Abstract
Cyclooxygenase (COX) is the rate-limiting enzyme in the metabolic conversion of arachidonic acid to prostaglandins (PGs), including prostaglandin E2 (PGE2), a major mediator of inflammation and angiogenesis. Herein, we report that macrophage migration inhibitory factor (MIF), a potent proinflammatory and growth-promoting factor found at elevated concentrations in the peritoneal fluid of women with endometriosis and active endometriosis lesions, acts directly on ectopic endometrial cells to stimulate the synthesis of COX-2, the inducible form of COX, and the release of PGE2. MIF treatment strongly activated p38 and ERK MAPK, and specific inhibitors of both pathways completely blocked basal and MIF-induced PGE2 synthesis. Whereas p38 inhibitors negatively affected the stimulated synthesis of COX-2 and that of PGE2, ERK inhibitors only decreased the production of PGE2. These findings show for the first time a direct role for MIF in the up-regulation of COX-2 synthesis and PGE2 secretion in ectopic endometrial cells. They further indicate that whereas p38 and ERK MAPK signaling pathways both play a significant role in the regulation of basal and MIF-induced synthesis of PGE2 by ectopic endometrial cells, only p38 kinase is involved in the regulation of COX-2 expression in these cells. This suggests that MIF acts at more than one level to stimulate the synthesis of PGE2 and triggers the coordinate activation of multiple enzymes in the biosynthesis pathway. Our data provide evidence for a novel mechanism by which MIF can induce a proinflammatory phenotype in ectopic endometrial cells, and favor the establishment of endometriosis and its related clinical symptoms.
Migration inhibitory factor distinctly acts via p38 and ERK MAPK to stimulate COX-2 expression and PGE2 secretion in ectopic endometrial cells, which may provide a novel mechanism underlying endometriosis development.
Endometriosis, one of the most common gynecological disorders affecting 10% of reproductive-aged women, is defined by the presence of endometrial tissue outside the uterus, mainly on the pelvic peritoneum. Various symptoms are associated with this disease, including chronic pelvic pain, dyspareunia, dysmenorrhea, and infertility, with an estimated prevalence of 30–50% in women with pain, infertility, or both (1).
Endometriosis is most probably a multifactorial disease, but available evidence points to a key role for immune dysfunctions in endometriosis pathogenesis and the manifestation of the disease’s clinical symptoms (1). For instance, and apart from numerous systemic immunological changes, patients with endometriosis show reduced natural killer and T-lymphocyte cytotoxicity in the peritoneal fluid, i.e. locally where the disease is frequently found, and an elevated number of activated macrophages (2). Instead of eliminating misplaced endometrial cells, these immune cells secrete numerous growth and angiogenic factors that act directly on endometrial cells to stimulate their implantation and proliferation (3,4). On the other hand, ectopic endometrial cells appear to resist immunosuppression and further activate the immune system, thereby exacerbating the local inflammatory reaction and favoring their own ectopic growth (5,6,7).
Prostaglandins (PGs) represent a family of lipid mediators, which also comprises leukotrienes and thromboxanes, and consist of four members, named prostaglandin D2 (PGD2) prostaglandin E2 (PGE2), prostaglandin F2α (PGF2α), and prostacyclin (8,9). In humans, PGs are deeply involved in reproductive functions such as ovulation, menstruation, fertilization, implantation, and parturition (10,11). They also appear to play a role in many endometrial pathologies, including menorrhagia, dysmenorrhea, and endometriosis (12). The biosynthesis of PGs is under the control of cyclooxygenase (COX), the first rate-limiting enzyme that converts free arachidonic acid into PGH2, the common precursor of PGs, which is further metabolized by a series of specific synthases to various PGs (13). Among the PGs, PGE2 is best known as a major regulator of the immune response, and plays an important role in chronic inflammatory diseases (14). Although studies have shown that PGE2 is implicated in the induction of inflammatory symptoms, such as edema, pain, and fever, there is growing evidence that it also exerts strong immunosuppressive effects, including shifting of the T-helper (Th) cell response from Th1 to Th2 cytokine production, and inhibition of leukocyte chemotaxis and phagocytosis (15,16,17).
Increased PG concentrations in the peritoneal fluid of infertile women with endometriosis have been reported (12). COX-2 expression was up-regulated in endometriotic tissue (18). Abnormal peritoneal levels of PGs may have adverse effects on spermatozoa, oocyte fertilization, implantation, and embryonic growth (12). Well known as a potent vasodilator, PGE2 shows angiogenic properties during implantation and endometrial stromal cell decidualization (19,20), and may, therefore, play a role in endometriosis-associated angiogenesis and further contribute to ectopic endometrial cell growth. Moreover, PGE2 appeared to stimulate the expression of aromatase, an essential enzyme in estrogen synthesis in ectopic endometrium, which may favor the ectopic implantation and growth of endometrial tissue (12,21).
Our previous studies showed a significant elevation of macrophage migration inhibitory factor (MIF) in the peritoneal fluid of women with endometriosis and a marked expression in active ectopic endometrial implants (22,23). Initially discovered as a product of activated T lymphocytes that inhibits the random migration of cultured macrophages, MIF is now known as a major regulator of the immune system (24). MIF showed various direct and indirect effects on tissue remodeling, proliferation, and angiogenesis as well, and appeared to play a critical role in the inflammatory reactions observed in many inflammatory disorders (24,25,26,27,28). Therefore, the purpose of the present study was to assess whether MIF may influence the expression of COX-1 and COX-2 and the production of PGE2 by ectopic endometrial cells, and elucidate possible underlying mechanisms.
Materials and Methods
Subjects and collection of tissue
Endometriotic biopsies used in this study (n = 11) were obtained from women who had given an informed consent for a research protocol approved by Saint-François d’Assise Hospital Ethics Committee on Human Research. These patients were between 29 and 36 yr old, consulted for infertility and/or pelvic pain, were found to have endometriosis during laparoscopy or laparotomy, had no endometrial hyperplasia or neoplasia, and had not received any antiinflammatory or hormonal medication during a period of at least 3 months before surgery. Endometriosis was staged according to the revised American Fertility Society classification system (Table 1). Endometriotic biopsies were immediately placed at 4 C in sterile Hanks’ Balanced Salt Solution (Sigma-Aldrich Corp., St. Louis, MO) containing 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin (Invitrogen Corp., Grand Island, NY) to be used for further studies.
Table 1.
Clinical characteristics of patients, sources of endometriotic tissue biopsies, and laparoscopic findings
| Patient no. | Age (yr) | Cycle phase | Stage of endometriosis | Location of endometriotic tissue |
|---|---|---|---|---|
| 1 | 17 | Unknown | II | Cul-de-sac and uterosacral ligament, deep lesions |
| 2 | 32 | Secretory | II | Ovary, brownish lesions |
| 3 | 30 | Unknown | II | Ovary and cul-de-sac |
| 4 | 36 | Proliferative | III | Uterosacral nodules |
| 5 | 33 | Secretory | II | Uterosacral brownish lesions and cul-de-sac superficial lesions |
| 6 | 27 | Secretory | II | Ovary and cul-de-sac |
| 7 | 32 | Proliferative | I | Cul-de-sac superficial lesion |
| 8 | 33 | Secretory | II | Cul-de-sac, ovarian fossae, superficial and deep red lesions |
| 9 | 32 | Secretory | III | Cul-de-sac superficial white, uterosacral ligament, ovarian fossae lesions |
| 10 | 31 | Proliferative | II | Cul-de-sac and ovarian fossae |
| 11 | 30 | Proliferative | II | Cul-de-sac blue typical lesions |
Immunohistofluorescence
Five-micrometer cryosections of endometrial tissue were fixed during 20 min in a 10% buffered formalin phosphate solution, treated at room temperature for 30 min with PBS containing 3% wt/vol BSA, 0.1% wt/vol saponin, and 0.1% Tween 20, and incubated during 1 h without (negative control) or with primary antibody diluted in PBS/BSA/saponin/Tween as follows: mouse monoclonal COX-2 antibody (1:20; catalog no. sc-19999; Santa Cruz Biotechnology, Inc., Santa Cruz, CA); mouse monoclonal CD74 antibody (1:50, catalog no. 555612; BD Biosciences, Mississauga, Ontario, Canada); rabbit polyclonal vimentin antibody (1:100, kindly provided by Dr. Normand Marceau, Laval University, Quebec, Canada); and chicken polyclonal cytokeratin-8 antibody (1:200, catalog no. 15-288-22752; GenWay Biotech, Inc., San Diego, CA). After rinsing with PBS containing 0.1% Tween, tissue sections were incubated 45 min with Alexa Fluor 568 goat-antimouse IgG antibody (1:1000; Molecular Probes, Inc., Eugene OR) for COX-2 and CD74, with Alexa Fluor 488 goat-antichicken for cytokeratin-8 (1:100; Invitrogen), and with a biotin-conjugated goat-antirabbit IgG antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) (1:2000 dilution in PBS containing 3% wt/vol BSA, 0.1% wt/vol saponin, and 0.1% Tween 20), followed by 45 min incubation with Alexa Fluor 488-streptavidin (1:100; Molecular Probes) for vimentin. 4′,6-Diaminido-2-phenyl-indole (DAPI) (1:2000 dilution in PBS/0.1% Tween 20) was used for counterstaining. The slides were then washed with PBS containing 0.1% Tween and mounted in Mowiol (Calbiochem, San Diego, CA) containing 10% of para-phenylenediamine (Sigma-Aldrich).
