Substantial clinical and experimental data over the years have supported the conclusion that estradiol-17β is the ovarian hormone primarily responsible for growth and progression of breast cancer (1). Progesterone was assumed to have negligible effects on breast tumorigenesis, in part because of its well established role to inhibit the proliferative effect of estrogen in the epithelial compartments of the endometrium and promote differentiation of the uterine glands (2). However, much publicized observational and clinical studies have more recently implicated progesterone as a proliferative hormone in the normal human breast and as a life-long risk factor for breast cancer, suggesting progesterone also contributes to early stages of breast tumorigenesis (3,4,5,6,7,8,9). Paradoxically, in established breast cancers, the presence of progesterone receptor (PR) in the primary tumor is an independent marker of favorable prognosis and is associated with a more differentiated tumor phenotype, suggesting progesterone has a protective role against progression and invasion once a tumor has developed (10,11,12). Indeed, in most PR-positive breast cancer cell lines, progestins induce a transient proliferation followed by cell cycle arrest such that the long-term effect is growth inhibition (13). This apparent dichotomy suggests that progesterone signaling is altered during disease progression. Uncertainties concerning the role of progesterone in the normal human breast and tumorigenesis have been due in part to the absence of a suitable PR-positive progesterone-responsive experimental system for the normal human breast.
Because there are many common developmental and hormonal features in rodent and human mammary morphogenesis and tumorigenesis, the mouse has served as an important in vivo surrogate to model the normal human breast. The PR knockout mouse has clearly established a proliferative role for progesterone in the mammary epithelium (2,14). In the postpubertal nulliparous animal, progesterone stimulates ductal side branching and is required for the massive epithelial cell expansion and alveologenesis that occurs during early pregnancy (2,14,15). The PR knockout mouse has also underscored the critical role of PR-mediated signaling in mammary tumorigenesis (16,17), a finding supported by independent rodent mammary tumor models (18,19,20). Despite significant advancements in our understanding of the cellular and molecular principles that underlie progesterone action in the rodent mammary epithelium, translating these findings to the human has also been difficult without a suitable experimental system for the normal human breast.
In this issue of Endocrinology, Graham et al. (21) report on an improved basement membrane-embedded system for three-dimensional (3D) culture of primary normal human breast cells that retains estrogen receptor (ER) and PR expression and responsiveness to progesterone. This for the first time establishes an in vitro system for sex-steroid hormone action in the normal human breast. This builds on the pioneering work of Bissell and colleagues (22) and Debnath and Brugge (23), who showed that primary and functionally normal mammary epithelial cell lines, when grown in growth factor-reduced reconstituted basement membrane (Matrigel), form acini with a single layer of polarized epithelial cells and a hollowed out lumen that recapitulates lobuloalveolar structures of the normal breast. Furthermore, signals transmitted from components of the reconstituted basement membrane were found to be sufficient and required to maintain ER expression and responsiveness to estradiol-17β in 3D cultures of primary mouse mammary epithelial cells (22). By immunohistochemical analysis of human 3D breast acini, ER and PR were found to be heterogeneously coexpressed in luminal cells but not in myoepithelial cells. After progesterone administration, a subset of luminal epithelial cells were found to undergo proliferation, and the majority of the proliferating cells were PR negative, localized nearby PR-positive cells that were largely nonproliferating. This patterning of PR-positive and PR-negative epithelial cells recapitulates the rodent normal mammary gland and earlier immunohistochemical findings with human breast biopsy samples (24,25,26) (Fig. 1). Together these studies suggest an evolutionary conserved paracrine mechanism of progesterone regulation of mammary epithelial proliferation and that the 3D culture system is suitable to study paracrine mechanisms in the normal human breast.
Figure 1.
