Abstract
Lipids have been shown to play a dual role in pancreatic β-cells: a lipid-derived signal appears to be necessary for glucose-stimulated insulin secretion, whereas lipid accumulation causes impaired insulin secretion and apoptosis. The ability of the protein perilipin to regulate lipolysis prompted an investigation of the presence of perilipin in the islets of Langerhans. In this study evidence is presented for perilipin expression in rat, mouse, and human islets of Langerhans as well as the rat clonal β-cell line INS-1. In rat and mouse islets, perilipin was verified to be present in β-cells. To examine whether the development of lipotoxicity could be prevented by manipulating the conditions for lipid storage in the β-cell, INS-1 cells with adenoviral-mediated overexpression of perilipin were exposed to lipotoxic conditions for 72 h. In cells exposed to palmitate, perilipin overexpression caused increased accumulation of triacylglycerols and decreased lipolysis compared with control cells. Whereas glucose-stimulated insulin secretion was retained after palmitate exposure in cells overexpressing perilipin, it was completely abolished in control β-cells. Thus, overexpression of perilipin appears to confer protection against the development of β-cell dysfunction after prolonged exposure to palmitate by promoting lipid storage and limiting lipolysis.
Perilipin is expressed in both clonal and primary β-cells and its overexpression in INS-1 cells protects against lipotoxicity by decreasing lipolysis and promoting triglyceride storage.
Several cooperative signaling pathways regulate insulin secretion from the islets of Langerhans (1). Glucose constitutes the primary extracellular signal triggering insulin secretion. Secondary signals augmenting insulin secretion have subsequently been demonstrated, one such being a lipid-derived intracellular signal, shown to be essential for normal glucose-stimulated insulin secretion (GSIS) (2,3,4,5). Externally added nonesterified fatty acids (NEFAs) have equally been shown to correlate with enhanced insulin secretion (6). However, the mediators of this effect as well as its molecular targets remain to be identified. Among candidates for the lipid-derived signal are long chain acyl-CoAs. These accumulate in the cytoplasm after glucose stimulation of β-cells due to inhibition of carnitine palmitoyl transferase 1 by malonyl CoA (7). Alternatively, NEFAs, and hence acyl-CoA, can be derived from plasma or from hydrolysis of the intracellular triacylglycerol (TAG) stores through the action of hormone-sensitive lipase (HSL) or other lipases.
Besides the positive effects of NEFAs, i.e. enhanced insulin secretion, prolonged exposure of islets/β-cells to elevated levels of NEFAs in the presence of high levels of glucose causes β-cell dysfunction, a process termed lipotoxicity or glucolipotoxicity to emphasize the fact that lipids are toxic only under hyperglycemic conditions. Islet lipotoxicity manifests itself as increased basal insulin secretion (8) and blunted GSIS (8,9) followed by β-cell apoptosis (10). The importance of intracellular NEFAs for the development of islet lipotoxicity has been emphasized through studies showing that NEFAs converted to TAG and stored in lipid droplets are less toxic than NEFAs (11,12) and that nonmetabolized NEFAs are more toxic than more readily metabolized NEFAs (11).
Perilipin is a protein found tightly associated to lipid droplets (13) and has to date been found in adipocytes (14,15,16), steroidogenic tissues (17), and atherosclerotic plaques (18,19). It was first detected as the most prevalent protein kinase A (PKA) phosphorylated protein in adipocytes with a molecular weight of 65/67 kDa on SDS-PAGE gels (16). Since then, three different isoforms have been detected: perilipin A (57 kDa), perilipin B (46 kDa), and perilipin C (38 kDa). Perilipin A and B have been detected in adipocytes, whereas perilipin C is expressed exclusively in steroidogenic cells. A fourth isoform, perilipin D, has been described as an mRNA transcript but not yet detected as a translated protein. All isoforms of perilipin originate from a single gene and are produced through alternative splicing (20).
Perilipin has been proposed to play a dual role in the regulation of lipolysis. On the one hand, the protein has been shown to decrease basal lipolysis, presumably by limiting the access for lipases to their substrate (21,22,23,24,25,26). On the other hand, perilipin has been shown to be essential for a full lipolytic response to catecholamine stimulation (21,22,23,24,25). A common denominator of the cell types known to express perilipin is the presence of a lipolytic machinery tightly regulated by PKA phosphorylation, including activation of the lipolytic enzyme HSL (27,28,29). Besides activating HSL, phosphorylation of perilipin by PKA is also accompanied by changes in the lipid droplet morphology (30) and perilipin distribution (30,31,32). These events could permit greater accessibility for TAG lipases to their substrate, favoring an increased lipid hydrolysis.
Here we provide evidence for expression of perilipin in pancreatic β-cells and show that overexpression of perilipin in β-cells increases the ability of the β-cell to effectively store lipids, which in turn protects against the adverse effects of lipotoxic conditions.
Materials and Methods
Cell culture, incubation conditions, and adenoviral infection
INS-1 cells at passage 90–100 were used in all experiments and cultured according to Wollheim and colleagues (33). Cells used in experiments including adenovirus were infected in suspension at a multiplicity of infection of approximately 1.5 and subsequently cultivated for 72 h with or without 0.75 mm palmitate and 1% BSA in RPMI 1640 with 16.7 mm glucose, with one routine change of medium.
Isolation of rat pancreatic islets
Islets from Wistar rats were isolated by collagenase digestion of the pancreas followed by handpicking under a stereomicroscope. All experiments including animals were approved by the local animal ethics committee.
Homogenization and fractionation of INS1 cells and pancreatic islet
INS-1 cells were harvested when confluent, washed with PBS (Sigma, St. Louis, MO) and homogenized in homogenization medium (HM): 0.25 m sucrose, 1 mm EDTA, 1 mm dithioerytrithol, 20 μg g/liter antipain, 20 μg/liter leupeptin, and 1 μg/liter pepstatin A (pH 7.4), followed by centrifugation at 100,000 × g for 1 h at 4 C. The supernatant was saved and the pellet washed in HM, resuspended in an equal volume of HM supplemented with 1% C13E12 (a detergent from the alkyl polyoxyethylene ether group), and then sonicated briefly and once more centrifuged as above. The supernatant with detergent was saved and the resulting pellet was resuspended in an equal volume of HM. Protein concentrations were determined using the bicinchoninic assay protein kit (Pierce, Rockford, IL).
Preparation of white adipose tissue (WAT) homogenates
Visceral WAT was harvested from female Wistar rats and homogenized in 4 vol of HM supplemented with 10% sodium dodecyl sulfate (Merck, Whitehouse Station, NJ) followed by centrifugation at 15,000 × g for 30 min at room temperature. The infranatant was saved and the protein concentration determined using the two-dimensional Quant kit (Amersham Biosciences, Stockholm, Sweden).
Phosphatase treatment
Aliquots of homogenates corresponding to 2 μg of total protein for WAT and 10 μg of total protein for islets and INS-1 cells were treated or not with calf intestinal alkaline phosphatase (Fermentas, Hanover, MD) for 1 h at room temperature using the reaction buffer supplied by the manufacturer, supplemented with a cocktail of protease inhibitors (Roche Complete; Roche Diagnostics, Mannheim, Germany).
