were introduced using the QuikChange site-directed mutagenesis kit (Stratagene, San Diego, CA). All mutations were confirmed by nucleotide sequencing. Although the vector is designed for stable transfections, because of difficulty in attaining this goal, we transiently transfected Chinese hamster ovary (CHO) cells with FuGENE HD according to the protocol of the manufacturer (Roche Molecular Biochemicals, Indianapolis, IN). Cells were cultured in Ham’s F12 medium supplemented with 10% fetal calf serum, penicillin (100 U/ml), gentamycin (50 μg/ml), and Fungizone (2.5 μg/ml; University of California San Francisco cell culture facility, San Francisco, CA), passaged the day after transfection and tested the following day.
Flow cytometry
CHO cells were harvested from six-well plates using 1 mm EDTA, 1 mm EGTA in PBS. After washing twice with PBS containing 10 mm HEPES (pH 7.4), 2% fetal bovine serum, and 0.05% NaN3, the cells were incubated for 30 min at room temperature in 100 μl of the same buffer containing 1 μg of either normal mouse IgG or the indicated mAb. After rinsing, the cells were incubated for 45 min with 100 μl fluorescein isothiocyanate-conjugated goat-antimouse IgG (1:100) (Caltag, Burlingame, CA), washed, and analyzed using a Beckman FACScan flow cytofluorimeter (Beckman Coulter, Inc., Fullerton, CA). Cells stained with propidium iodide (1 μg/ml final concentration) were excluded from analysis.
Cultured cell cAMP assays
CHO cells transiently transfected with the wild-type TSHR or TSHR mutants were transferred into 96-well plates approximately 24 h after transfection and 24 h before assay. For bioassay, the culture medium described previously was replaced with F12 medium supplemented with 1 mm isobutyl methylxanthine, 10 mm HEPES, and, where indicated in the text, bovine TSH (bTSH) (Sigma-Aldrich Corp., St. Louis, MO). Mock-transfected CHO cells were included as controls. After 60 min at 37 C, the medium was aspirated, and intracellular cAMP was extracted with 0.2 ml 95% ethanol. The extracts were evaporated to dryness, resuspended in 0.1 ml PBS (pH 7.5), and samples (12 ml) were assayed using the LANCE cAMP kit according to the protocol of the manufacturer (PerkinElmer, Shelton CT). The effective dose of TSH required for half-maximal stimulation of intracellular cAMP levels (EC50) was calculated using GraphPad Prism software (GraphPad Software Inc., San Diego, CA).
TSHR mAb
The generation and purification of murine mAb CS-17 have been described previously (10,13). Nonfunctional murine mAb 2C11 and 4C1 (18) were purchased from Serotec (Oxford, UK).
Results
Contribution of the TSHR hinge region to the CS-17 epitope
Previously, based on CS-17 recognizing the human but not the pig TSHR, TSHR LRD amino acids 195, and to a lesser extent 235 and 243, were identified as contributing to the CS-17 epitope (13). However, identity in the human and pig TSHR amino acid sequences further downstream of residue 243 (up to residue 289, the C terminus of the immunogen) precluded further analysis of the CS-17 epitope based on a human-pig TSHR difference. On the other hand, the inability of CS-17 to recognize chimeric receptor TSH-LHR-6 (10) (Fig. 1A) provided an alternative approach to identifying potential CS-17 epitopic residues between TSHR residues 261 and 289. Within this segment, homology between the rat LHR and human TSHR is relatively poor, with 13 mismatches in 29 amino acid residues (Fig. 1B). It is not known which, or how many, of these residues are responsible for the loss of CS-17 binding to the chimeric receptor. This region is also of particular interest because it represents the N-terminal component of the TSHR hinge region.
Figure 1.