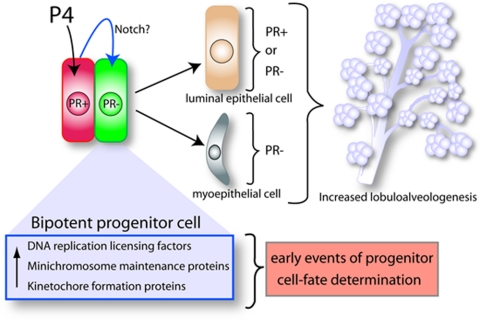
An emerging model for progesterone action in the human breast shares common features with a proposed paracrine mechanism of action model for progesterone in the rodent mammary gland (24,25,26). Based on findings of Graham et al., (21), the schematic outlines a hypothetical model to explain the role of progesterone (P4) signaling in the expansion and morphogenesis of the epithelial compartment in the human breast. A subset of mammary epithelial cells (red), which are PR positive, receive, transduce, and subsequently relay, in a paracrine fashion, the proliferative signal imparted by P4 hormone to a juxtacrine cell subgroup (green) that is PR negative. Gene profiling studies implicate the notch pathway as possibly one of a number of paracrine signals that project the proliferative effects of progesterone from PR-positive cells to a subset of PR-negative cells (green) that exhibit bipotent progenitor properties. Upon receiving paracrine signal inputs, the progenitor cell subtype reprograms and proliferates to generate both luminal and myoepithelial cell lineages. Luminal epithelial descendants can be positive or negative for PR expression, whereas myoepithelial cell are PR negative. Together, the expansion and differentiation of these cell lineages contribute to increased lobuloalveologenesis. Induction of DNA replication licensing factors, minichromosome maintenance proteins, and kinetochore formation proteins are possible early molecular changes that precede progesterone-induced progenitor cell reprogramming and subsequent proliferation.
Gene profiling of progesterone-induced genes in these 3D acini revealed an enrichment of targets associated with proliferation including the cell cycle and components of the DNA replication licensing machinery, minichromosome maintenance, and kinetochore formation. Interestingly, minimal overlap was observed with progesterone-induced gene targets in a breast cancer cell line grown as a 3D culture, suggesting the presence of distinct progesterone/PR signaling pathways in normal breast and breast cancer. Gene targets (Wnt-4, receptor of activated nuclear factor-κB ligand, and amphiregulin) known to be functionally important paracrine mediators of progesterone proliferation in the rodent mammary gland (27,28,29), however, were not affected by progesterone in human breast 3D cultures, suggesting either a species difference or that the microenvironment in vivo is required for progesterone regulation of these paracrine factors.
Using various immunohistochemical markers of progenitor cells and a mammosphere assay that selects for survival of progenitor cells as spheres in suspension, Graham et al. (21) further showed that a bipotent progenitor cell, which is capable of generating both luminal and myoepithelial descendants in these 3D cultures, is a likely cell type that receives the progesterone paracrine signal(s) to direct expansion of the mammary epithelial compartment (Fig. 1). Although previously suspected, these studies are the first to provide a functional link between progesterone exposure and mammary progenitor cell-fate determination (30). From molecular profiling, genes in the Notch signaling pathway were also identified as progesterone up-regulated targets, suggesting that Notch is a potential paracrine mediator of progesterone in the human breast epithelium. This proposal is significant because Notch signaling has been associated with control of tissue stem/progenitor cell fate decisions (31) and deregulation of Notch signaling has been suggested to underlie a number of epithelial cancers (32).
The progesterone-responsive human breast epithelial 3D culture system developed by Graham et al. (21) provides a promising new tool to address a number of important issues and questions. First is the influence of genetic alterations on individual and combined roles of ER- and PR-mediated signaling during the early stages of mammary epithelial neoplastic transformation and progression, including the role of the A and B isoforms of PR. Changes in normal 1:1 ratios of PR-A to PR-B have been observed to occur frequently in certain benign breast disease lesions and breast cancer, suggesting that a switch in PR isoform signaling could contribute to breast disease progression (33,34). Second is the identification of paracrine factors and mechanisms that mediate progesterone control of proliferation and how deregulation of this system contributes to transformation. A switch from a paracrine to an autocrine mode for ER/PR signaling in the human breast has been previously postulated to herald a breakdown in normal hormone responses that can lead to tumorigenesis (24,25,26). Also, these steroid signaling pathways have been predicted to influence cancer stem cell fate (30). Third is a comparison of the progesterone-induced target gene network, or transcriptome, in the normal human breast with that of the recently identified progesterone transcriptome in the normal mouse mammary gland (15) to disclose common and divergent pathways between these two species. The fourth issue is to determine whether cross talk occurs between progesterone and prolactin signaling pathways in the human breast as has been observed in the murine mammary gland (15,35). Prolactin has been shown to exert an important role in not only murine mammary morphogenesis but also breast cancer susceptibility (36). The final issue is to investigate the role of extranuclear actions of PR in mediating progestin-induced activation of cytoplasmic/cell membrane signal transduction pathways that has been studied so far almost exclusively in breast cancer cells and established immortalized cell lines (37,38). Are the signaling pathways and role of extranuclear PR the same or different in normal breast and cancer cells? Because breast cancer risk is associated with the cumulative exposure of the breast epithelium to ovarian steroids (1), the above studies promise to not only broaden our mechanistic understanding of the role of progesterone (and estrogens) in normal breast proliferation and cancer progression but also may well accelerate the formulation of more effective diagnostic, prognostic, and/or therapeutic approaches for hormone-dependent breast cancers.