Western blot analysis
Samples were separated by SDS-PAGE and electroblotted onto nitrocellulose membranes (Hybond-C Extra; Amersham Biosciences). To detect perilipin, a rabbit antibody made against the N terminus of mouse perilipin, thus recognizing all known isoforms of perilipin, was used and to detect phosphorylated hormone-sensitive lipase an antibody recognizing phosphorylation on Ser-565 was used (Cell Signaling, Beverly, MA). As secondary antibody, a horseradish peroxidase-linked whole-donkey antirabbit antibody (Amersham Biosciences) was used. The blots were developed using a system for enhanced chemoluminescence.
Immunohistochemistry
Pancreata from adult Sprague Dawley rats, perilipin-deficient mice on a C57/BL6 background (22), and sex- and age-matched wild-type controls were processed and stained for immunohistochemical analysis according to (34) with primary antibodies as stated in (Table 1) and appropriate secondary antibodies coupled to either fluorescein isothiocyanate Texas-Red (Jackson ImmunoResearch, West Grove, PA), or 7-amino-4-methyl coumarin-3-acetic acid (Jackson ImmunoResearch). The specificity of immunostaining was tested using primary antisera preabsorbed with homologous antigen (100 μg of peptide per milliliter antiserum) or by omission of the primary antibodies. The controls also included testing for inappropriate binding of the secondary antibodies. Immunofluorescence was examined in an epifluorescence microscope (BX60; Olympus, Tokyo, Japan). Images were captured with a digital camera (DP50; Olympus).
Table 1.
Specifications for the antibodies used in immunohistochemical staining
| Antigen | Code | Raised against | Raised in | Dilution | Source |
|---|---|---|---|---|---|
| Perilipin | α-PAT | Amino terminal peptide of perilipin | Rabbit | 1:800 | Brasaemle et al. (44) |
| Proinsulin | 9003 | Human proinsulin | Guinea pig | 1:2560 | Eurodiagnostica (Malmö, Sweden) |
| Glucagon | 8708 | Porcine glucagon | Guinea pig | 1:5210 | Eurodiagnostica |
| Somatostatin | V 1169 | Human somatostatin | Mouse monoclonal | 1:400 | Biomeda (Foster City, CA) |
| PP | AHP 515 | Human PP | Sheep | 1:640 | Serotec (Oxford, UK) |
For further details see Materials and Methods.
RT-PCR, Southern blot analysis, and sequencing
Total RNA was extracted from WAT, adrenals, isolated islets, and INS-1 cells according to Chomczynski and Sacchi (35), treated with deoxyribonuclease (Life Technologies, Grand Island, NY), and followed by reverse transcriptase reactions using Superscript II (Life Technologies) and random hexamers (Amersham Pharmacia, Uppsala, Sweden). In negative controls the reverse transcriptase was excluded. PCR was then performed on all samples using Dynazyme (Finnzymes Oy, Espoo, Finland), which was added during the first cycle of PCR and with the addition of 5% dimethyl sulfoxide. The PCR program used was 94 C for 5 min followed by a cycle of 20 sec at 94 C, 20 sec at 50 C, and 20 sec at 72 C, which was repeated 35 times with a final 7 min at 72 C. The primer pairs used to amplify the perilipin isoforms were: perilipin A, B, C, and D: ACAGCACCAAAGAAGCCCAC and CGTCTTTTGCCGTCCTGAA; perilipin B, GACGGCAAAAGACGCAGAA and GCCATGAAAGCCTATTGAACAG; perilipin C and D, AATATGCTGCCAACACCCG and TACCCCATTTCCCTCCTTCTC; and perilipin D, AACAGCACCAAAGAAGCCCA and GACATTCCCTATCCCAGAGCAG. After electrophoresis on 1% agarose gels, nucleic acids were passively blotted to nylon membranes (Hybond-N+; Amersham Biosciences) and UV cross-linked using standard techniques. Hybridization with radioactive probes was carried out in ExpressHyb (CLONTECH, Palo Alto, CA) according to the manufacturer’s protocol. Detection was performed by digital imaging (Fujix BAS 2000). Probes against the different isoforms of perilipin were produced by RT-PCR on RNA from WAT according to the same protocol as described previously. The primers used were: probe AD, CTCTGTGTGCAATGCCTA and CTTCTCGATGCTTCCCAA; probe BC, TTTTGAGGAGGGTCAGCA and CTTCTTCTTCTTCCTCCTCA. The PCR products were run on a 1% agarose gel, excised, and purified with Qiaex II gel extraction kit (QIAGEN, Valencia, CA). The purified probes were then 32P-labeled using an oligolabeling kit (Pharmacia, Uppsala, Sweden) and subsequently purified on Nick columns (Pharmacia). To confirm the identity of RT-PCR products, these were purified and sequenced with BigDye 3.1 (Applied Biosystems, Foster City, CA) using the same primers as for the southern blot analysis.
Quantitative real-time PCR on INS1 cells, rat islets, and human islets
Human pancreatic islets were prepared by collagenase digestion followed by density gradient purification (36,37) from five multiorgan donors (age: 47.8 ± 21.0 yr; gender: three males and two females; body mass index: 24.8 ± 1.8 kg/m2). Total RNA was extracted and cDNA synthesis performed as previously detailed (36,37). Extraction was accomplished by using the RNasy protect minikit (QIAGEN) and quantification by absorbance at A260-A280 (ratio > 1.65) nm in a PerkinElmer spectrophotometer (Waltham, MA). RNA integrity was assessed by electrophoresis in 1% agarose gel by ethidium bromide staining. cDNA synthesis was performed from 2 μg RNA. Islets and epididymal WAT tissue from female Wistar rats were snap frozen in liquid nitrogen and stored at −80 C until RNA extraction. Total RNA was extracted from rat islets using the RNeasy Plus minikit (QIAGEN) or, for WAT samples, the RNeasy lipid tissue minikit (QIAGEN). Human sc WAT total RNA was from BioCAT (Heidelberg, Germany). Subsequent cDNA synthesis was performed as described above.
Quantitative real-time PCR analysis was performed on 25 or 12.5 ng of cDNA using TaqMan chemistry (Applied Biosystems) on a 7900HT Fast real-time PCR system (Applied Biosystems). The mRNA expression levels of perilipin A and B (TaqMan assays on demand Mm00558672_m1 for rat islets and Hs00160173_m1 for human islets), adipose differentiation-related protein (ADRP) (Rn01472318_m1 for rat islets and Hs00605340_m1 for human islets), and, as reference gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Rn99999916_s1 and Hs02758991_g1) were determined using the following program for the amplification: 2 min at 50 C, 10 min at 95 C; followed by 40 cycles (15 sec at 95 C, 1 min at 60 C); and finally 15 sec at 95 C, 15 sec at 60 C, and 15 sec at 95 C. The efficiency of the perilipin mouse TaqMan probe was confirmed to be 102% on rat WAT cDNA samples (reference range 95–105%).