Cell culture and stimulation
Endometriotic stromal cells were isolated, characterized, and cultured in DMEM-Ham’s F-12 (HyClone, Logan, UT) containing 10% fetal bovine serum and 1% antibiotics (Invitrogen) (complete medium) according to a well-established protocol (29). At confluence, cells were starved for 16 h in DMEM-Ham’s F-12 and stimulated for several periods of time (0–24 h) with various concentrations of human recombinant MIF (0–50 ng/ml) (provided by C.N.M.), considering peritoneal MIF concentrations (22). In some cultures, cells were coincubated with 50 μmol/liter (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester (ISO-1), a specific inhibitor of MIF (30), or with NS-398 (50 μmol/liter) (Cayman Chemical Co., Ann Arbor, MI), a specific inhibitor of COX-2 activity. For signaling experiments, cells were first preincubated during 45 min with or without PD98059, (50 μmol/liter) (Biosource International, Camarillo, CA), a specific inhibitor of ERK MAPK pathway, or with or without SB203580 (50 μmol/liter) (Sigma-Aldrich), a specific inhibitor of p38-MAPK pathway. MIF was then added to the medium at different concentrations (0–50 ng/ml), and incubation continued for an additional 30 min, 12 h, or 24 h.
Transfection of small interfering RNA (siRNA)
Endometriotic stromal cells were cultured as previously described, and transiently transfected with a mixture of 33 nmol/ml CD74 siRNA or Silencer negative control no. 1 siRNA (Ambion Applied BioSystems, Foster City, CA) and Lipofectamine 2000 (Invitrogen) in Opti-MEM I Reduced Serum Medium (Invitrogen) for 48 h. Cells were then starved for 16 h in DMEM-Ham’s F-12 and stimulated with MIF (50 ng/ml) during 24 h.
Immunocytofluorescence
Endometriotic stromal cells were plated on chamber slides (BD Biosciences) and cultured in complete medium. Cells were then starved for 16 h before being stimulated for 24 h with MIF (0–50 ng/ml). Cells were then fixed for 20 min in formaldehyde (3.7% vol/vol in PBS), rinsed in PBS, treated for 90 min with PBS containing 3% wt/vol BSA and 0.1% wt/vol saponin, incubated for 1 h at 37 C with monoclonal mouse anti-COX-1, anti-COX-2, or anti-CD74 antibody (1:100, 1:75, or 1:50 dilution, respectively, in PBS containing 3% BSA, 0.1% saponin, and 0.05% Tween 20) (catalog nos. sc-19998 and sc-19999, respectively for COX-1 and COX-2; Santa Cruz Biotechnology; and catalog no. 555612 for CD74; BD Biosciences), rinsed with PBS/0.05% Tween 20, incubated with Alexa Fluor 568 goat-antimouse IgG antibody (1:1000 dilution in PBS containing 3% BSA, 0.1% saponin, and 0.05% Tween 20) during 1 h at 37 C, rinsed in PBS/0.05% Tween 20, and mounted in glycine medium containing 10% of para-phenylenediamine. Slides were observed by fluorescence microscopy (Model BX51; Olympus Corp., Tokyo, Japan). Cells incubated without the primary antibody or with isotype control mouse IgG were included as negative controls.
Real-time RT-PCR
Endometriotic stromal cells were cultured and stimulated with MIF as described earlier. Total RNA was extracted using the TRIzol reagent (Invitrogen) and reverse transcribed in the presence of random hexamers. Quantitative real-time PCRs were performed in an ABI 7000 Thermal Cycler (Applied Biosystems, Foster City, CA). Each standard PCR reaction contained 2 μl reverse transcriptase (RT) product, 0.5 μl of each primer (final concentration, 0.1 mm), 12.5 μl SYBR Green PCR Master Mix (Invitrogen) consisting of Taq DNA polymerase reaction buffer, deoxynucleotide triphosphate mix, SYBR green I, MgCl2, and Taq DNA polymerase. After a 95 C denaturation for 2 min, the reactions were cycled 40 times with a 95 C denaturation for 15 sec and a 60 C annealing for 60 sec. Primers for COX-1 (forward, 5′-GACCCGCCTCATCCTCATAG-3′; reverse, 5′-TTGGAACTGGACACCGAACA-3′; amplimer size, 124 bp), COX-2 (forward, 5′-TCCCTTGGGTGTCAAAGGTAA-3′; reverse, 5′-TCCCTTGGGTGTCAAAGGTAA-3′; amplimer size, 146 bp), CD74 (forward, 5′-GAGCTGTCGGGAAGATCAGA-3′; reverse, 5′-AGGAAGTAGGCGGTGGTG-3′; amplimer size, 192 bp), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward, 5′-TGATGACATCAAGAAGGTGGTGAAG-3′; reverse, 5′-TCCTTGGAGGCCATGTGGGCCAT-3′; amplimer size, 240 bp) were designed using Primer Express 2.0 (Applied Biosystems) to span intron-exon boundaries to avoid amplification of genomic DNA and selected to have compatible Tm values (59–61 C). Quantification of COX-1, COX-2, and CD74 mRNA was performed using a relative quantification method. For each experimental sample, COX-1, COX-2, and CD74 mRNA levels were normalized to GAPDH mRNA levels. After each run, melting curve analysis (55–95 C) was performed to verify the specificity of the PCR. All samples were tested in triplicate, and each run included no-template and no-RT controls.
Western blotting
For COX-1 and COX-2 expression, endometriotic stromal cell cultures were stimulated with MIF as described earlier, and cells were trypsinized and divided into two aliquots for protein extraction. One aliquot was processed using 0.5% Triton X-100, 10 mmol/liter HEPES, 150 mmol/liter NaCl, 2 mmol/liter ethylene glycol-bis (2-aminoethylether-N,N,N′,N′-tetraacetic acid, 2 mmol/liter ethylenediamine tetra-acetic acid, 0.02% NaN3, 1 mmol/liter phenylmethylsulfonylfluoride, and protease inhibitors to analyze COX-1 protein expression, and the second aliquot was processed using a lysis buffer containing 10 mmol/liter Tris-HCl, 1% sodium dodecyl sulfate, 1 mmol/liter phenylmethylsulfonylfluoride, and 1 mmol/liter dithiothreitol to analyze COX-2 expression. Equal amounts of proteins (10 μg) were separated by electrophoresis on a 10% polyacrylamide gel under denaturing conditions and were electro-transferred onto nitrocellulose membranes (Pall Corp., Pensacola FL). Membranes were incubated for 1 h with 5% dry nonfat skimmed milk powder in Tris-buffered saline [20 mm Tris base and 150 mm NaCl (pH 7.4)] containing 0.1% Tween 20 (TBS-Tween) (Sigma-Aldrich). After washes in TBS-Tween, membranes were incubated for 1 h with primary monoclonal anti COX-1 or COX-2 antibody in TBS-Tween, washed in TBS-Tween, and incubated with horseradish peroxidase-conjugated goat antimouse IgG (catalog no. 115–035-146; Jackson ImmunoResearch Laboratories). Detection was made using a chemiluminescence detection kit (ECL; Amersham Bioscience Corp., Baie d’Urfé, Quebec, Canada) following the manufacturer’s instructions. To normalize the expression of COX-1 and COX-2, the same membranes were blotted with a monoclonal α-tubulin antibody (Sigma-Aldrich).