A, Schematic depiction of present information on the CS-17 epitope. Because mAb CS-17 was generated by immunization with TSHR 1–289 (amino acid residues 1–21 being the signal peptide), its epitope must lie between residues 22 and 289. CS-17 recognition of the wild-type TSHR is diminished by mutation of residue 195, and to a much lesser extent by the combined mutation of residues 235 and 243 (13). Mutation of all three residues abolishes CS-17 binding. There is an N-linked glycan at N198 adjacent to Y195. CS-17 also does not recognize chimeric receptor TSH-LHR-6, in which arbitrary TSHR domains D and E (residues 261–418) are replaced by the homologous region of the rat LHR. Therefore, there are likely to be additional components to the CS-17 epitope between residues 261 and 289. B, Homology between human TSHR amino acid residues 261–289 and the rat LHR. Amino acid identity is depicted by a dot. C, Flow cytometric detection of TSHR mutants by mAb CS-17. CHO cells transiently transfected with plasmids expressing TSHR with the indicated mutations [on a background of TSHR Q235Y/S243P (13); modified wild-type TSHR (Mod-wt)] were tested in the same experiment with mAb CS-17 and, as a control, mAb 4C1, whose epitope includes residues 381–384 (18), far downstream of TSHR residue 289 (18) (Materials and Methods). mock, Transfection with empty plasmid. In the bottom right panel, the TSHR with the T273L and R274V mutations was tested with mAb 2C11. The dashed ovals highlight the lack of mAb recognition. This experiment was repeated three times with similar results.
Because an antibody epitope on a large protein typically comprises 15–20 discontinuous amino acid residues, mutation of individual amino acids on the antigen may not result in a readily detectable change in antibody recognition, as was the case for TSHR residues 235 and 243 (13). Therefore, we chose to replace TSHR residues in a slightly “compromised” substrate, namely TSHR-Q235Y/S243P. On this modified wild-type background, we generated a series of seven TSHR mutants in which all 13 nonhomologous amino acid residues were replaced by the corresponding residues in the rat LHR (Fig. 1B). We tested these “mini-chimeric” receptors (six double and one single amino acid substitutions) for CS-17 recognition using flow cytometry (Fig. 1C). Of these substitutions, only the double substitution T273L/R274V markedly reduced CS-17 recognition relative to that of control mAb 4C1, whose epitope includes residues 381–384 (18), far downstream of TSHR residue 289. Because the 4C1 signal was relatively weak with the TSHR T273L/R274V mutant, we confirmed this finding using another mAb, 2C11 (19), whose epitope includes residues 355–358 (18) (Fig. 1C, bottom right panel). Unlike the dual mutation, individual mutations of T273L and R274V did not affect CS-17 recognition (data not shown). The apparent difference between 4C1 and 2C11 recognition of TSHR T273L/R274V is of interest and requires further evaluation. The identified component of the 4C1 epitope is adjacent to Y385, a residue known to be important for TSH binding (20,21). It is possible that other, discontinuous components of the 4C1 epitope lie further upstream and are influenced by the T273L/R274V mutation.
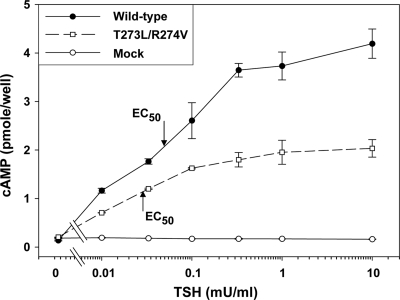
The foregoing data provide evidence that the discontinuous CS-17 epitope includes a portion of the SSD, novel information for the two known TSHR inverse agonist antibodies. Unlike its effect on CS-17 binding, the TSHR T273L/R274V mutation did not reduce the effective dose of TSH required for half-maximal stimulation of intracellular cAMP levels (EC50) (Fig. 2). Neither did any of the other mutations within the TSHR segment 261–289 (Fig. 1B) reduce TSH responsiveness (data not shown). Therefore, although CS-17 is a also competitor for TSH binding and function (10), some of the amino acids deduced to be within the CS-17 epitope are not significantly involved in TSH function.
Figure 2.
TSH stimulation of intracellular cAMP in CHO cells expressing TSHR mutants within the N-terminal portion of the hinge region (amino acid residues 261–289). After transient transfection, aliquots of the same cells used in the experiment shown in Fig. 1C were transferred to 96-well plates and stimulated with the indicated concentrations of bTSH (Materials and Methods). The effective dose of TSH required for half-maximal stimulation of intracellular cAMP levels (EC50) is indicated by the arrows. Each point represents the mean plus range of determinations in duplicate wells. This experiment was repeated twice with similar results.