Adenovirus construction, amplification, and purification
Adenovirus were constructed according to Sztalryd et al. (14), amplified, and purified according to protocol for Adeno-X virus purification kit (BD Biosciences, Franklin Lakes, NJ). Titers were obtained through plaque assay and OD determination.
Proliferation assay
The level of proliferation in INS-1 cells was determined using CellTiter 96 AQueous One Solution cell proliferation assay (Promega, Madison WI) according to the manufacturer’s protocol.
Triglyceride determination
INS-1 cells were washed with PBS, harvested, and homogenized in HM. Lipids were extracted according to Folch et al. (38). After solubilization in aqueous medium containing 20% Thesit (Fluka, Buchs, Switzerland), the triglyceride content was determined with ThermoTrace triglyceride reagent (Thermo Scientific, West Sussex, UK).
Insulin secretion assay and lipolysis
INS-1 cells were plated at a density of 1 × 106 per well in a 24-well plate, infected, and maintained as described above. After 72 h the cells were washed with PBS and the medium replaced with serum-free RPMI 1640 with 2.8 mm glucose. The cells were incubated for 2 h after which the serum-free medium was removed and used for determination of basal lipolysis. After washing the cells with PBS, Krebs Ringer buffer [KRB; 120 mm NaCl, 5 mm NaHCO3, 5 mm KCl, 1.2 mm KH2PO4, 2.5 mm CaCl2, 1.2 mm MgSO4, 0.2% BSA, and 10 mm HEPES (pH 7.2)] with 2.8 mm and 16.7 mm glucose were added and the cells incubated for an additional 60 min. The KRB was then removed and assayed for insulin by ELISA (Mercodia, Uppsala, Sweden) and glycerol content using a kinetic luminometric method according to Hellmer et al. (39). Attached cells were washed with PBS, homogenized in HM, and total protein content determined as above. Insulin content was measured after sonication of cells in acid ethanol (2% H2SO4) followed by three freeze/thaw cycles and centrifugation for 5 min at 10,000 × g. Insulin was measured in the supernatant by RIA (Linco, St. Charles, MO).
Fatty acid oxidation
INS-1 cells were infected and plated as described above using 12-well plates. After incubation in the absence or presence 0.75 mm palmitate for 72 h, cells were rinsed with PBS and trypsinized. Palmitate oxidation was determined as production of 14CO2 from [1-14C]palmitate under conditions of low (2.8 mm) or high (16.7 mm) glucose as described (40).
Statistics
Data are presented as mean ± sem. The statistical significance of the differences between experimental groups was assessed by unpaired Student’s t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Results
Detection of perilipin and ADRP in INS-1 cells and rat islets of Langerhans
To investigate whether perilipin is expressed in β-cells, Western blot analyses of INS-1 cell homogenates, rat islets, and rat WAT were performed. In INS-1 cell homogenates, bands corresponding to the described amino acid chain sizes of perilipin A, i.e. 62, 65, and 67 kDa, were detected (Fig. 1A). Upon fractionation of the homogenate, perilipin was recovered in the pellet fraction, but solubilization of the pellet with a nonionic detergent caused the major portion of perilipin to redistribute to the supernatant fraction (Fig. 1A). Western blot analysis of fractionated islets confirmed the findings in clonal β-cells. Although Western blot analysis is only a semiquantitative technique, estimations and back calculations from the blots shown in Fig. 1, A and B, indicate that the level of perilipin expression is around 500 times lower in islets than in WAT.
Figure 1.
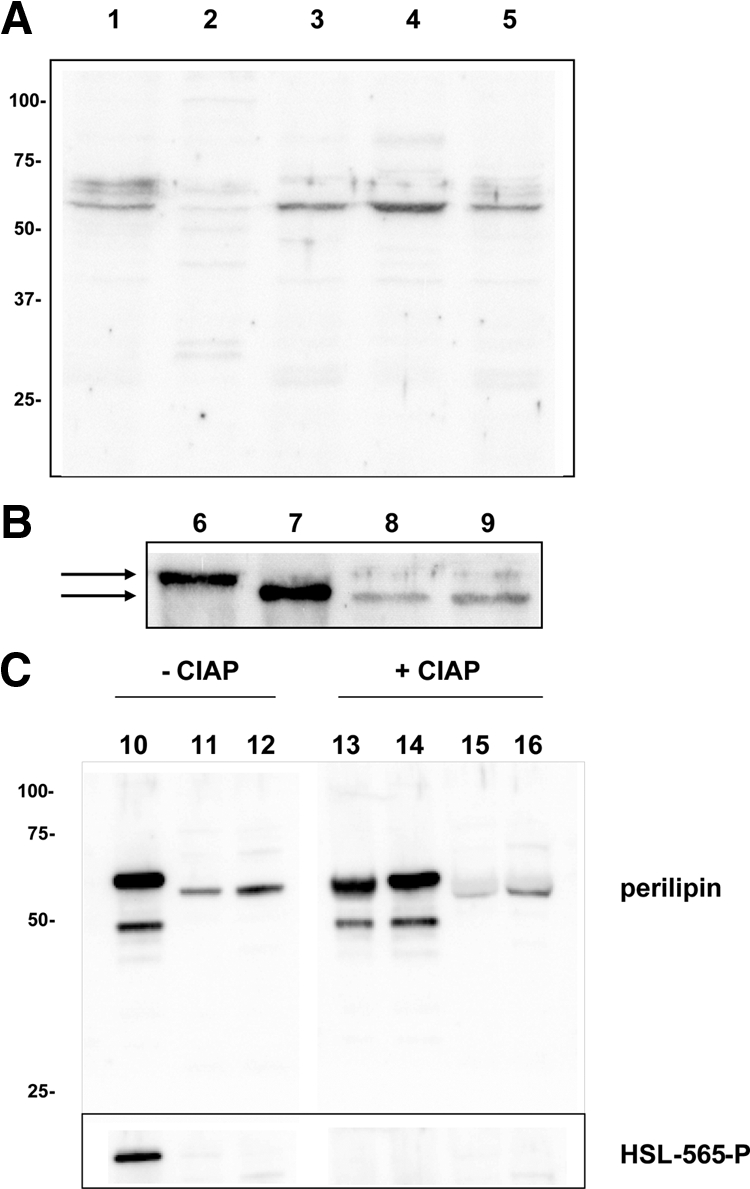
INS-1 cells express perilipin protein. A, Western blot analysis for perilipin after fractionation of INS-1 cells. Twenty micrograms of protein were loaded in each lane. Lane 1, INS-1 crude homogenate; lane 2, supernatant after 1 h of centrifugation at 100,000 × g; lane 3, the pellet to the supernatant in lane 2; lane 4, supernatant after detergent solubilization of the pellet from lane 3 and subsequent centrifugation; lane 5, the pellet to the supernatant in lane 4. B, Western blot analysis for perilipin in rat WAT, rat pancreatic islets, and INS-1 cells. Four micrograms of total protein were loaded in lanes 6 and 7 and 20 μg in lane 8 and 9. Lane 6, Visceral WAT; lane 7, INS-1 cells overexpressing perilipin A; lane 8, detergent-solubilized supernatant from homogenized and fractionated pancreatic islets; lane 9: detergent-solubilized supernatant from homogenized and fractionated INS-1 cells. The arrows indicate the difference in apparent molecular size observed between perilipin from WAT and islet cells. C, Western blot analysis for perilipin and for Ser-565 phosphorylated HSL in rat WAT, rat pancreatic islets, and INS-1 cells after treatment or not with calf intestinal alkaline phosphatase (CIAP). A homogenate of WAT corresponding to 2 μg of total protein was treated with either 10 U (lane 13; 5 U/μg protein; 0.1 U/μl) or 2 U CIAP (lane 14; 1 U/μg protein; 0.02 U/μl) and homogenates of islets (lane 15) and INS-1 cells (lane 16) corresponding to 10 μg of total protein were treated with 10 U CIAP (1 U/μg protein; 0.1 U/μl) and analyzed together with samples not treated with CIAP (lane 10, WAT; lane 14, rat islets; lane 15, INS-1 cells) by Western blot. To confirm the efficiency of the CIAP treatment, the blot was also probed with a phosphospecific HSL antibody, which recognized Ser-565 phosphorylation on HSL in WAT in samples not treated with CIAP but not in CIAP-treated samples. HSL expression in islets and INS-1 cells was not detected using this antibody.