The phosphorylation level of ERK-1/2 and p38 MAPKs was assessed using antibodies specific to the phosphorylated or the total forms of ERK-1/2 and p38 MAPKs. After stimulation with MIF as described earlier, proteins extracted using 0.5% Triton X-100, 10 mmol/liter HEPES, 150 mmol/liter NaCl, 2 mmol/liter ethylene glycol-bis (2-aminoethylether-N,N,N′,N′-tetraacetic acid, 2 mmol/liter ethylenediamine tetra-acetic acid, 0.02% NaN3, 1 mmol/liter phenylmethylsulfonylfluoride, and protease inhibitors were separated by electrophoresis on a 10% polyacrylamide gel with denaturing conditions and were electro-transferred onto nitrocellulose membranes. For ERK-1/2 or p38, membranes were first blocked 1 h with 5% dry nonfat skimmed milk powder in TBS-Tween and then incubated overnight at 4 C with mouse monoclonal antiphospho-p44/p42 (ERK-1/2) MAPK (catalog no. 9106; Cell Signaling Technology, Inc., Danvers, MA) or with rabbit polyclonal antiphospho-p38 antibody (catalog no. 9211; Cell Signaling Technology) in blocking buffer. After washes in TBS-Tween, membranes were incubated with horseradish peroxidase-conjugated goat antimouse IgG or with horseradish peroxidase-conjugated goat antirabbit IgG (catalog no. 111–035-144; Jackson ImmunoResearch Laboratories) in blocking buffer during 45 min for ERK-1/2 and p38, respectively, followed by washes in TBS-Tween and chemiluminescent detection. Membranes were then stripped, reprobed with rabbit polyclonal anti-p44/p42 MAPK antibody (catalog no. 9102; Cell Signaling Technology) in TBS-Tween containing 5% BSA for ERK-1/2, or with rabbit polyclonal anti-p38 MAPK antibody (catalog no. sc-728; Santa Cruz Biotechnology) for p38, and incubated for 45 min with horseradish peroxidase-conjugated goat-antirabbit IgG in blocking buffer before detection. The ratio of COX-1 or COX-2 signal to that of α-tubulin, phospho-p44/p42 signal to that of p44/p42, and phospho-p38 signal to that of p38 was determined, and data were expressed as percentage of control (0 ng/ml MIF). COX-1, COX-2, phospho-p44/p42, p44/p42, phospho-p38, p38, and α-tubulin proteins were quantified by densitometric analysis using Quantity One The Discovery series software (Bio-Rad Laboratories, Inc., Hercules, CA).
PGE2 assay
PGE2 concentration in cell culture supernatants was measured using a PGE2 Colorimetric Enzyme Immunoassay (EIA) kit (Assay Designs Inc., Ann Arbor, MI) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using one-way ANOVA, followed by the Bonferroni’s multiple comparison test (Prism 3.0; GraphPad Software Inc., San Diego, CA). Differences were considered as significant for P values less than 0.05.
Results
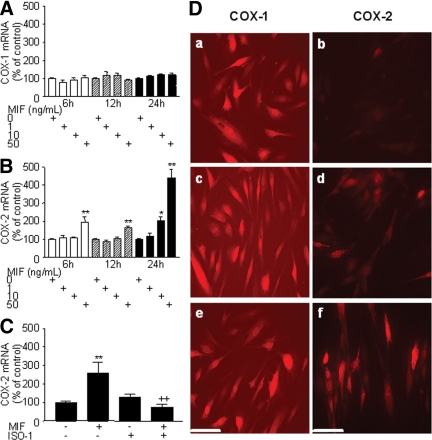
Dual-immunofluorescence experiments showed that COX-2, the inducible form of COX, was expressed throughout endometriotic tissue, either in vimentin-positive stromal or cytokeratin-positive epithelial cells (Fig. 1). In the present study, endometriotic stromal cells were used to investigate in vitro in cell culture the effects of MIF on COX-2 expression and PGE2 secretion. Our data showed that MIF had no statistically significant effect on the expression of COX-1, the constitutive form of COX, as first evaluated by quantitative real-time PCR analysis of mRNA steady-state levels (n = 4) (Fig. 2A). However, MIF appeared to up-regulate COX-2 mRNA levels in a time- and dose-dependent manner. A statistically significant up-regulation of COX-2 mRNA levels was observed in cells exposed to 50 ng/ml MIF for 6 (P < 0.001) and 12 h (P < 0.001), and to 10 and 50 ng/ml MIF for 24 h (P < 0.05 and P < 0.001, respectively) (n = 4) (Fig. 2 B). Interestingly, blockade of MIF with its specific inhibitor ISO-1 (50 μmol/liter; optimal predetermined concentration that efficiently inhibits MIF without damaging cells) significantly inhibited the MIF-induced expression of COX-2 during 6 h treatment (P < 0.001, n = 3) (Fig. 2C).
Figure 1.
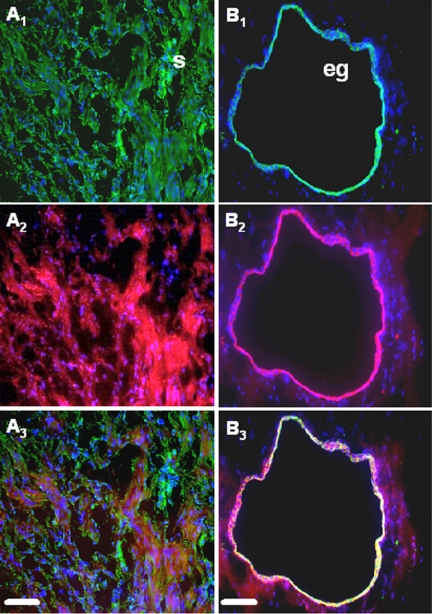
Simultaneous immunofluorescent staining of COX-2, vimentin, and cytokeratin-8 in ectopic endometrial tissue. COX-2, vimentin, and cytokeratin-8 were immunostained as described in Materials and Methods, and DAPI (blue) was used for counterstaining. Note the green fluorescence corresponding to vimentin (A1) or cytokeratin-8 (B1) and the red fluorescence corresponding to COX-2 (A2 and B2). Superposition of the green and red signals shows simultaneous immunostaining between COX-2 and vimentin (A3) and COX-2 and cytokeratin-8 (B3). Scale bar, 50 μm. eg, Epithelial gland; s, stroma.
Figure 2.
Analysis of COX-1 and COX-2 mRNA expression by quantitative real-time PCR and immunocytofluorescence. Cells were treated with 0, 1, 10, or 50 ng/ml MIF during 6, 12, or 24 h. After total RNA extraction and RT, COX-1 and COX-2 mRNAs were quantified using GAPDH as an internal control. MIF did not affect COX-1 mRNA expression in human endometriotic cells (A) but increased COX-2 mRNA expression in a dose-dependent manner (B). Data are from four different endometriotic cell cultures for COX-1 (patient nos. 2, 3, 6, and 7, Table 1) and from four different endometriotic cell cultures for COX-2 (patient nos. 2, 3, 7, and 10, Table 1). Cells were treated in parallel with 0 or 50 ng/ml MIF in the absence or presence of 50 μmol/liter ISO-1, a specific MIF inhibitor, during 6 h. C, Note the significant inhibition of MIF-induced COX-2 mRNA expression after MIF blockade by ISO-1. Data are from three different endometriotic cell cultures (patient nos. 2, 3, and 6, Table 1). *, P < 0.05; and **, P < 0.01 are significantly different from the control medium, which was devoid of stimuli (0 ng/ml MIF). ++, P < 0.001 is significantly different from cells incubated with the same concentration of MIF, using ANOVA and the Bonferroni’s multiple comparison test post hoc. D, For immunocytofluorescence, cells were incubated for 24 h in the culture medium without stimuli (a and b), or with 10 ng/ml (c and d) and 50 ng/ml (e and f) MIF. COX-1 and COX-2 were detected using specific monoclonal mouse antibodies. COX-1 immunostaining was detectable in cells incubated with the culture medium alone (a), and no difference with MIF-treated cultures (c and e) could be seen. Note the weak COX-2 immunostaining in endometriotic cells that were incubated with the culture medium alone (b) and the marked increase in the intensity of staining in the presence of MIF (d and f). Data are from patient no. 9 and representative of cultures from three different patients (nos. 3, 4, and 9, Table 1). Scale bar, 50 μm.