Potential role of the TSHR N-linked glycan in the CS-17 epitope
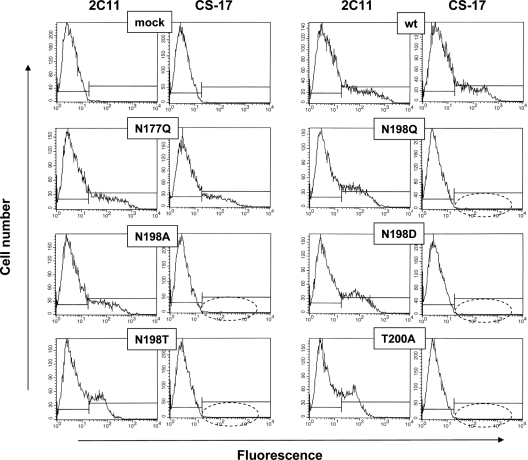
TSHR-289, the immunogen used to generate mAb CS-17, is heavily glycosylated, with glycan comprising nearly half of its mass (22). Of the six N-linked glycans in the TSHR ectodomain, that at N198 is adjacent to Y195 on the convex surface of the LRD (Fig. 3). As mentioned previously, mutation of Y195 substantially reduces CS-17 binding, as detected by flow cytometry without affecting TSH binding (13). Therefore, the question occurred whether a glycan moiety could contribute to the CS-17 epitope. To address this question, we mutated wild-type TSHR residue N198, as well as N177 slightly further upstream, to eliminate the N-linked glycans at these sites. On flow cytometry with transfected CHO cells, TSHR mutants N177Q and N198Q were expressed on the cell surface when detected with mAb 2C11 (Fig. 4). However, CS-17 recognized only N177Q and not N198Q, suggesting that the latter residue contributed to the CS-17 epitope.
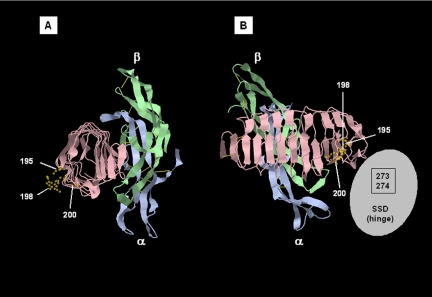
Figure 3.
Ribbon diagram of homologous TSHR residues shown on the three-dimensional structure of FSH bound to the LRD of the FSHR. The FSHR structure is used because, although the TSHR LRD structure has been reported (16), the coordinates have not been entered into the NCBI Structure database but are only available in a patent application (40) that we are unable to use. The FSHR is shown in pink, and the FSH α and β-chains are blue and green, respectively. The data are from Fan and Hendrickson (15), NCBI, Molecular Modeling DataBase no. 31464, and Protein Data Base 1XWD. The structure is shown using Jmol (http://molvis.sdsc.edu/fgij/fg.htm?mol=http://opm.phar.umich.edu/pdb/1xwd.pdb). A, Lateral view looking into the LRD tube from its C-terminal side. B, Posterior view from the convex side of the slightly curved, tubular LRD, the ligand binding on the opposite convex surface. The SSD (or hinge region), whose structure and spatial relationship to the LRD is unknown, is depicted as a gray oval. TSHR amino acid residues 273 and 274 within the SSD comprise a discontinuous component to the CS-17 epitope.
Figure 4.
Flow cytometric detection of TSHR mutants involved the N-linked glycan motif at amino acid residues 198–200. CHO cells transiently transfected with plasmids expressing the wild-type TSHR with the indicated mutations were tested in the same experiment with mAb CS-17 and, as a control, mAb 2C11 whose epitope includes residues 355–358 (18), downstream of the CS-17 epitope (Materials and Methods). mock, Transfection with empty plasmid; wt, wild-type TSHR. The dashed ovals highlight the lack of mAb recognition. This experiment was repeated three times with similar results.
The N198Q mutation could have its effect by altering the TSHR polypeptide backbone or by abolition of the N-linked glycan. To distinguish between these two possibilities, we mutated TSHR N198 to a number of other residues; N198A, N198D, and N198T. All of these mutations behaved identically to N198Q, namely loss of CS-17, but not 2C11 recognition on flow cytometry (Fig. 4). Mutation of T200, the third residue in the N-linked glycan motif to a residue other than serine would also abolish N-linked glycosylation at N198 while leaving the N198 residue intact. Indeed, a T200A mutation in the TSHR abolished CS-17 but not 2C11 recognition (Fig. 4). The EC50 for TSH stimulation of intracellular cAMP levels was unaffected by the N198 and T200 mutations relative to the wild-type TSHR (Fig. 5), as were the EC50 values for the other mutations shown in Fig. 4 (data not shown).