In both INS-1 cells and in rat islets, the major perilipin band detected appeared slightly smaller in size (i.e. 62 kDa) than the major band observed in the WAT homogenate (Fig. 1B). To investigate whether the three different bands identified with the perilipin antibody corresponded to protein in different phosphorylation states, in which the 65/67 kDa band would correspond to the doublet observed in earlier publications (14,16), samples were treated with phosphatase and then analyzed in parallel to samples not treated with phosphatase. As shown in Fig. 1C, even after phosphatase treatment, a small size difference in electrophoretic mobility remained when comparing WAT perilipin A with perilipin in islets and β-cells. The efficiency of the phosphatase treatment was confirmed by the finding that an antibody recognizing Ser-565 phosphorylation of HSL recognized an HSL band in nontreated WAT samples but not treated WAT samples. Thus, islets/β-cells appear to express a variant of perilipin A that is slightly smaller than the perilipin A variant in WAT.
Earlier studies have shown a correlation between the intracellular TAG level and the amount of perilipin present in the cell (41). To investigate whether the same correlation could be observed in clonal β-cells, INS-1 cells were incubated with 0.5 mm oleate for 48 h, and the level of perilipin was subsequently determined by Western blot analysis. There was no detectable change in the perilipin expression level before compared with that after the incubation (data not shown).
Immunohistochemical staining of rat islets demonstrated weak to moderate perilipin immunoreactivity (PIR) in all islets examined (Fig. 2). To assess the cellular identity of PIR cells, double immunostainings for the main islet hormones [insulin, glucagon, somatostatin, and pancreatic polypeptide (PP)] were performed, demonstrating that a large proportion of the β-cells expressed perilipin, albeit to a variable degree. A small subpopulation of the α-cells also contained perilipin, whereas PP cells and δ-cells were devoid of PIR (Fig. 2). To further confirm the expression of perilipin in β-cells, islets from perilipin knockout mice were subjected to immunohistochemical analysis. As shown in Fig. 3, islets of perilipin knockout mice were almost completely devoid of PIR, whereas islets of wild-type mice demonstrated weak to moderate PIR as the rat islets. Double immunostainings demonstrated no PIR in β-cells of perilipin-knockout islets, whereas α-cells showed some PIR, although weaker than in wild-type islets (Fig. 3). Taken together, the Western blot and immunohistochemistry analyses show that β-cells definitely express perilipin. It is possible that also α-cells express perilipin, but this must be regarded more uncertain due to the fact that some, apparently unspecific, staining was found in α-cells of perilipin-deficient islets.
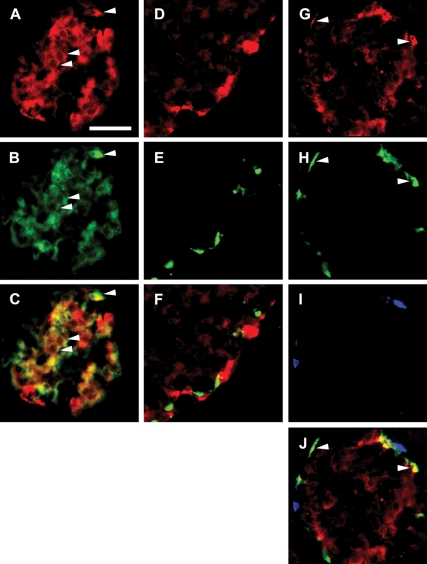
Figure 2.
Perilipin is expressed in β-cells and α-cells of rat islets of Langerhans. Fluorescence photomicrographs of double- and triple-immunostained rat islets. A, Staining for perilipin (red). B, Staining for insulin (green). C, Merged image of A and B, demonstrating that the majority of the islet β-cells harbor perilipin. Arrowheads exemplify colocalization (yellow) of perilipin and insulin. D, Staining for perilipin (red). E, Staining for somatostatin (green). F, Merged image of D and E demonstrating that islet δ-cells are devoid of perilipin. Staining for perilipin (G; red), glucagon (H; green), and PP (I; blue) is shown. J, Merged image of G–I demonstrating that perilipin is not present in PP cells but is expressed in a subpopulation of α-cells. Colocalization of perilipin and glucagon (yellow) is exemplified by arrowheads. Scale bar (A, valid for A–J), 50 μm. Details of staining procedure and antibodies used are given in Materials and Methods and Table 1, respectively.
Figure 3.
Perilipin is expressed in β-cells of wild-type mouse islets (A–C) but lacking in islets of perilipin knockout mice (D–F). Fluorescence photomicrographs of islets double immunostained for perilipin (green; A and D) and insulin (red; B and E), merged in C and F. Arrowheads exemplify colocalization (yellow) of perilipin and insulin. Scale bar (A, valid for A–F), 50 μm.