The expression of COX-1 and COX-2 in endometriotic cell cultures in response to MIF was then determined by immunocytofluorescence. As shown in Fig. 2D, COX-1 immunostaining was intense in endometriotic cells that were incubated in the basal serum-free culture medium alone, and did not show any noticeable change in cells exposed to MIF (10 and 50 ng/ml). In contrast, COX-2 immunostaining was rather weak in the absence of stimulation but markedly increased in the presence of MIF (10 and 50 ng/ml). No immunostaining was detected in cells incubated with PBS instead of the primary anti-COX-1 or anti-COX-2 antibody (data not shown).
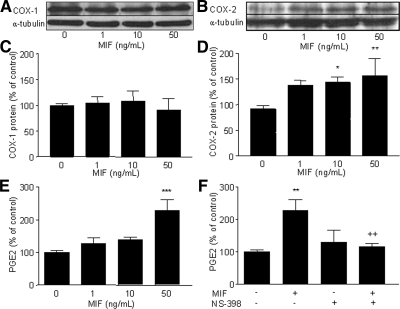
Western blot analysis corroborated the immunocytofluorescence data because MIF had no perceptible effect on COX-1 protein expression in endometriotic cells (Fig. 3A) but appeared to up-regulate COX-2 protein expression in a dose-dependent manner (Fig. 3B). Densitometric analysis of COX-2 relative to α-tubulin bands showed a significant increase in cells stimulated with 10 and 50 ng/ml MIF compared with control nonstimulated cells (P < 0.05 and P < 0.01, respectively, n = 4) (Fig. 3D), whereas no significant change in COX-1 levels in response to MIF was noted (Fig. 3C) (n = 3).
Figure 3.
Western blot analysis of COX-1 and COX-2 expression in human endometriotic cells and detection of PGE2 secretion in response to MIF. After starving, cells were incubated 24 h with 0, 1, 10, or 50 ng/ml MIF. Protein extraction and Western blotting were performed as described in Materials and Methods. Western blotting of α-tubulin was performed on the same membranes to normalize COX protein expression. PGE2 concentration was measured by EIA. Representative Western blots of COX-1 (A) and COX-2 (B) are from patient nos. 3 and 1, respectively (Table 1). Densitometric analysis data are from three different endometriotic cell cultures for COX-1 (patient nos. 1, 3, and 4, Table 1) and from four different endometriotic cell cultures for COX-2 (patients 1, 3, 4, and 5, Table 1). COX-1 protein level did not vary significantly in endometriotic cells stimulated with MIF (C), whereas COX-2 protein level was increased in endometriotic cells in response to MIF (D). MIF significantly induced PGE2 secretion by endometriotic cells (E), and such an effect was inhibited by NS-398 (50 μmol/liter), a specific inhibitor of COX-2 activity (F); data are from four different endometriotic cell cultures (patient nos. 2–5, Table 1). *, P < 0.05; **, P < 0.01; and ***, P < 0.001 are significantly different from the control medium, which was devoid of stimuli (0 ng/ml MIF). ++, P < 0.01 is significantly different from cells incubated with the same concentration of MIF (50 ng/ml), using ANOVA and the Bonferroni’s multiple comparison test post hoc.
COXs are key enzymes in the biosynthesis of PGs, and PGE2 is believed to be implicated in endometriosis development and symptoms (12,31). Therefore, we further assessed whether the MIF-induced COX-2 expression in endometriotic cells leads to an elevated secretion of PGE2. Our data summarized in Fig. 3E showed that MIF induced the release of significant amounts of PGE2 at 50 ng/ml (P < 0.001, n = 4), and that NS-398, a specific inhibitor of COX-2 activity, completely reversed MIF’s effects and significantly inhibited MIF-induced secretion of PGE2 (P < 0.01, n = 4) (Fig. 3F). This indicates that MIF’s up-regulatory effect on PGE2 is mediated by COX-2.
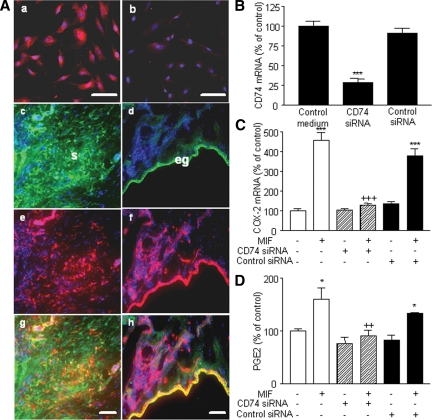
CD74 has been recently described as the putative receptor of MIF (32). Dual-immunofluorescent staining with vimentin or cytokeratin-8 showed the expression of CD74 in endometriotic stromal cell cultures (Fig. 4Aa), as well as in both stromal and epithelial cells in situ in ectopic endometrial tissue (Fig. 4A, c–h). To assess whether the exogenously added recombinant MIF acts via CD74 to induce COX-2 expression and PGE2 secretion in endometriotic stromal cells, CD74 expression in these cells was knocked down using specific CD74 siRNA. Only CD74 siRNA and not negative control siRNA significantly inhibited CD74 mRNA expression (P < 0.001, n = 3) (Fig. 4B). Moreover, knockdown of CD74 significantly inhibited MIF-induced mRNA expression of COX-2 (Fig. 4C) and PGE2 secretion (Fig. 4D) (P < 0.001 and P < 0.01, respectively, n = 3).
Figure 4.
Detection of CD74 in endometriotic cells and effect of CD74 knockdown on COX-2 expression and PGE2 secretion. CD74 was immunostained in endometriotic stromal cell culture (A) as described in Materials and Methods. CD74 was detectable in endometriotic cells using a specific monoclonal mouse antibody (a), whereas a weak immunostaining was observed in cells incubated with isotype control mouse IgG (b). A, CD74, vimentin, and cytokeratin-8 were immunostained in ectopic endometrial tissue as described in Materials and Methods, and DAPI (blue) was used for counterstaining. Note the green fluorescence corresponding to vimentin (c) or cytokeratin-8 (d) and the red fluorescence corresponding to CD74 (e and f). Superposition of the green and red signals shows simultaneous immunostaining between CD74 and vimentin (g) and CD74 and cytokeratin-8 (h); eg, Epithelial gland; s, stroma. Scale bar, 50 μm. Endometriotic stromal cells were transfected with 33 nm CD74 siRNA or negative control siRNA for 48 h and then incubated or not with 50 ng/ml MIF for 24 h. After total RNA extraction and RT, CD74 and COX-2 mRNA levels were quantified by quantitative real-time PCR, and PGE2 secretion was measured in the culture supernatants using EIA. CD74 siRNA significantly inhibited CD74 mRNA expression (B), and prevented MIF stimulation of COX-2 mRNA expression (C) and PGE2 secretion (D). Data are from three different endometriotic cells cultures (patient nos. 2, 8, and 11, Table 1). *, P < 0.05; ***, P < 0.001 vs. control medium using ANOVA and the Bonferroni’s multiple comparison test post hoc. ++, P < 0.01; +++, P < 0.001 are significantly different from cells incubated with the same concentration of MIF, using ANOVA and the Bonferroni’s multiple comparison test post hoc.