Figure 5.
TSH stimulation of intracellular cAMP in CHO cells expressing TSHR mutated to eliminate the N-linked glycan motif at amino acid residues 198–200. After transient transfection, aliquots of the same cells used in the experiment shown in Fig. 4 were transferred to 96-well plates and stimulated with the indicated concentrations of bTSH (Materials and Methods). The effective dose of TSH required for half-maximal stimulation of intracellular cAMP levels (EC50) is indicated by the arrows. Each point represents the mean plus range of determinations in duplicate wells. This experiment was repeated twice with similar results. Mock, Transfection with empty plasmid.
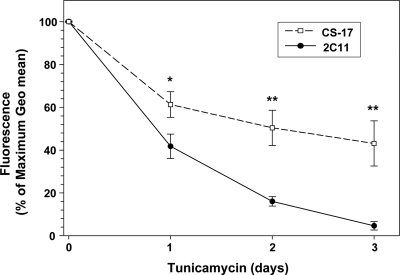
Despite this evidence that a glycan moiety at N198 contributed to the CS-17 epitope, the possibility remained that the N198 and T200 residues themselves, and not glycan, were part of the CS-17 epitope. Therefore, we incubated CHO cells stably expressing the wild-type TSHR for 1, 2, or 3 d with a high concentration of tunicamycin (5 μg/ml), conditions known to greatly reduce or abolish N-linked glycosylation in cells expressing glycoprotein hormone receptors (23,24). A higher concentration or longer duration was not attempted because at the end of 3 d, cells were clearly sick. As expected there was a time-dependent decrease in flow cytometric detection of TSHR on the cell surface using mAb 2C11 as a control (epitope without a glycan component) (Fig. 6). Contrary to providing evidence for glycan epitopic component, the CS-17 flow cytometric signal did not decrease more rapidly over 3 d than the signal of 2C11. Indeed, we consistently observed the reverse (more rapid decline in the 2C11 signal).
Figure 6.
Effect of tunicamycin on mAb recognition of TSHR on the cell surface. CHO cells stably expressing the wild-type TSHR were incubated for up to 3 d in medium supplemented with 5 μg/ml tunicamycin. Medium was replaced each day. Intact cells were then subjected to flow cytometry using mAb CS-17 and 2C11. To combine data from multiple experiments, arbitrary fluorescence units [geometrical (Geo) means] were normalized with maximum values at d 0 expressed as 100%. Each point represents the mean + sd of values from three experiments. *, P < 0.003; **, P < 0.001.
Discussion
TSHR mAb CS-17 is a remarkable reagent in that it represents one of the few antibodies known to possess inverse agonist activity for members of the superfamily of G protein-coupled receptors. Such antibodies have also been described for the b2-adrenergic (25) and M2-muscarinic acetylcholine (26) receptors. Because of the very small extracellular regions of the latter receptors, these inverse agonist antibodies interact with the extracellular loops. In contrast, both mouse mAb CS-17 (10) and human mAb 5C9 (11) bind to the very large ectodomains of the TSHR (397 amino acid residues after signal peptide removal). Because of the importance of TSHR constitutive activity in disease (reviewed in Refs. 10 and 27), characterization of the CS-17 and 5C9 epitopes is of mechanistic interest.
The present data carry the novel message that an inverse agonist for the TSHR, CS-17, interacts with a portion of the receptor’s SSD that links the LRD to the membrane-spanning region. We prefer the term SSD rather than hinge, the former coined by Moyle et al. (14) because it conveys a ligand binding and signaling (not inert) role for this vital component of the glycoprotein hormone receptors. The SSD has largely been ignored in recent years, perhaps because its three-dimensional structure is unknown, unlike the LRDs for the FSH receptor (FSHR) (15) and TSHR (16), whose crystal structures have been determined, and their serpentine transmembrane domains can be modeled on the crystal structures of other G protein-coupled receptors (reviewed in Ref. 28). Indeed, based on the crystal structure of FSH in complex with the FSHR LRD (15), and TSH binding modeled on the crystal structure of the TSHR LRD (29), the glycoprotein hormone receptor SSDs have been largely overlooked despite much experimental data implicating this region in ligand binding and function (reviewed in Refs. 30 and 31). Fortunately, the immunogen for CS-17 included the proximal portion of the TSHR SSD, and we were consequently able to identify TSHR residues T273 and R274 as contributing to the CS-17 epitope. Although the present lack of a structural model for the TSHR SSD precludes the topographical localization of these residues, our data are consistent with increasing evidence that the SSD plays an important role in modulating TSHR constitutive activity (e.g. Refs. 30 and 32). We suggest that CS-17 binding (at least in part) to the SSD stabilizes the TSHR, thereby suppressing constitutive activity.