Footnotes
This work was supported by National Institutes of Health Grants CA77530 (to J.P.L.) and HD038129, CA46938, and DK49030 (to D.P.E.).
Disclosure Summary: The authors have nothing to disclose.
For article see page 3318
Abbreviations: 3D, Three-dimensional; ER, estrogen receptor; PR, progesterone receptor.
References
- Pike MC, Spicer DV, Dahmoush L, Press MF 1993 Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev 15:17–35 [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Mukherjee A, Mulac-Jericevic B, Conneely OM, DeMayo FJ, Amato P, Lydon JP 2005 Revealing progesterone’s role in uterine and mammary gland biology: insights from the mouse. Semin Reprod Med 23:22–37 [DOI] [PubMed] [Google Scholar]
- Anderson E 2002 The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res 4:197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofseth LJ, Raafat AM, Osuch JR, Pathak DR, Slomski CA, Haslam SZ 1999 Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J Clin Endocrinol Metab 84:4559–4565 [DOI] [PubMed] [Google Scholar]
- Beral V 2003 Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362:419–427 [DOI] [PubMed] [Google Scholar]
- Lee S, Kolonel L, Wilkens L, Wan P, Henderson B, Pike M 2006 Postmenopausal hormone therapy and breast cancer risk: the multiethnic cohort. Int J Cancer 118:1285–1291 [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J 2002 Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Ross RK, Paganini-Hill A, Wan PC, Pike MC 2000 Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst 92:328–332 [DOI] [PubMed] [Google Scholar]
- Clarke CA, Glaser SL, Uratsu CS, Selby JV, Kushi LH, Herrinton LJ 2006 Recent declines in hormone therapy utilization and breast cancer incidence: clinical and population-based evidence. J Clin Oncol 24:e49–e50 [DOI] [PubMed] [Google Scholar]
- Bast Jr RC, Ravdin P, Hayes DF, Bates S, Fritsche Jr H, Jessup JM, Kemeny N, Locker GY, Mennel RG, Somerfield MR 2001 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 19:1865–1878 [DOI] [PubMed] [Google Scholar]
- Creighton CJ, Kent Osborne C, van de Vijver MJ, Foekens JA, Klijn JG, Horlings HM, Nuyten D, Wang Y, Zhang Y, Chamness GC, Hilsenbeck SG, Lee AV, Schiff R 2009 Molecular profiles of progesterone receptor loss in human breast tumors. Breast Cancer Res Treat 114:287–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Schiff R, Arpino G, Osborne CK, Lee AV 2005 Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol 23:7721–7735 [DOI] [PubMed] [Google Scholar]
- Sutherland RL, Prall OW, Watts CK, Musgrove EA 1998 Estrogen and progestin regulation of cell cycle progression. J Mamm Gland Biol Neoplasia 3:63–72 [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Mukherjee A, Creighton CJ, Buser AC, DeMayo FJ, Edwards DP, Lydon JP 2008 Transcriptional response of the murine mammary gland to acute progesterone exposure. Endocrinology 149:6236–6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton Jr RT, Lydon JP, Mehta RG, Mateo ET, Pletz A, Jordan VC 2002 Role of the progesterone receptor (PR) in susceptibility of mouse mammary gland to 7,12-dimethylbenz[a]anthracene-induced hormone-independent preneoplastic lesions in vitro. Cancer Lett 188:47–52 [DOI] [PubMed] [Google Scholar]
- Lydon JP, Ge G, Kittrell FS, Medina D, O'Malley BW 1999 Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res 59:4276–4284 [PubMed] [Google Scholar]
- Carnevale RP, Proietti CJ, Salatino M, Urtreger A, Peluffo G, Edwards DP, Boonyaratanakornkit V, Charreau EH, Bal de Kier Joffé E, Schillaci R, Elizalde PV 2007 Progestin effects on breast cancer cell proliferation, proteases activation, and in vivo development of metastatic phenotype all depend on progesterone receptor capacity to activate cytoplasmic signaling pathways. Mol Endocrinol 21:1335–1358 [DOI] [PubMed] [Google Scholar]