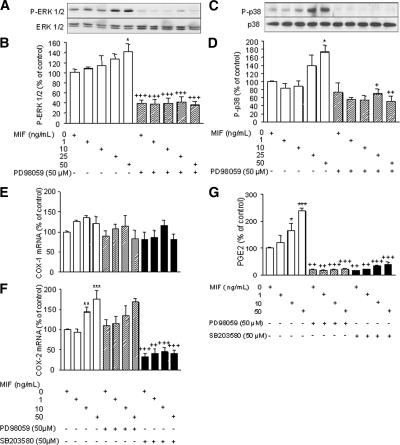
MAPKs are activated by diverse stimuli and can in turn phosphorylate numerous substrates to activate many signaling pathways (33,34). Therefore, we examined the effects of MIF on the phosphorylation of ERK-1/2 and p38 MAPKs by immunoblotting. Endometriotic cells were pretreated with or without PD98059, a specific inhibitor of the ERK MAPK pathway, or SB203580, a specific inhibitor of the p38-MAPK pathway, during 45 min and then stimulated with 0–50 ng/ml MIF for 30 min. Our data showed that MIF induced the phosphorylation of ERK-1/2 (p44/p42 MAPK) and p38 MAPKs in endometriotic cells (Fig. 5, A and C). Densitometric analysis of p44/p42 MAPK phosphorylation showed a significant increase in cells exposed to 50 ng/ml MIF compared with control nonstimulated cells (P < 0.05, n = 3). Furthermore, pretreatment with PD98059 considerably reduced the MIF-induced stimulation of ERK-1/2 MAPK phosphorylation (P < 0.001, n = 3) (Fig. 5B). Similarly, p38 MAPK phosphorylation was induced under MIF stimulation at 50 ng/ml (P < 0.05), and pretreatment with SB203580 completely inhibited the MIF-induced stimulation of p38 MAPK phosphorylation (P < 0.01) (n = 3) (Fig. 5D).
Figure 5.
ERK-1/2 and p38 MAPK phosphorylation in human endometriotic stromal cells in response to MIF, and effect of MAPK inhibition on COX-1 and COX-2 mRNA expression and PGE2 secretion. After starving, cells were preincubated for 45 min with or without PD98059 (50 μm), a specific inhibitor of ERK-1/2 MAPK, or SB203580 (50 μm), a specific inhibitor of p38 MAPK, before MIF addition at a final concentration of 0, 1, 10, 25, or 50 ng/ml. Thirty minutes later, protein extraction and Western blotting were performed as described in Materials and Methods. Representative Western blot of ERK-1/2 MAPK (A) and p38 MAPK (C) are from patient nos. 6 and 3, respectively (Table 1). Densitometric analysis data are from three different endometriotic cell cultures for ERK-1/2 MAPK (nos. 6–8, Table 1) and p38 MAPK (nos. 3, 4, and 10, Table 1). MIF increased ERK-1/2 MAPK (B) and p38 MAPK (D) phosphorylation, and pretreatment with PD98059 or SB203580 completely abolished MIF-induced activation of ERK-1/2 and p38 MAPKs, respectively. *, P < 0.05 vs. control medium. +, P < 0.05; ++, P < 0.01; and +++, P < 0.001 are significantly different from cells incubated with the same concentration of MIF, using ANOVA and the Bonferroni’s multiple comparison test post hoc. To evaluate the effect of MAPK inhibition on COX-1 and COX-2 mRNA expression and PGE2 secretion, endometriotic cells were preincubated for 45 min with or without PD98059 (50 μm) or SB203580 (50 μm) before incubation with MIF (0, 1, 10, or 50 ng/ml) for an additional 12 or 24 h. COX-1 and COX-2 mRNA levels were quantified by quantitative real-time PCR using GAPDH as an internal control, and PGE2 secretion was measured in the culture supernatants using EIA. Data are from three different endometriotic cell cultures (patient nos. 4, 8, and 10, Table 1). Whereas no statistically significant change in COX-1 mRNA expression was noted (E), MIF significantly induced COX-2 mRNA expression, and pretreatment with SB203580 but not PD98059 significantly reversed MIF stimulatory effects (F). G, MIF increased PGE2 secretion, and MIF-induced PGE2 liberation was completely abolished by pretreatment of PD98059 or SB203580. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 are significantly different from the control medium, which was devoid of stimuli (0 ng/ml MIF); +, P < 0.01; ++, P < 0.001 are significantly different from cells incubated with the same concentration of MIF, using ANOVA and the Bonferroni’s multiple comparison test post hoc.
The involvement of ERK-1/2 and p38 MAPK pathways in COX-1 and COX-2 expression and PGE2 secretion after MIF stimulation was then assessed. Endometriotic cells were pretreated with or without PD98059 or SB203580 during 45 min, MIF (0–50 ng/ml) was then added to cultures, and incubation continued during 12 or 24 h. Our data showed no statistically significant change in COX-1 mRNA levels in response to MIF (n = 3). Moreover, neither pretreatment with PD98059 nor with SB203580 had any effect on COX-1 mRNA expression (Fig. 5E). However, COX-2 mRNA expression was induced in cells exposed to 10 and 50 ng/ml MIF for 12 h (P < 0.01 and P < 0.001, respectively; n = 3) (Fig. 5F). In the presence of PD98059, the increased expression of COX-2 induced by 10 and 50 ng/ml MIF was no longer significant, but no statistically significant difference in COX-2 expression between cells pretreated or not with PD98059 was noted. However, addition of an inhibitor of p38 MAPK, SB203580, significantly abolished basal (P < 0.001) and MIF-induced COX-2 expression at 10 and 50 ng/ml (P < 0.001) (Fig. 5F). As shown in Fig. 5G, MIF significantly induced PGE2 secretion at 10 and 50 ng/ml (P < 0.05 and P < 0.001, respectively: n = 3). Moreover, pretreatment with PD98059 or SB203580 significantly reduced both basal (P < 0.01) and MIF-induced PGE2 secretion at 10 and 50 ng/ml (P < 0.001) (n = 3).
Discussion
In the present study, we provide evidence that MIF, a multifunctional growth-promoting and proinflammatory factor (24,25,26), exerted a direct significant stimulatory effect on COX-2 mRNA and protein expression in human ectopic endometrial cells, and had no noticeable regulatory effect on COX-1. Furthermore, ISO-1, a specific chemical inhibitor of MIF (30), completely blocked the MIF-induced expression of COX-2, thereby indicating a specific effect of the cytokine. Further analyses showed that endometriotic cell exposure to MIF resulted in a significant increase in PGE2 secretion. Preincubation of endometriotic cells with NS-398, a specific inhibitor of COX-2 activity, significantly inhibited MIF-induced PGE2 secretion, thereby linking COX-2 expression with the production of the eicosanoid. It is interesting to note that MIF appeared to act via its CD74 receptor (32), which, according to our present data, is expressed in these cells in vitro as well as in situ in endometriotic tissue. Actually, knockdown of CD74 in endometriotic cells using specific MIF siRNA markedly inhibited the MIF-induced COX-2 expression and PGE2 secretion.
To elucidate further the mechanisms by which MIF up-regulates COX-2 expression and PGE2 secretion by ectopic endometrial cells, we assessed the role of MAPK pathways. Phosphorylation of MAPK leads to expression of target genes important in inflammation and proliferation. The ERK MAPK cascade appears in general to mediate signals promoting cell proliferation, differentiation, or survival, whereas p38 MAPK often seems to be involved in cell responses to stresses and cytokines (35).
Our study showed for the first time that MIF activates ERK and p38 MAPKs in ectopic endometrial cells. Specific inhibition of p38 and ERK MAPK activation markedly inhibited basal and MIF-induced secretion of PGE2 in these cells. Inhibition of p38 led to a significant down-regulation of basal and MIF-induced COX-2 expression as well. However, inhibition of ERK MAPK had minor and nonstatistically significant inhibitory effects on MIF-induced COX-2 expression. These findings indicate that p38 and ERK MAPK signaling pathways are both required for the ultimate release of basal and MIF-induced PGE2 by ectopic endometrial cells, whereas ERK MAPK is less involved than p38 in mediating the induction of COX-2 by MIF. They further suggest that MIF may act at different levels to induce PGE2 synthesis in human endometriotic cells, which requires the coordinate induction of multiple enzymes in the biosynthesis pathway. Activation of the expression of COX-2, the rate-limiting enzyme for PGE2 production, is likely one of the underlying mechanisms and appears to be under the control of p38. However, activation of downstream late-acting terminal enzyme PG synthases (PGESs) might represent another possible complementary mechanism, probably regulated by ERK1/2.