In addition to implicating the TSHR SSD as contributing to the CS-17 epitope, the present data provide additional insight into its epitopic component in the TSHR LRD. Previous data provided evidence for the involvement of TSHR LRD residues Y195, and to a much lesser extent residues Q235 and S243 (13). These data for CS-17 raised questions regarding the present concept of the glycoprotein hormone receptor binding sites. Besides being an inverse agonist, CS-17 (as well as its Fab fragment) is also an antagonist for TSH binding (10), and TSHR Y195 is present on the convex surface of the TSHR LRD (Fig. 3). This observation requires reconciliation with the present concept of the TSH binding site (the concave surface of the LRD) deduced from the crystal structure of FSH in complex with the FSHR LRD (15), from molecular modeling of TSH in combination with the crystallography-determined TSHR LRD (29), as well as from mutagenesis studies (33). Adding to the uncertainly is a recent report that another TSHR inverse agonist mAb, 5C9 (unlike CS-17), binds to the concave surface of the TSHR LRD (11).
Fortuitously, our focus on a potential glycan component to the CS-17 epitope strengthens the evidence that CS-17 does, indeed, bind to the convex surface of the TSHR LRD. Thus, mutation of either TSHR residues N198 or T200, both on the convex surface of the LRD and adjacent to Y195 (Fig. 3), abrogates CS-17 binding. Nevertheless, TSHR N198 (and even more so T200) is not diametrically opposite the convex surface but is clustered toward the edge of the convex facet. Therefore, it is possible that as yet unidentified residues in the CS-17 epitope do overlap with the TSH binding site. Alternatively, there is a greater likelihood of steric hindrance between the CS-17 Fab and TSH. However, it should be appreciated that the epitope of a Fab, including M22 (a stimulatory TSHR antibody whose crystal structure in complex with the TSHR LRD has been determined), contains approximately 16–24 amino acid residues (12,16). In contrast, the FSH (15) and (modeled) TSH (29) “footprints” on their respective receptors are much larger (45–50 residues). Furthermore, whereas a Fab is an elongated cylindrical molecule that binds via its smaller end facet (e.g. Fig. 3A in Ref. 16), the elongated glycoprotein hormones bind via their flat surfaces. Therefore, despite a Fab having nearly twice the mass of TSH, the binding site of the former is more “focused” and may not readily result in steric hindrance with TSH. Unlike for CS-17, inverse agonist activity for 5C9 has not, to our knowledge, been tested using its Fab component. Therefore, such activity may occur indirectly, e.g. via its Fc region. Assuming that further studies confirm 5C9 binding to the concave surface of the LRD, it could then be inferred that suppression of constitutive activity need not require antibodies with similar epitopes. In this case it will also be of interest to determine whether the 5C9 epitope, like CS-17, has an epitopic component in the TSHR SSD.
TSHR residues N198 and T200 comprise an N-linked glycan motif (NXS/T), and TSHR N198 is known to be glycosylated (34). Whether the glycan at N198 does, indeed, contribute to the CS-17 epitope remains uncertain. Besides a glycan epitope being a rare phenomenon, eliminating N-linked glycosylation by mutagenesis also alters the amino acid structure. It is not possible to enzymatically remove N-linked glycan from mature TSHR on the cell surface without destroying the cells and denaturing the protein. Enzymatically inhibiting glycan incorporation during TSHR synthesis with tunicamycin greatly reduces or abolishes the trafficking of functional TSHR to the cell surface (24). With this background, our goal in using tunicamycin was to determine whether the time course of TSHR disappearance from the cell surface would be more rapid when assessed using CS-17 vs. a mAb (2C11), whose epitope does not contain glycan. Such an observation would support an N-linked glycan contributing to the CS-17 epitope. Indeed, the opposite occurred. The decay in flow cytometric detection of cell surface TSHR was more rapid with 2C11 than CS-17. This observation is consistent with the visible toxicity of tunicamycin on the cells. TSHR cleavage and loss of the C-peptide region (the 2C11 epitope) are accelerated with “unhealthy” cells in culture (35).