Detection of perilipin and ADRP mRNA in rat and human islets
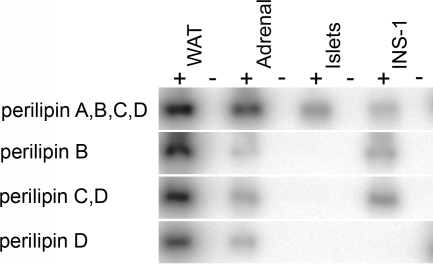
The perilipin gene is extremely complex, with multiple transcription start sites, multiple splice sites, and multiple poly A sites, which generate four major mRNA species with overlapping organizations. Using a limited set of primers for RT-PCR, followed by hybridization with specific probes, we could evaluate the expression of perilipin mRNAs A, B, C, and D. RT-PCR was performed on RNA extracted from WAT, adrenals, islets, and INS-1 cells followed by Southern blot analysis. The experiments (Fig. 4) were solely designed to amplify and detect regions corresponding to only a single perilipin mRNA species (B or D) or combinations of the perilipin mRNAs (A+B+C+D or C+D). These qualitative results indicate that only the perilipin A isoform is expressed in islets of Langerhans; perilipin mRNAs B, C, and D are not detected, regardless of the extent of amplification. INS-1 cells were positive for perilipin mRNAs B, C and presumably A because the perilipin protein isoform expressed in INS-1 cells (Fig. 1) is too large to be encoded by mRNAs B or C. (Fig. 4). Data for WAT and adrenal cells is consistent with that previously reported, in which all four mRNAs are detected (20). Next we quantified the perilipin A mRNA levels in rat and human islets. Quantitative real-time PCR analysis of total RNA extracts from rat islets, rat WAT, human islets, and human WAT demonstrated a very low level of expression of perilipin A in rat islets and a low but readily detectable perilipin A mRNA level in human islets (Table 2). ADRP mRNA levels were detectable in all samples from rat and human islets. When normalized to the expression of the reference gene GAPDH in each tissue, the expression level of perilipin A in human islets was found to be 750 times lower than in human WAT, whereas the mRNA expression level of ADRP was 32 times lower in islets than in WAT. In rat islets ADRP mRNA was detected at an expression level of 0.5 times the level in rat WAT, but the mRNA level of perilipin A was more than 3500 times lower in rat islets than in the WAT total RNA extracts. However, the very low level of perilipin mRNA in rat islets makes the absolute numbers in this analysis difficult to assess.
Figure 4.
Perilipin mRNA pattern in rat islets of Langerhans and INS-1 cells. Southern blot performed on amplified DNA derived from RT-PCR performed on RNA isolated from WAT, adrenals, islets of Langerhans, and INS-1 cells with primers designed to amplify the sequences from the different perilipin mRNAs as indicated. Detection was performed with two different probes complementary to shorter internal sequences of the different perilipin isoforms. Negative controls were included for all samples (−) and show no DNA amplification.
Table 2.
Quantification of perilipin and ADRP mRNA levels in rat and human islets and WAT
| Gene | Rat
|
WAT to islet ratio | Human
|
WAT to islet ratio | ||
|---|---|---|---|---|---|---|
| Islets | WAT | Islets | WAT | |||
| Perilipin A+B | 0.000079 | 0.300 | 3791 | 0.00211 | 1.650 | 783 |
| ADRP | 0.0195 | 0.0382 | 1.96 | 0.0313 | 1.014 | 32 |
Mean values are arbitrary units. Relative mRNA quantities are normalized to the internal control gene GAPDH.
Overexpression of perilipin in INS-1 cells using a recombinant adenoviral expression vector
To assess the role of perilipin in β-cells, especially its putative role under exposure to glucolipotoxic conditions, INS-1 cells were cultured at high glucose conditions (16.7 mm) for 72 h with or without 0.75 mm palmitate. Before the 72-h cultivation period, the cells were infected with either an adenovirus expressing perilipin A (Adperi) or a control virus expressing β-galactosidase (Adβ-gal).
After 72 h, the Adβ-gal-infected cells had a lower proliferation rate when exposed to palmitate and high levels of glucose compared with high glucose alone (Fig. 5A). By contrast, the Adperi-infected cells were nearly unsusceptible to the deleterious effects of palmitate exposure. Very similar results to those shown in Fig. 5A, obtained using a commercially available proliferation assay, were obtained by simply measuring the protein content of each of the wells (286.1 μg protein/well vs. 142.6 μg/well for palmitate exposed cells, P < 0.001, n = 12) and 239.5 μg protein/well vs. 225.2 μg/well for palmitate exposed cells, n = 12).
Figure 5.
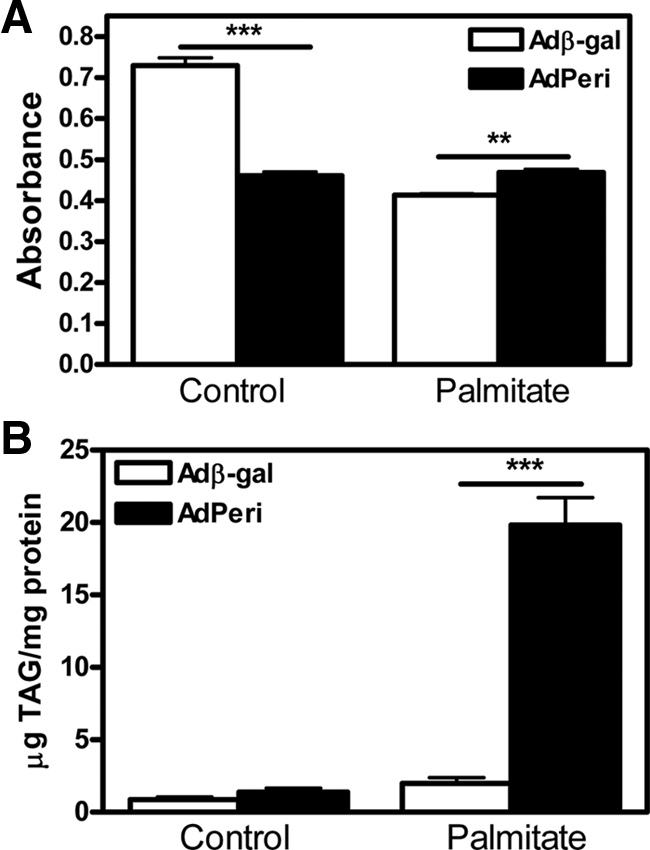
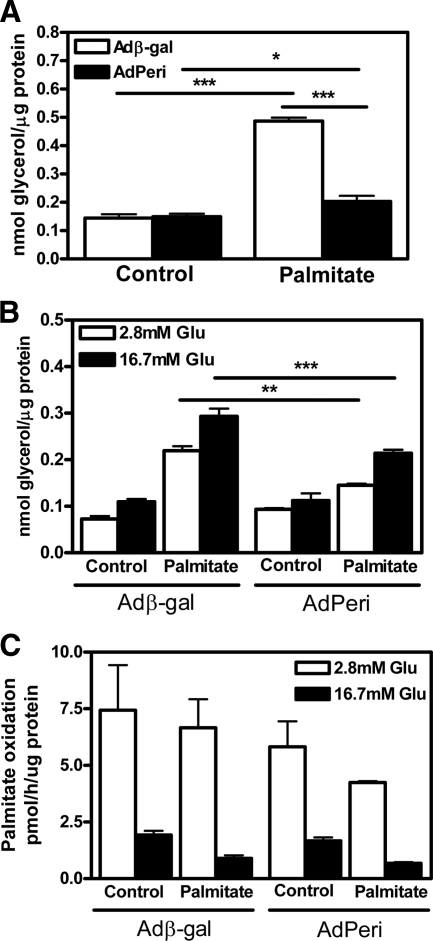
Impact of perilipin overexpression on cell proliferation rate (A) and cellular TAG stores (B). INS-1 cells were infected with Adperi or Adβ-gal virus (control) and preincubated for 72 h with or without palmitate in the presence of 16.7 mm glucose. Cell proliferation rate (A) was measured using a commercially available assay and TAG content (B) was measured after Folch extraction. Data shown are mean ± sem (n = 8 in A and n = 4 in B). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To establish the impact of perilipin overexpression on the cellular TAG stores in β-cells, INS-1 cells were cultivated in the presence of palmitate for 72 h followed by subsequent determination of intracellular TAG content. The 72-h exposure to palmitate did not cause a significant change in the TAG stores of Adβ-gal-infected cells (Fig. 5B). However, cells infected with Adperi had approximately the same level of TAG as the Adβ-gal infected cells in the absence of palmitate, whereas in the presence of palmitate, the Adperi-infected cells stored about 10 times more TAG than cells infected with Adβ-gal (Fig. 5B).