PGE2 synthesis is a complex process involving mobilization of arachidonic acid from membrane phospholipids by phospholipase A2 (PLA2), the conversion to PGH2 by COX-1 and COX-2, and the conversion to PGE2 by PGES, of which one cytosolic and two microsomal (mPGES-1 and mPGES-2) forms are currently described. Of these enzymes, COX-1, cytosolic PGES, and mPGES-2 are known as constitutively expressed, whereas COX-2 as well as mPGES-1 are inducible by inflammatory stimuli (36,37). Nothing is known about the regulation of PGES by MIF. An extensive literature has evolved suggesting a complex array of pathway utilization upstream from COX-2 gene activation in several cell types. Prominent among these are the p38 and ERK MAPKs (38). However, little is known about the signaling pathways involved in the regulation of PGES. Available literature indicates that cytokines such as IL-1 stimulate PGE2 by up-regulating mPGES or specifically mPGES-1, and point to a significant role for MAPK signaling cascades (39,40,41,42,43). p38 and ERK MAPK signaling pathways both appeared to play important roles in the induction of mPGES-1 (38), which makes possible mPGES up-regulation by MIF in ectopic endometrial cells.
Furthermore, research is underway to elucidate the possible involvement of PLA2 in the signaling cascade leading to the induction of PGE2 by MIF. This is all the more quite plausible because MIF activated PLA2 in synoviocytes (44). On the other hand, the available literature indicates that other signaling pathways such as Jun N-terminal kinase, activating protein-1, and nuclear factor-κ B pathways mediate PGE2 synthesis in various cell types and pathophysiological states (37,39). Activation of these pathways by MIF has previously been reported (45,46,47), but their involvement in mediating COX-2 and PGE2 stimulation by MIF in endometriotic cells needs further investigation.
These findings may have a considerable relevance for endometriosis pathophysiology.
Actually, recent data showed a significant increase in the level of p38 and ERK MAPK phosphorylation in vivo in ectopic endometrial implants (48,49), which, together with our present findings, suggest that MIF may contribute in vivo to the activation of these signaling pathways in the ectopic implantation site.
Originally described as a product of activated T lymphocytes that inhibit the migration of macrophages, MIF is now known for being a multifunctional factor with a wide spectrum of effects and cell targets. In addition to its potent proinflammatory and immunological functions, MIF plays an essential role in tumorigenesis by inhibiting apoptosis and stimulating cell proliferation, tissue remodeling, and angiogenesis (50,51,52,53,54). Previous studies from our laboratory found a marked expression of MIF in endometriotic lesions, particularly in active, highly vascularized, and early stage implants, and high levels of this factor in the peritoneal fluid of patients suffering from endometriosis (22,23). Therefore, our data showing that MIF strongly stimulates the production of PGE2 in ectopic endometrial cells via up-regulation of COX-2 and activates p38 and ERK MAPK provide evidence for a novel mechanism by which MIF can induce a proinflammatory and proliferative phenotype in endometriotic cells, and contribute to the pathogenesis of endometriosis and its major associated symptoms.
Angiogenesis, the growth of newly formed blood vessels, is of pivotal importance for the ectopic survival and growth of endometrial implants, and, consequently, for the development of the disease (55,56,57). Once viewed as the prototypical mediator of inflammation, PGE2 is now regarded as a promoter of neoplastic growth (58) and tumor angiogenesis (59,60). PGE2 exerts angiogenic properties during implantation and endometrial stromal cell decidualization (20). It appears to act either directly by increasing endometrial vascular permeability (20) and inducing endothelial cell proliferation, migration, and tube formation (61,62), or indirectly by stimulating vascular endothelial growth factor expression (63) and activating fibroblast growth factor receptor-1 (64). Our previous studies identified MIF as a potent mitogenic factor for endothelial cells released by ectopic endometrial cells (54). Further in vitro and in vivo studies from our and other laboratories revealed an important role for MIF in stimulating endothelial cell proliferation, and promoting tumor and embryonic growth-associated angiogenesis (53,65,66). Thus, the capacity of endometriotic cells to generate high levels of PGE2 after activation by MIF could impact substantially on immune responses occurring at the implantation site, and could direct the pattern of angiogenesis and tissue remodeling into the host tissue.
Endometriosis is considered as an estrogen-dependent disorder (1,21,67). Aromatase, the rate-limiting enzyme for the synthesis of estrogen, is aberrantly expressed in endometriotic implants (21). Aberrant expression of COX-2 and PGE2 secretion by ectopic endometriotic implants has been reported, although the underlying mechanism is not clearly understood (12,31). PGE2 was shown to induce aromatase expression and estrogen production in endometriotic lesions (68). Therefore, considering the marked up-regulation of MIF in the peritoneal fluid of endometriosis women and active ectopic endometrial implants, MIF may act in paracrine and autocrine manners to increase COX-2 expression and PGE2 release in endometriotic cells, and potentiate, therefore, the positive feedback loop between PGE2 and estrogen in endometriotic lesions. This may represent a new mechanism by which MIF may promote the ectopic growth of endometrial tissue.
COX-derived PGs are physiologically implicated in various female reproductive functions (8). PGE2 was involved in the regulation of human endometrial stromal cell decidualization and embryo implantation (69). However, increased peritoneal fluid PGs in endometriosis (70) have been related to ovulation interference and to increase in tubal motility because PGs are known for being potent mediators of smooth muscle contraction, such that the embryo may reach the uterine cavity at a suboptimum time for implantation (71). According to our present data, MIF may up-regulate PG levels in the peritoneal fluid of women with endometriosis by inducing COX-2 expression and PGE2 synthesis in endometriotic cells, and may consequently have deleterious effects on the reproductive process. This adds to our previous findings showing a particular increase of MIF concentrations in the peritoneal fluid and the eutopic endometrium of women with endometriosis who suffer from infertility and a direct negative impact of such high MIF levels on sperm capacitation (72). Furthermore, it may represent another mechanism by which increased levels of MIF may adversely affect endometriosis women fertility.
In conclusion, the present study is the first to demonstrate that MIF up-regulates COX-2 expression and PGE2 secretion in ectopic endometrial cells. The study further demonstrates that both ERK and p38 MAPK are activated by MIF in these cells and likely involved in MIF-induced PGE2 production. COX-2 appeared as a possible target of MIF’s action. However, mechanisms involving downstream activation of terminal PGES and/or upstream effects on PLA2 may likely be involved and required further investigations. In view of the wide spectrum of PGE2 inflammatory, growth-promoting and angiogenic properties and implication in various female reproductive functions, these data point to a new pathway by which MIF, whose levels are markedly elevated in ectopic endometriotic lesions, may participate in endometriosis establishment and associated infertility.
Acknowledgments
We thank Drs. Mathieu Leboeuf and Rodolphe Maheux for patient evaluation and providing endometriotic tissue samples, and Johanne Pelletier and Sylvia Pleau for technical assistance.
Footnotes
This work was supported by Canadian Institutes for Health Research (Grant MOP-77737).
Disclosure Summary: C.C. and P.H.N. have nothing to declare. C.N.M. has stock options in a company interested in macrophage migration inhibitory factor (Cytokine PharmaSciences, Inc.); she is an inventor on a patent related to macrophage migration inhibitory factor and received royalty payment. Y.A.-A. is an inventor on patents covering (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester and analogs. A.A. is an inventor on a patent related to macrophage migration inhibitory factor and its potential use for diagnosis of endometriosis, and received Canadian Institutes for Health Research grants related to macrophage migration inhibitory factor. A.A. is Chercheur National from the Fonds de la Recherche en Santé du Québec.
First Published Online March 12, 2009
Abbreviations: COX, Cyclooxygenase; DAPI, 4′,6-diaminido-2-phenyl-indole; EIA, enzyme immunoassay; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ISO-1, (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester; MIF, macrophage migration inhibitory factor; mPGES, microsomal enzyme prostaglandin synthase; PG, prostaglandin; PGE2, prostaglandin E2; PGES, enzyme prostaglandin synthase; PLA2, phospholipase A2; RT, reverse transcriptase; siRNA, small interfering RNA; TBS-Tween, 20 mm Tris base and 150 mm NaCl (pH 7.4) containing 0.1% Tween 20; Th, T-helper.