The TSHR, like other glycoprotein hormone receptors, can exist as multimers (probably dimers) in the plasma membrane (36,37). There are conflicting data as to whether TSH binding influences receptor multimerization (37,38). However, TSH binding to TSHR homodimers can generate allosteric changes, leading to reduced affinity for ligand (negative cooperativity) (37). Previously, even in the absence of ligand, a progressive increase in the number of TSHR expressed on the cell surface was observed to be associated with a reciprocal decrease in the affinity for ligand. Because this phenomenon suggested allosteric effects consequent to TSHR-TSHR interactions, it was also termed negative cooperativity (39). With this background it is interesting to consider whether CS-17 antagonism of TSH binding and inverse agonist properties are related to TSHR multimerization. It should be noted that monomeric CS-17 Fab and divalent IgG molecules have equimolar potency for both properties (13). However, we cannot exclude the possibility that CS-17 competition for TSH binding or suppression of constitutive activity are, by an unknown mechanism, related to TSHR dimerization.
Parenthetically, an explanation is required as to why we have depicted relevant TSHR amino acids on the three-dimensional structure of the FSHR rather than the TSHR LRD (Fig. 3). Although the latter structure has been reported (16), the coordinates have only been provided in a patent application (40) rather than in the National Center for Biotechnology Information (NCBI) Structure database. From this information, we (and others) are unable to generate a three-dimensional model of the TSHR LRD. Nevertheless, the highly similar structures of the TSHR and FSHR LRD regions (15,16) make TSHR amino acid localization using the FSHR structure quite accurate.
In conclusion, the present study provides novel information on the epitope of mAb CS-17, a TSHR inverse agonist and TSH antagonist. Interaction between an inverse agonist antibody with the TSHR SSD (or hinge region) has not been previously reported. Furthermore, our data provide additional strong evidence that the CS-17 epitope includes a discontinuous segment on the convex surface of the TSHR LRD, raising questions regarding the present concept of glycoprotein hormone binding and function.
Acknowledgments
We are grateful for contributions to our laboratory by Dr. Boris Catz (Los Angeles, CA).
Footnotes
This work was supported by National Institutes of Health Grants DK 19289 (to B.R.) and DK 54684 (to S.M.M.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 19, 2009
Abbreviations: CHO, Chinese hamster ovary; FSHR, FSH receptor; LHR, LH receptor; LRD, leucine-rich domain; mAb, monoclonal antibody (or antibodies); NCBI, National Center for Biotechnology Information; SSD, signaling specificity domain; TSHR, TSH receptor.
References
- Vassart G, Dumont JE 1992 The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev 13:596–611 [DOI] [PubMed] [Google Scholar]
- Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM 1998 The thyrotropin receptor: interaction with thyrotropin and autoantibodies. Endocr Rev [Erratum (1999) 20:100] 19:673–716 [DOI] [PubMed] [Google Scholar]
- Adams DD, Purves HD 1956 Abnormal responses in the assay of thyrotropins. Proc Univ Otago Med Sch 34:11–12 [Google Scholar]
- Kriss JP, Pleshakov V, Chien JR 1964 Isolation and identification of the long-acting thyroid stimulator and its relation to hyperthyroidism and circumscribed pretibial myxedema. J Clin Endocrinol Metab 24:1005–1028 [DOI] [PubMed] [Google Scholar]
- Konishi J, Iida Y, Kasagi K, Misaki T, Nakashima T, Endo K, Mori T, Shinpo S, Nohara Y, Matsuura N 1985 Primary myxedema with thyrotrophin-binding inhibitor immunoglobulins. Clinical and laboratory findings in 15 patients. Ann Intern Med 103:26–31 [DOI] [PubMed] [Google Scholar]
- Cetani F, Tonacchera M, Vassart G 1996 Differential effects of NaCl concentration on the constitutive activity of the thyrotropin and the luteinizing hormone/chorionic gonadotropin receptors. FEBS Lett 378:27–31 [DOI] [PubMed] [Google Scholar]