The rate of lipolysis, measured as glycerol release into the medium, was affected by both the overexpression of perilipin and the amount of stored TAG. Basal lipolysis was increased in all cells loaded with palmitate, but although TAG levels were much higher in cells infected with Adperi after palmitate exposure, the Adβ-gal-infected cells had twice as high basal lipolytic rate compared with Adperi-infected cells under the same conditions (Fig. 6A). The glycerol release was also monitored during the insulin secretion experiments, both at high (16.7 mm) and low (2.8 mm) glucose levels (Fig. 6B). Again, an increase in the level of lipolysis was observed in cells exposed to palmitate, regardless of whether they were infected with Adβ-gal or Adperi. Furthermore, lipolysis was increased under high glucose conditions compared with low glucose conditions for all cells. Notably, lipolysis was lower in Adperi-infected cells compared with Adβ-gal-infected cell at both high- and low-glucose concentrations in the presence of palmitate despite the demonstrated difference in intracellular TAG stores. It has previously been demonstrated that ectopic overexpression of perilipin increases TAG storage by decreasing the rate of lipolysis, whereas the rate of TAG synthesis was unaffacted (26). Possible effects of ectopic perilipin expression on fatty acid oxidation were not reported. Thus, we compared fatty acid oxidation in Adβ-gal infected cells and Adperi-infected cells. No significant differences were found, neither under normal incubation conditions nor after long-term exposure to palmitate (Fig. 6C).
Figure 6.
Overexpression of perilipin decreases lipolysis in INS-1 cells but has no effect on fatty acid oxidation. A, Determination of basal lipolytic activity (release of glycerol). INS-1 cells were infected with Adperi or Adβ-gal virus (control) and preincubated for 72 h with or without palmitate in the presence of 16.7 mm glucose. Basal lipolysis was subsequently determined during 2 h of incubation in serum-free medium under nonstimulatory conditions (2.8 mm glucose). Data shown are mean ± sem (n = 12). B, After determination of basal lipolysis, the lipolytic activity during acute stimulation with glucose (Glu) was determined. The Adperi- and Adβ-gal-infected cells were incubated for an additional hour in KRB containing low (2.8 mm) or high (16.7 mm) glucose and the amount of glycerol released into the cell culture medium was determined. Data shown are mean ± sem (n = 8). C, Palmitate oxidation was measured under the same experimental conditions as under A and B. Data shown are mean ± sem (n = 3). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
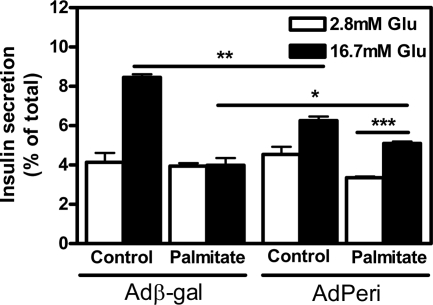
Finally, we assessed the impact of perilipin overexpression on β-cell function after exposure to lipotoxic conditions for 72 h. INS-1 cells infected with Adperi had a lower insulin response compared with Adβ-gal-infected cells when incubated under standard conditions, but after exposure to palmitate the insulin response in Adperi-infected cells was significantly higher than the response observed in palmitate-cultivated Adβgal-infected cells (Fig. 7). Thus, these results demonstrate a retained GSIS function in the perilipin-overexpressing cells under conditions that cause loss of β-cell function in the Adβ-gal infected control cells.
Figure 7.
INS-1 cells overexpressing perilipin are protected against lipotoxicity. INS-1 cells were infected and incubated for 72 h with or without 0.75 mm palmitate in the presence of 16.7 mm glucose (Glu; for more details, see Materials and Methods). Insulin secretion was thereafter measured during 60 min at low (2.8 mm) or high glucose (16.7 mm) conditions and related to insulin content. Data shown are mean ± sem (n = 4). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Discussion
In this study the presence of perilipin A in pancreatic β-cells has been shown for the first time. The presence of the protein was demonstrated in both clonal and primary cells and was in both instances recovered in the insoluble pellet fraction after centrifugation. Upon solubilization with a mild nonionic detergent, the protein redistributed to the supernatant fraction, as expected for a protein associated with membranes/lipids rather than a protein inserted into hydrophobic structures via a transmembrane region. Upon fractionation of primary adipocytes, perilipin distributes to the fat cake rather than the pellet fraction. However, a similar lipid-rich phase does not form upon fractionation of β-cells and the discrepancy in the localization of perilipin between adipocytes and β-cells most likely reflects that perilipin in β-cells either resides in small, dense lipid droplets as suggested by Moore et al. (42) or in the plasma membrane (43).
The apparent molecular size of the perilipin protein detected in both clonal and primary β-cells after SDS-PAGE separation was slightly smaller than the 62 kDa perilipin A detected in WAT. This appears not to reflect differences in phosphorylation state, although this possibility is difficult to completely rule out for multiphosphorylated proteins like perilipin. The expression level of perilipin in INS-1 cells was shown to be independent of the lipid content of the cells because it did not change upon cultivation of the cells with oleate, which is incorporated into cellular TAG stores much more readily than palmitate. This is in contrast to what has previously been reported for adrenal cortical cells, in which the perilipin expression was induced on oleate loading (44) but in accordance with a report showing that lipid loading did not affect the expression of perilipin in macrophages (45). In parallel with the analysis of perilipin expression, ADRP expression was also investigated. For both these proteins mRNA was detected in rat as well as human islets. In rat islets the ADRP mRNA level was comparatively high, whereas the perilipin mRNA level was very low. In human islets, however, ADRP mRNA levels were lower than in rat islets relative to the expression levels in WAT from the same species, whereas the perilipin mRNA levels were comparatively higher in human than in rat islets. The relatively higher expression of perilipin in human than rat islets may indicate that larger sized lipid droplets are more readily formed in human islets than rat islets. There is an apparent discrepancy in the relative expression of perilipin between WAT and perilipin, depending on whether the comparison is made at the mRNA level or the protein level, i.e. the level of expression at the mRNA level was found to be around 3500 times lower in islets than in WAT (Table 2), whereas the corresponding figure at the protein level was estimated to 500 (Fig. 1, A and B). The reason for this discrepancy is not known, but it has previously been shown that protein perilipin levels do not always correlate with perilipin mRNA levels (41,46,47). Thus, one possibility is that perilipin protein stability differs between the two tissues. Perilipin has been reported to have a half-life exceeding 70 h when bound to adipocyte lipid droplets (48), whereas it is rapidly degraded once dissociated from lipids (41). It can be speculated that a consequence of the low level of perilipin expression in islets is that a larger fraction of the perilipin is lipid bound.