References
- Giudice LC, Kao LC 2004 Endometriosis. Lancet 364:1789–1799 [DOI] [PubMed] [Google Scholar]
- Siristatidis C, Nissotakis C, Chrelias C, Iacovidou H, Salamalekis E 2006 Immunological factors and their role in the genesis and development of endometriosis. J Obstet Gynaecol Res 32:162–170 [DOI] [PubMed] [Google Scholar]
- Agic A, Xu H, Finas D, Banz C, Diedrich K, Hornung D 2006 Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest 62:139–147 [DOI] [PubMed] [Google Scholar]
- Minici F, Tiberi F, Tropea A, Fiorella M, Orlando M, Gangale MF, Romani F, Catino S, Campo S, Lanzone A, Apa R 2007 Paracrine regulation of endometriotic tissue. Gynecol Endocrinol 23:574–580 [DOI] [PubMed] [Google Scholar]
- Braun DP, Gebel H, Rana N, Dmowski WP 1998 Cytolysis of eutopic and ectopic endometrial cells by peripheral blood monocytes and peritoneal macrophages in women with endometriosis. Fertil Steril 69:1103–1108 [DOI] [PubMed] [Google Scholar]
- Dmowski WP, Gebel H, Braun DP 1998 Decreased apoptosis and sensitivity to macrophage mediated cytolysis of endometrial cells in endometriosis. Hum Reprod Update 4:696–701 [DOI] [PubMed] [Google Scholar]
- Ulukus M, Arici A 2005 Immunology of endometriosis. Minerva Ginecol 57:237–248 [PubMed] [Google Scholar]
- Wang H, Dey SK 2005 Lipid signaling in embryo implantation. Prostaglandins Other Lipid Mediat 77:84–102 [DOI] [PubMed] [Google Scholar]
- Achache H, Revel A 2006 Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update 12:731–746 [DOI] [PubMed] [Google Scholar]
- Poyser NL 1995 The control of prostaglandin production by the endometrium in relation to luteolysis and menstruation. Prostaglandins Leukot Essent Fatty Acids 53:147–195 [DOI] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK 1997 Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91:197–208 [DOI] [PubMed] [Google Scholar]
- Sales KJ, Jabbour HN 2003 Cyclooxygenase enzymes and prostaglandins in pathology of the endometrium. Reproduction 126:559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral GA 2005 Lipids as bioeffectors in the immune system. Life Sci 77:1699–1710 [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Narumiya S 2007 Prostaglandin receptor signaling in disease. ScientificWorldJournal 7:1329–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SB 2006 Prostaglandins in health and disease: an overview. Semin Arthritis Rheum 36:37–49 [DOI] [PubMed] [Google Scholar]
- Li W, Xu RJ, Zhang HH, Jiang LH 2006 Overexpression of cyclooxygenase-2 correlates with tumor angiogenesis in endometrial carcinoma. Int J Gynecol Cancer 16:1673–1678 [DOI] [PubMed] [Google Scholar]
- Harris HA 2007 Estrogen receptor-β: recent lessons from in vivo studies. Mol Endocrinol 21:1–13 [DOI] [PubMed] [Google Scholar]
- Ota H, Igarashi S, Sasaki M, Tanaka T 2001 Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod 16:561–566 [DOI] [PubMed] [Google Scholar]
- Chakraborty I, Das SK, Wang J, Dey SK 1996 Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J Mol Endocrinol 16:107–122 [DOI] [PubMed] [Google Scholar]
- Frank GR, Brar AK, Cedars MI, Handwerger S 1994 Prostaglandin E2 enhances human endometrial stromal cell differentiation. Endocrinology 134:258–263 [DOI] [PubMed] [Google Scholar]
- Attar E, Bulun SE 2006 Aromatase and other steroidogenic genes in endometriosis: translational aspects. Hum Reprod Update 12:49–56 [DOI] [PubMed] [Google Scholar]
- Kats R, Collette T, Metz CN, Akoum A 2002 Marked elevation of macrophage migration inhibitory factor in the peritoneal fluid of women with endometriosis. Fertil Steril 78:69–76 [DOI] [PubMed] [Google Scholar]
- Kats R, Metz CN, Akoum A 2002 Macrophage migration inhibitory factor is markedly expressed in active and early-stage endometriotic lesions. J Clin Endocrinol Metab 87:883–889 [DOI] [PubMed] [Google Scholar]
- Calandra T, Roger T 2003 Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol 3:791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javeed A, Zhao Y, Zhao Y 2008 Macrophage-migration inhibitory factor: role in inflammatory diseases and graft rejection. Inflamm Res 57:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub S, Hickey MJ, Morand EF 2008 Mechanisms of disease: macrophage migration inhibitory factor in SLE, RA and atherosclerosis. Nat Clin Pract Rheumatol 4:98–105 [DOI] [PubMed] [Google Scholar]
- Ohkawara T, Nishihira J, Takeda H, Asaka M, Sugiyama T 2005 Pathophysiological roles of macrophage migration inhibitory factor in gastrointestinal, hepatic, and pancreatic disorders. J Gastroenterol 40:117–122 [DOI] [PubMed] [Google Scholar]
- Metz CN, Bucala R 1997 Role of macrophage migration inhibitory factor in the regulation of the immune response. Adv Immunol 66:197–223 [DOI] [PubMed] [Google Scholar]
- Akoum A, Lemay A, Brunet C, Hébert J 1995 Cytokine-induced secretion of monocyte chemotactic protein-1 by human endometriotic cells in culture. The Groupe d’Investigation en Gynecologie. Am J Obstet Gynecol 172(2 Pt 1):594–600 [DOI] [PubMed] [Google Scholar]
- Al-Abed Y, Dabideen D, Aljabari B, Valster A, Messmer D, Ochani M, Tanovic M, Ochani K, Bacher M, Nicoletti F, Metz C, Pavlov VA, Miller EJ, Tracey KJ 2005 ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J Biol Chem 280:36541–36544 [DOI] [PubMed] [Google Scholar]
- Wu MH, Wang CA, Lin CC, Chen LC, Chang WC, Tsai SJ 2005 Distinct regulation of cyclooxygenase-2 by interleukin-1β in normal and endometriotic stromal cells. J Clin Endocrinol Metab 90:286–295 [DOI] [PubMed] [Google Scholar]
- Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R 2003 MIF signal transduction initiated by binding to CD74. J Exp Med 197:1467–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos LL, Lacey D, Yang Y, Leech M, Morand EF 2004 Activation of synovial cell p38 MAP kinase by macrophage migration inhibitory factor. J Rheumatol 31:1038–1043 [PubMed] [Google Scholar]
- Wu GS 2007 Role of mitogen-activated protein kinase phosphatases (MKPs) in cancer. Cancer Metastasis Rev 26:579–585 [DOI] [PubMed] [Google Scholar]
- Firestein GS, Manning AM 1999 Signal transduction and transcription factors in rheumatic disease. Arthritis Rheum 42:609–621 [DOI] [PubMed] [Google Scholar]
- Murakami M, Kudo I 2006 Prostaglandin E synthase: a novel drug target for inflammation and cancer. Curr Pharm Des 12:943–954 [DOI] [PubMed] [Google Scholar]
- Jüngel A, Distler O, Schulze-Horsel U, Huber LC, Ha HR, Simmen B, Kalden JR, Pisetsky DS, Gay S, Distler JH 2007 Microparticles stimulate the synthesis of prostaglandin E(2) via induction of cyclooxygenase 2 and microsomal prostaglandin E synthase 1. Arthritis Rheum 56:3564–3574 [DOI] [PubMed] [Google Scholar]
- Han R, Tsui S, Smith TJ 2002 Up-regulation of prostaglandin E2 synthesis by interleukin-1β in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and glutathione-dependent prostaglandin E2 synthase expression. J Biol Chem 277:16355–16364 [DOI] [PubMed] [Google Scholar]
- Degousee N, Angoulvant D, Fazel S, Stefanski E, Saha S, Iliescu K, Lindsay TF, Fish JE, Marsden PA, Li RK, Audoly LP, Jakobsson PJ, Rubin BB 2006 c-Jun N-terminal kinase-mediated stabilization of microsomal prostaglandin E2 synthase-1 mRNA regulates delayed microsomal prostaglandin E2 synthase-1 expression and prostaglandin E2 biosynthesis by cardiomyocytes. J Biol Chem 281:16443–16452 [DOI] [PubMed] [Google Scholar]