- Parma J, Duprez L, Van Sande J, Cochaux P, Gervy C, Mockel J, Dumont J, Vassart G 1993 Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature 365:649–651 [DOI] [PubMed] [Google Scholar]
- Duprez L, Parma J, Van Sande J, Allgeier A, Leclère J, Schvartz C, Delisle MJ, Decoulx M, Orgiazzi J, Dumont J, Vassart G 1994 Germline mutations in the thyrotropin receptor gene cause non-autoimmune autosomal dominant hyperthyroidism. Nat Genet 7:396–401 [DOI] [PubMed] [Google Scholar]
- Bond RA, Ijzerman AP 2006 Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci 27:92–96 [DOI] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, Rapoport B 2007 Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology 148:2375–2382 [DOI] [PubMed] [Google Scholar]
- Sanders J, Evans M, Betterle C, Sanders P, Bhardwaja A, Young S, Roberts E, Wilmot J, Richards T, Kiddie A, Small K, Platt H, Summerhayes S, Harris R, Reeve M, Coco G, Zanchetta R, Chen S, Furmaniak J, Smith BR 2008 A human monoclonal autoantibody to the thyrotropin receptor with thyroid stimulating blocking activity. Thyroid 18:735–746 [DOI] [PubMed] [Google Scholar]
- Davies DR, Padlan EA, Sheriff S 1990 Antibody-antigen complexes. Annu Rev Biochem 59:439–473 [DOI] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, Rapoport B 2008 Identification of key amino acid residues in a thyrotropin receptor monoclonal antibody epitope provides insight into its inverse agonist and antagonist properties. Endocrinology 149:3427–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle WR, Xing Y, Lin W, Cao D, Myers RV, Kerrigan JE, Bernard MP 2004 Model of glycoprotein hormone receptor ligand binding and signaling. J Biol Chem 279:44442–44459 [DOI] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA 2005 Structure of human follicle-stimulating hormone in complex with its receptor. Nature 433:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J, Chirgadze DY, Sanders P, Baker S, Sullivan A, Bhardwaja A, Bolton J, Reeve M, Nakatake N, Evans M, Richards T, Powell M, Miguel RN, Blundell TL, Furmaniak J, Smith BR 2007 Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid 17:395–410 [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Kaufman KD, Seto P, Rapoport B 1989 Molecular cloning, sequence and functional expression of the cDNA for the human thyrotropin receptor. Biochem Biophys Res Commun 165:1184–1190 [DOI] [PubMed] [Google Scholar]
- Johnstone AP, Cridland JC, Da Costa CR, Nussey SS, Shepherd PS 2003 A functional site on the human TSH receptor: a potential therapeutic target in Graves’ disease. Clin Endocrinol (Oxf) 59:437–441 [DOI] [PubMed] [Google Scholar]
- Johnstone AP, Cridland JC, DaCosta CR, Harfst E, Shepherd PS 1994 Monoclonal antibodies that recognize the native human thyrotropin receptor. Mol Cell Endocrinol 105:R1–R9 [DOI] [PubMed] [Google Scholar]
- Kosugi S, Ban T, Akamizu T, Kohn LD 1991 Site-directed mutagenesis of a portion of the extracellular domain of the rat thyrotropin receptor important in autoimmune thyroid disease and nonhomologous with gonadotropin receptors. Relationship of functional and immunogenic domains. J Biol Chem 266:19413–19418 [PubMed] [Google Scholar]
- Costagliola S, Panneels V, Bonomi M, Koch J, Many MC, Smits G, Vassart G 2002 Tyrosine sulfation is required for agonist recognition by glycoprotein hormone receptors. EMBO J 21:504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazenbalk GD, Jaume JC, McLachlan SM, Rapoport B 1997 Engineering the human thyrotropin receptor ectodomain from a non-secreted form to a secreted, highly immunoreactive glycoprotein that neutralizes autoantibodies in Graves’ patients’ sera. J Biol Chem 272:18959–18965 [DOI] [PubMed] [Google Scholar]
- Liu X, Davis D, Segaloff DL 1993 Disruption of potential sites for N-linked glycosylation does not impair hormone binding to the lutropin/choriogonadotropin receptor if Asn-173 is left intact. J Biol Chem 268:1513–1516 [PubMed] [Google Scholar]