An emerging notion regarding islet lipotoxicity is that sequestration of cytosolic NEFAs in cellular TAG stores represents a cytoprotective mechanism (11,12). As mentioned above, palmitate is known to be incorporated into the TAG stores to a lesser extent and imposing a greater lipotoxic effect on cells than oleate (8,11,12,49). Furthermore, when cells are exposed to oleate and palmitate simultaneously, oleate facilitates the incorporation of palmitate into the TAG stores and thereby decreases the lipotoxic action of palmitate (12). On the other hand, in cells unable to store TAG, oleate has been shown to be lipotoxic to the same degree as palmitate (12). Also, bromopalmitate, a nonmetabolizable fatty acid, which is incorporated into TAG with low efficiency, is more lipotoxic than palmitate. The results obtained in this study demonstrating a retained GSIS function in INS-1 cells with an increased capacity for storing TAG from perilipin overexpression support the concept that if β-cells are capable of efficiently storing excess fatty acids as TAG, the lipotoxic effects of chronic exposure to NEFAs are to a large extent abolished. The data further show that overexpression of perilipin can protect β-cells from lipotoxicity by limiting lipolysis. This results in sequestration of NEFAs in TAG stores, thus preventing them from inducing lipotoxic events. It should be emphasized that although these experiments, using forced overexpression of perilipin, demonstrate the potential of perilipin, other type of experiments, e.g. silencing of perilipin expression in β-cells/islets, are needed to elucidate the role of endogenous perilipin.
When INS-1 cells were incubated without palmitate, the insulin response was lower for Adperi-infected cells than for control-infected cells. This could be a consequence of the slightly lower viability observed after Adperi infection compared with Adβ-gal infection. An alternative explanation could be that overexpression of perilipin reduces lipolytic rate to such an extent that generation of the lipid-derived signal that appears to be essential for GSIS (2,3,4,5) is hampered.
In summary, this study demonstrates that perilipin is expressed at low levels in clonal and primary β-cells and that increased lipid storage through overexpression of perilipin protects β-cells from the development of lipotoxicity. Whether this mechanism is indeed used as a protective mechanism in vivo remains to be determined. The results identify perilipin as a potential target for development of drugs aiming to reduce lipid-induced β-cell dysfunction. They also indicate that perilipin may be involved in regulating the generation of the lipid coupling factor shown to be important for GSIS.
Acknowledgments
We thank Birgitta Danielsson and Doris Persson for excellent technical assistance.
Footnotes
This work was supported by the Swedish Research Council Project 112 84 (to C.H.); the European Union (Eurodia LSHM-CT-2006-518153); the Swedish Diabetes Association; Cell Factory for Functional Genomics, a program funded by the Swedish Foundation for Strategic Research; the intramural research program of National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; and the following foundations: Novo Nordisk, A. Påhlsson, Salubrin/Druvan, and Torsten and Ragnar Söderberg.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 19, 2009
Abbreviations: Adβ-gal, Control virus expressing β-galactosidase; ADRP, adipose differentiation-related protein; Adperi, adenovirus expressing perilipin A; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GSIS, glucose-stimulated insulin secretion; HM, homogenization medium; HSL, hormone-sensitive lipase; KRB, Krebs Ringer buffer; NEFA, nonesterified fatty acid; PIR, perilipin immunoreactivity; PKA, protein kinase A; PP, pancreatic polypeptide; TAG, triacylglycerol; WAT, white adipose tissue.
References
- Henquin JC 2000 Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49:1751–1760 [DOI] [PubMed] [Google Scholar]
- Mulder H, Yang S, Winzell MS, Holm C, Ahrén B 2004 Inhibition of lipase activity and lipolysis in rat islets reduces insulin secretion. Diabetes 53:122–128 [DOI] [PubMed] [Google Scholar]
- Stein DT, Esser V, Stevenson BE, Lane KE, Whiteside JH, Daniels MB, Chen S, McGarry JD 1996 Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest 97:2728–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama K, Chen G, Wang MY, Lee Y, Shimabukuro M, Newgard CB, Unger RH 1997 β-Cell function in normal rats made chronically hyperleptinemic by adenovirus-leptin gene therapy. Diabetes 46:1276–1280 [DOI] [PubMed] [Google Scholar]
- Masiello P, Novelli M, Bombara M, Fierabracci V, Vittorini S, Prentki M, Bergamini E 2002 The antilipolytic agent 3,5-dimethylpyrazole inhibits insulin release in response to both nutrient secretagogues and cyclic adenosine monophosphate agonists in isolated rat islets. Metabolism 51:110–114 [DOI] [PubMed] [Google Scholar]
- Warnotte C, Gilon P, Nenquin M, Henquin JC 1994 Mechanisms of the stimulation of insulin release by saturated fatty acids. A study of palmitate effects in mouse β-cells. Diabetes 43:703–711 [DOI] [PubMed] [Google Scholar]
- Prentki M, Vischer S, Glennon MC, Regazzi R, Deeney JT, Corkey BE 1992 Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem 267:5802–5810 [PubMed] [Google Scholar]
- Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY 2001 Distinct effects of saturated and monounsaturated fatty acids on β-cell turnover and function. Diabetes 50:69–76 [DOI] [PubMed] [Google Scholar]
- Zhou YP, Grill V 1995 Long term exposure to fatty acids and ketones inhibits β-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab 80:1584–1590 [DOI] [PubMed] [Google Scholar]
- Shimabukuro M, Zhou YT, Levi M, Unger RH 1998 Fatty acid-induced β-cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA 95:2498–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG 2001 Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes 50:1771–1777 [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese Jr RV, Ory DS, Schaffer JE 2003 Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 100:3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C 1991 Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 266:11341–11346 [PubMed] [Google Scholar]
- Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C 2003 Peripilin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol 161:1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AS, Egan JJ, Wek SA, Moos Jr MC, Londos C, Kimmel AR 1993 Isolation of cDNA for perilipin A and B: sequence and expression of lipid droplet-associated proteins of adipocytes. Proc Natl Acad Sci USA 90:12035–12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan JJ, Greenberg AS, Chang MK, Londos C 1990 Control of endogenous phosphorylation of the major cAMP-dependent protein kinase substrate in adipocytes by insulin and β-adrenergic stimulation. J Biol Chem 265:18769–18775 [PubMed] [Google Scholar]