- Kojima F, Naraba H, Miyamoto S, Beppu M, Aoki H, Kawai S 2004 Membrane-associated prostaglandin E synthase-1 is upregulated by proinflammatory cytokines in chondrocytes from patients with osteoarthritis. Arthritis Res Ther 6:R355–R365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stichtenoth DO, Thorén S, Bian H, Peters-Golden M, Jakobsson PJ, Crofford LJ 2001 Microsomal prostaglandin E synthase is regulated by proinflammatory cytokines and glucocorticoids in primary rheumatoid synovial cells. J Immunol 167:469–474 [DOI] [PubMed] [Google Scholar]
- Pascual RM, Carr EM, Seeds MC, Guo M, Panettieri Jr RA, Peters SP, Penn RB 2006 Regulatory features of interleukin-1β-mediated prostaglandin E2 synthesis in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 290:L501–L508 [DOI] [PubMed] [Google Scholar]
- Bradbury DA, Corbett L, Knox AJ 2004 PI 3-kinase and MAP kinase regulate bradykinin induced prostaglandin E(2) release in human pulmonary artery by modulating COX-2 activity. FEBS Lett 560:30–34 [DOI] [PubMed] [Google Scholar]
- Sampey AV, Hall PH, Mitchell RA, Metz CN, Morand EF 2001 Regulation of synoviocyte phospholipase A2 and cyclooxygenase 2 by macrophage migration inhibitory factor. Arthritis Rheum 44:1273–1280 [DOI] [PubMed] [Google Scholar]
- Pakozdi A, Amin MA, Haas CS, Martinez RJ, Haines 3rd GK, Santos LL, Morand EF, David JR, Koch AE 2006 Macrophage migration inhibitory factor: a mediator of matrix metalloproteinase-2 production in rheumatoid arthritis. Arthritis Res Ther 8:R132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin MA, Haas CS, Zhu K, Mansfield PJ, Kim MJ, Lackowski NP, Koch AE 2006 Migration inhibitory factor up-regulates vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 via Src, PI3 kinase, and NFκB. Blood 107:2252–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh ML, Aeberli D, Lacey D, Yang Y, Santos LL, Clarkson M, Sharma L, Clyne C, Morand EF 2006 Regulation of IL-1 and TNF receptor expression and function by endogenous macrophage migration inhibitory factor. J Immunol 177:4818–4825 [DOI] [PubMed] [Google Scholar]
- Murk W, Atabekoglu CS, Cakmak H, Heper A, Ensari A, Kayisli UA, Arici A 2008 Extracellularly signal-regulated kinase activity in the human endometrium: possible roles in the pathogenesis of endometriosis. J Clin Endocrinol Metab 93:3532–3540 [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Hirota Y, Koga K, Hirata T, Harada M, Morimoto C, Yano T, Nishii O, Tsutsumi O, Taketani Y 2004 Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am J Reprod Immunol 52:306–311 [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R 2002 Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci USA 99:345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihira J, Ishibashi T, Fukushima T, Sun B, Sato Y, Todo S 2003 Macrophage migration inhibitory factor (MIF): its potential role in tumor growth and tumor-associated angiogenesis. Ann NY Acad Sci 995:171–182 [DOI] [PubMed] [Google Scholar]
- Arcuri F, Cintorino M, Vatti R, Carducci A, Liberatori S, Paulesu L 1999 Expression of macrophage migration inhibitory factor transcript and protein by first-trimester human trophoblasts. Biol Reprod 60:1299–1303 [DOI] [PubMed] [Google Scholar]
- Bondza PK, Metz CN, Akoum A 2008 Postgestational effects of macrophage migration inhibitory factor on embryonic implantation in mice. Fertil Steril 90(Suppl):1433–1443 [DOI] [PubMed] [Google Scholar]
- Yang Y, Degranpré P, Kharfi A, Akoum A 2000 Identification of macrophage migration inhibitory factor as a potent endothelial cell growth-promoting agent released by ectopic human endometrial cells. J Clin Endocrinol Metab 85:4721–4727 [DOI] [PubMed] [Google Scholar]
- May K, Becker CM 2008 Endometriosis and angiogenesis. Minerva Ginecol 60:245–254 [PubMed] [Google Scholar]
- Van Langendonckt A, Donnez J, Defrère S, Dunselman GA, Groothuis PG 2008 Antiangiogenic and vascular-disrupting agents in endometriosis: pitfalls and promises. Mol Hum Reprod 14:259–268 [DOI] [PubMed] [Google Scholar]
- Wiegerinck MA, Van Dop PA, Brosens IA 1993 The staging of peritoneal endometriosis by the type of active lesion in addition to the revised American Fertility Society classification. Fertil Steril 60:461–464 [DOI] [PubMed] [Google Scholar]
- Eisinger AL, Prescott SM, Jones DA, Stafforini DM 2007 The role of cyclooxygenase-2 and prostaglandins in colon cancer. Prostaglandins Other Lipid Mediat 82:147–154 [DOI] [PubMed] [Google Scholar]
- Wang MT, Honn KV, Nie D 2007 Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev 26:525–534 [DOI] [PubMed] [Google Scholar]
- Mehrotra S, Morimiya A, Agarwal B, Konger R, Badve S 2006 Microsomal prostaglandin E2 synthase-1 in breast cancer: a potential target for therapy. J Pathol 208:356–363 [DOI] [PubMed] [Google Scholar]
- Rao R, Redha R, Macias-Perez I, Su Y, Hao C, Zent R, Breyer MD, Pozzi A 2007 Prostaglandin E2-EP4 receptor promotes endothelial cell migration via ERK activation and angiogenesis in vivo. J Biol Chem 282:16959–16968 [DOI] [PubMed] [Google Scholar]
- Namkoong S, Lee SJ, Kim CK, Kim YM, Chung HT, Lee H, Han JA, Ha KS, Kwon YG, Kim YM 2005 Prostaglandin E2 stimulates angiogenesis by activating the nitric oxide/cGMP pathway in human umbilical vein endothelial cells. Exp Mol Med 37:588–600 [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Sales KJ, Smith OP, Battersby S, Boddy SC 2006 Prostaglandin receptors are mediators of vascular function in endometrial pathologies. Mol Cell Endocrinol 252:191–200 [DOI] [PubMed] [Google Scholar]
- Finetti F, Solito R, Morbidelli L, Giachetti A, Ziche M, Donnini S 2008 Prostaglandin E2 regulates angiogenesis via activation of fibroblast growth factor receptor-1. J Biol Chem 283:2139–2146 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Nishihira J, Sato Y, Kondo M, Takahashi N, Oshima T, Todo S 2000 An antibody for macrophage migration inhibitory factor suppresses tumour growth and inhibits tumour-associated angiogenesis. Cytokine 12:309–314 [DOI] [PubMed] [Google Scholar]
- Chesney J, Metz C, Bacher M, Peng T, Meinhardt A, Bucala R 1999 An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med 5:181–191 [PMC free article] [PubMed] [Google Scholar]
- Vercellini P, Frontino G, De Giorgi O, Pietropaolo G, Pasin R, Crosignani PG 2003 Endometriosis: preoperative and postoperative medical treatment. Obstet Gynecol Clin North Am 30:163–180 [DOI] [PubMed] [Google Scholar]
- Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE 1997 Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab 82:600–606 [DOI] [PubMed] [Google Scholar]
- Pierro E, Minici F, Alesiani O, Monica LD, Anna MM, Muncuso S, Lanzone A 2003 In vitro regulation of β1 and β3 integrin subunits in endometrial epithelial cells from normal endometrium. Am J Reprod Immunol 49:373–376 [DOI] [PubMed] [Google Scholar]
- Wu MH, Sun HS, Lin CC, Hsiao KY, Chuang PC, Pan HA, Tsai SJ 2002 Distinct mechanisms regulate cyclooxygenase-1 and -2 in peritoneal macrophages of women with and without endometriosis. Mol Hum Reprod 8:1103–1110 [DOI] [PubMed] [Google Scholar]
- Oral E, Olive DL, Arici A 1996 The peritoneal environment in endometriosis. Hum Reprod Update 2:385–398 [DOI] [PubMed] [Google Scholar]
- Carli C, Leclerc P, Metz CN, Akoum A 2007 Direct effect of macrophage migration inhibitory factor on sperm function: possible involvement in endometriosis-associated infertility. Fertil Steril 88:1240–1247 [DOI] [PubMed] [Google Scholar]