- Nagayama Y, Namba H, Yokoyama N, Yamashita S, Niwa M 1998 Role of asparagine-linked oligosaccharides in protein folding, membrane targeting, and thyrotropin and autoantibody binding of the human thyrotropin receptor. J Biol Chem 273:33423–33428 [DOI] [PubMed] [Google Scholar]
- Peter JC, Eftekhari P, Billiald P, Wallukat G, Hoebeke J 2003 scFv single chain antibody variable fragment as inverse agonist of the β2-adrenergic receptor. J Biol Chem 278:36740–36747 [DOI] [PubMed] [Google Scholar]
- Peter JC, Wallukat G, Tugler J, Maurice D, Roegel JC, Briand JP, Hoebeke J 2004 Modulation of the M2 muscarinic acetylcholine receptor activity with monoclonal anti-M2 receptor antibody fragments. J Biol Chem 279:55697–55706 [DOI] [PubMed] [Google Scholar]
- Duprez L, Parma J, Van Sande J, Rodien P, Dumont JE, Vassart G, Abramowicz M 1998 TSH receptor mutations and thyroid disease. Trends Endocrinol Metab 9:133–140 [DOI] [PubMed] [Google Scholar]
- Kobilka B, Schertler GF 2008 New G-protein-coupled receptor crystal structures: insights and limitations. Trends Pharmacol Sci 29:79–83 [DOI] [PubMed] [Google Scholar]
- Núñez Miguel R, Sanders J, Jeffreys J, Depraetere H, Evans M, Richards T, Blundell TL, Rees Smith B, Furmaniak J 2004 Analysis of the thyrotropin receptor-thyrotropin interaction by comparative modeling. Thyroid 14:991–1011 [DOI] [PubMed] [Google Scholar]
- Mizutori Y, Chen CR, McLachlan SM, Rapoport B 2008 The thyrotropin receptor hinge region is not simply a scaffold for the leucine-rich domain but contributes to ligand binding and signal transduction. Mol Endocrinol 22:1171–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Kleinau G, Jaeschke H, Paschke R, Krause G 2008 Extended hormone binding site of the human thyroid stimulating hormone receptor: distinctive acidic residues in the hinge region are involved in bovine thyroid stimulating hormone binding and receptor activation. J Biol Chem 283:18048–18055 [DOI] [PubMed] [Google Scholar]
- Kleinau G, Jäschke H, Neumann S, Lättig J, Paschke R, Krause G 2004 Identification of a novel epitope in the thyroid-stimulating hormone receptor ectodomain acting as intramolecular signaling interface. J Biol Chem 279:51590–51600 [DOI] [PubMed] [Google Scholar]
- Smits G, Campillo M, Govaerts C, Janssens V, Richter C, Vassart G, Pardo L, Costagliola S 2003 Glycoprotein hormone receptors: determinants in leucine-rich repeats responsible for ligand specificity. EMBO J 22:2692–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama Y, Nishihara E, Namba H, Yamashita S, Niwa M 2000 Identification of the sites of asparagine-linked glycosylation on the human thyrotropin receptor and studies on their role in receptor function and expression. J Pharmacol Exp Ther 295:404–409 [PubMed] [Google Scholar]
- Tanaka K, Chazenbalk GD, McLachlan SM, Rapoport B 1999 The shed component of the TSH receptor is primarily a carboxyl terminal truncated form of the A subunit, not the entire A subunit. Mol Cell Endocrinol 150:113–119 [DOI] [PubMed] [Google Scholar]
- Latif R, Graves P, Davies TF 2001 Oligomerization of the human thyrotropin receptor. Fluorescent protein-tagged hRSHR reveals post-translational complexes. J Biol Chem 276:45217–45224 [DOI] [PubMed] [Google Scholar]
- Urizar E, Montanelli L, Loy T, Bonomi M, Swillens S, Gales C, Bouvier M, Smits G, Vassart G, Costagliola S 2005 Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J 24:1954–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif R, Graves P, Davies TF 2002 Ligand-dependent inhibition of oligomerization at the human thyrotropin receptor. J Biol Chem 277:45059–45067 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Kakinuma A, Jaume JC, McLachlan SM, Rapoport B 1996 Evidence for negative cooperativity among human thyrotropin receptors overexpressed in mammalian cells. Endocrinology 137:4586–4591 [DOI] [PubMed] [Google Scholar]
- Smith BR, Furmaniak J, Sanders J 2008 TSH receptor blocking antibodies. Thyroid 18:1239 [DOI] [PubMed] [Google Scholar]