- Servetnick DA, Brasaemle DL, Gruia-Gray J, Kimmel AR, Wolff J, Londos C 1995 Perilipin are associated with cholesteryl ester droplets in steroidogenic adrenal cortical and Leydig cells. J Biol Chem 270:16970–16973 [DOI] [PubMed] [Google Scholar]
- Forcheron F, Legedz L, Chinetti G, Feugier P, Letexier D, Bricca G, Beylot M 2005 Genes of cholesterol metabolism in human atheroma: overexpression of perilipin and genes promoting cholesterol storage and repression of ABCA1 expression. Arterioscler Thromb Vasc Biol 25:1711–1717 [DOI] [PubMed] [Google Scholar]
- Faber BC, Cleutjens KB, Niessen RL, Aarts PL, Boon W, Greenberg AS, Kitslaar PJ, Tordoir JH, Daemen MJ 2001 Identification of human genes potentially involved in rupture of human atherosclerotic plaques. Circ Res 89:547–554 [DOI] [PubMed] [Google Scholar]
- Lu X, Gruia-Gray J, Copeland NG, Gilbert DJ, Jenkins NA, Londos C, Kimmel AR 2001 The murine perilipin gene: the lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm Genome 12:741–749 [DOI] [PubMed] [Google Scholar]
- Martinez-Botas J, Anderson JB, Tessier D, Lapillonne A, Chang BH, Quast MJ, Gorenstein D, Chen KH, Chan L 2000 Absence of perilipin results in leaness and reverses obesity in Leprdb/db mice. Nat Genet 26:474–479 [DOI] [PubMed] [Google Scholar]
- Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C 2001 Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA 98:6494–6499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza SC, Muliro KV, Liscum L, Lien P, Yamamoto MT, Schaffer JE, Dallal GE, Wang X, Kraemer FB, Obin M, Greenberg AS 2002 Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J Biol Chem 277:8267–8272 [DOI] [PubMed] [Google Scholar]
- Tansey JT, Huml AM, Vogt R, Davis KE, Jones JM, Fraser KA, Brasaemle DL, Kimmel AR, Londos C 2003 Functional studies on native and mutated forms of perilipin. J Biol Chem 278:8401–8406 [DOI] [PubMed] [Google Scholar]
- Zhang HH, Souza SC, Muliro KV, Kraemer FB, Obin MS, Greenberg AS 2003 Lipase-selective functional domain of perilipin A differentially regulate constitutive and protein kinase A-stimulated lipolysis. J Biol Chem 278:51535–51542 [DOI] [PubMed] [Google Scholar]
- Brasaemle DL, Rubin B, Harten IA, Gruia-Gray J, Kimmel AR, Londos C 2000 Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J Biol Chem 275:38486–38493 [DOI] [PubMed] [Google Scholar]
- Egan JJ, Greenberg AS, Chang MK, Wek SA, Moos Jr MC, Londos C 1992 Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc Natl Acad Sci USA 89:8537–8541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C 2003 Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol 161:1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CL, Sztalryd C, Contreras JA, Holm C, Kimmel AR, Londos C 2003 Mutational analysis of the hormone-sensitive lipase translocation reaction in adipocytes. J Biol Chem 278:43615–43619 [DOI] [PubMed] [Google Scholar]
- Londos C, Brasaemle DL, Schultz CJ, Segrest JP, Kimmel AR 1999 Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Cell Dev Biol 10:51–58 [DOI] [PubMed] [Google Scholar]
- Clifford GM, Londos C, Kraemer FB, Vernon RG, Yeaman SJ 2000 Translocation of hormone-sensitive lipase and perilipin upon lipolytic stimulation of rat adipocytes. J Biol Chem 275:5011–5015 [DOI] [PubMed] [Google Scholar]
- Souza SC, de Vargas LM, Yamamoto MT, Lien P, Franciosa MD, Moss LG, Greenberg AS 1998 Overexpression of perilipin A and B blocks the ability of tumor necrosis factor α to increase lipolysis in 3T3-L1 adipocytes. J Biol Chem 273:24665–24669 [DOI] [PubMed] [Google Scholar]
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB 1992 Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130:167–178 [DOI] [PubMed] [Google Scholar]
- Wierup N, Kuhar M, Nilsson BO, Mulder H, Ekblad E, Sundler F 2004 Cocaine- and amphetamine-regulated transcript (CART) is expressed in several islet cell types during rat development. J Hisotochem Cytochem 52:169–177 [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N 1987 Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M 2007 The endoplasmatic reticulum in pancreatic β cells of type 2 diabetes patients. Diabetologia 50:2486–2494 [DOI] [PubMed] [Google Scholar]
- Del Guerra S, Lupi R, Marselli L, Masini M, Bugliani M, Sbrana S, Torri S, Pollera M, Boggi U, Mosca F, Del Prato S, Marchetti P 2005 Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes 54:727–735 [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH 1957 A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509 [PubMed] [Google Scholar]
- Hellmér J, Arner P, Lundin A 1989 Automatic luminometric kinetic assay of glycerol for lipolysis studies. Anal Biochem 177:132–137 [DOI] [PubMed] [Google Scholar]
- Antinozzi PA, Segall L, Prentki M, McGarry JD, Newgard CB 1998 Molecular or pharmacologic perturbation of the link between glucose and lipid metabolism is without effect on glucose-stimulated insulin secretion. A re-evaluation of the long-chain acyl-CoA hypothesis. J Biol Chem 273:16146–16154 [DOI] [PubMed] [Google Scholar]
- Brasaemle DL, Barber T, Kimmel AR, Londos C 1997 Post-translational regulation of perilipin expression. J Biol Chem 272:9378–9387 [DOI] [PubMed] [Google Scholar]
- Moore HP, Silver RB, Mottillo EP, Bernlohr DA, Granneman JG 2005 Perilipin targets a novel pool of lipid droplets for lipolytic attack by hormone-sensitive lipase. J Biol Chem 280:43109–43120 [DOI] [PubMed] [Google Scholar]
- Robenek H, Robenek MJ, Buers I, Lorkowski S, Hofnagel O, Troyer D, Severs NJ 2005 Lipid droplets gain PAT family proteins by interaction with specialized plasma membrane domains. J Biol Chem 280:26330–26338 [DOI] [PubMed] [Google Scholar]
- Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C 1997 Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 38:2249–2263 [PubMed] [Google Scholar]
- Larigauderie G, Bouhlel MA, Furman C, Jaye M, Fruchart JC, Rouis M 2006 Perilipin, a potential substitute for adipophilin in triglyceride storage in human macrophages. Atherosclerosis 189:142–148 [DOI] [PubMed] [Google Scholar]
- Wang Y, Sullivan S, Trujillo M, Lee MJ, Schneider SH, Brolin RE, Kang YH, Werber Y, Greenberg AS, Fried SK 2003 Perilipin expression in human adipose tissues: effects of severe obesity, gender, and depot. Obes Res 11:930–936 [DOI] [PubMed] [Google Scholar]
- Arvidsson E, Blomqvist L, Rydén M 2004 Depot-specific differences in perilipin mRNA but not protein expression in obesity. J Intern Med 255:595–601 [DOI] [PubMed] [Google Scholar]
- Kovsan J, Ben-Romano R, Souza SC, Greenberg AS, Rudich A 2007 Regulation of adipocyte lipolysis by degradation of the perilipin protein: nelfinavir enhances lysosome-mediated perilipin proteolysis. J Biol Chem 282:21704–21711 [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Ory DS, Schaffer JE 2001 Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem 276:14890–14895 [DOI] [PubMed] [Google Scholar]