- Medina D, Kittrell FS, Shepard A, Contreras A, Rosen JM, Lydon J 2003 Hormone dependence in premalignant mammary progression. Cancer Res 63:1067–1072 [PubMed] [Google Scholar]
- Poole AJ, Li Y, Kim Y, Lin SC, Lee WH, Lee EY 2006 Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science 314:1467–1470 [DOI] [PubMed] [Google Scholar]
- Graham J, Mote P, Salagame U, van Dijk J, Balleine R, Huschtscha L, Reddel RR, Clarke C 2009 DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology 150:3318–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novaro V, Roskelley CD, Bissell MJ 2003 Collagen-IV and laminin-1 regulate estrogen receptor alpha expression and function in mouse mammary epithelial cells. J Cell Sci 116:2975–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Brugge JS 2005 Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev 5:675–688 [DOI] [PubMed] [Google Scholar]
- Clarke RB, Howell A, Potten CS, Anderson E 1997 Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 57:4987–4991 [PubMed] [Google Scholar]
- Rosen JM 2003 Hormone receptor patterning plays a critical role in normal lobuloalveolar development and breast cancer progression. Breast Dis 18:3–9 [DOI] [PubMed] [Google Scholar]
- Russo J, Ao X, Grill C, Russo IH 1999 Pattern of distribution of cells positive for estrogen receptor α and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat 53:217–227 [DOI] [PubMed] [Google Scholar]
- Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA 2000 Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev 14:650–654 [PMC free article] [PubMed] [Google Scholar]
- Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK 1995 Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol 9:691–705 [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Mukherjee A, Ying Y, Li J, Paquet M, Demayo FJ, Lydon JP, The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev Biol 328:127–139 [DOI] [PubMed] [Google Scholar]
- LaMarca HL, Rosen JM 2008 Minireview: hormones and mammary cell fate—what will I become when I grow up? Endocrinology 149:4317–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS 2004 Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res 6:R605–R615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H, Wicha MS 2009 HER-2, Notch, and breast cancer stem cells: targeting an axis of evil. Clin Cancer Res 15:1845–1847 [DOI] [PubMed] [Google Scholar]
- Hopp TA, Weiss HL, Hilsenbeck SG, Cui Y, Allred DC, Horwitz KB, Fuqua SA 2004 Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res 10:2751–2760 [DOI] [PubMed] [Google Scholar]
- Mote PA, Bartow S, Tran N, Clarke CL 2002 Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat 72:163–172 [DOI] [PubMed] [Google Scholar]
- Buser AC, Gass-Handel EK, Wyszomierski SL, Doppler W, Leonhardt SA, Schaack J, Rosen JM, Watkin H, Anderson SM, Edwards DP 2007 Progesterone receptor repression of prolactin/signal transducer and activator of transcription 5-mediated transcription of the β-casein gene in mammary epithelial cells. Mol Endocrinol 21:106–125 [DOI] [PubMed] [Google Scholar]
- Oakes SR, Rogers RL, Naylor MJ, Ormandy CJ 2008 Prolactin regulation of mammary gland development. J Mamm Gland Biol Neoplasia 13:13–28 [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP 2007 The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol 21:359–375 [DOI] [PubMed] [Google Scholar]
- Faivre EJ, Daniel AR, Hillard CJ, Lange CA 2008 Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol 22:823–837 [DOI] [PMC free article] [PubMed] [Google Scholar]